Researchers have shed new light on how tissues in the body are repaired following the damage and premature death of tissue cells.
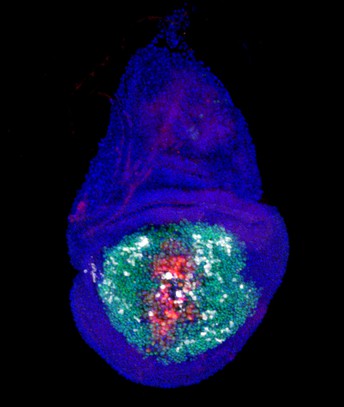
A necrotic Drosophila wing imaginal disc undergoing regeneration, showing NiA/NiCP (white) cells at a distance from the injury (green: GFP-labeled wing pouch cells, red: necrotic wound, white: caspase activity). Image credit: Robin Harris (CC BY 4.0)
Their study in fruit flies, which first appeared in eLife as a Reviewed Preprint and is now published as the final version, describes what the editors call fundamental discoveries with solid evidence for how dying (or necrotic) cells contribute to tissue regeneration through a previously uncharacterised mechanism. It suggests these cells play a role in signalling for the body to produce other types of cells that are involved in controlling natural cell death and inflammation, with findings that may have implications for wound repair and tissue regeneration.
As our bodies grow and develop, cells naturally die off where they are no longer needed, in a process called apoptosis. On the other hand, cells can be damaged and die prematurely due to injury, infectious diseases or other factors, in a process known as necrosis. In necrosis, the integrity of the cell membrane swells and is lost altogether, causing the cellular contents to be released into the body and resulting in a significant inflammatory response. As treatments for necrosis focus on invasive procedures that are often met with limited success, the researchers say it is crucial to better understand its effects.
“Little is known about necrosis and how it affects surrounding healthy tissue during wound healing, which is an essential consideration when developing effective methods to treat such injuries,” says corresponding author Robin Harris, Principal Investigator at Arizona State University, US. “Much of our understanding instead comes from models involving regulated forms of cell death like apoptosis. Our lab previously established a method to study necrosis-induced regeneration in the wing imaginal disc of Drosophila fruit flies, and revealed a phenomenon where cells at a distance from the site of injury signal for an increase in the activity of enzymes known as caspases that play essential roles in regulated cell death. This process, called Necrosis-induced Apoptosis, or NiA, is vital for regeneration, and we wanted to characterise it further in this study.”
To do this, Harris and colleagues conducted a series of studies in genetically modified lines of Drosophila melanogaster fruit flies. Their analyses involved looking at where the formation of NiA occurs, how it is regulated, and the role it plays in the tissue regeneration process following injury.
The Drosophila imaginal wing disc is a cluster of tissue cells in fly larvae that are a precursor to the wing. The cells comprise different identities reflecting the adult structures they ultimately create, including the pouch, hinge and notum. Previously, the team found that NiA occurs in the lateral pouch of the wing disc upon the start of necrosis in the distal pouch.
To gain more insights into formation and role of NiA in regeneration, they tested whether the occurrence of necrosis in different areas of the disc leads to NiA. Their analyses revealed a number of key findings. First, areas of the disc can be killed by necrosis and potentially release molecules called damage-associated molecular patterns (DAMPS) to trigger tissue repair. And secondly, NiA is limited to the pouch when local necrosis occurs, but may also be induced in the notum when multiple areas of the disc undergo necrosis. The ability to undergo NiA reflects the uneven regenerative capacity of the wing disc, with NiA occurring mainly in the highly regenerative wing pouch.
Next, the team explored how this positioning of NiA formation relates to its role in promoting regeneration. Their previous work showed that the appearance of NiA coincides with the localised production – or proliferation – of cells in the distal pouch at 18 hours following necrotic injury close to a wound, which persists at 24 hours following injury. However, their investigations at subsequent time points of recovery showed that regenerative proliferation continues to increase through 36 and 48 hours of regeneration, significantly later in the process than they previously observed. Using tools to trace the activity of caspases and cell death, they showed this is possible because a proportion of NiA survive caspase activation and persist late into the regeneration process.
“Unlike normal apoptotic cells that are rapidly cleared from the wing disc, NiA formations persist and increase in abundance when proliferation localises to the distal pouch, at 36, 48, and even up to 64 hours following injury, when regeneration is complete and the pouch tissue is restored,” says co-author Jacob Klemm, a former graduate student in the Harris Lab at Arizona State University, now postdoctoral researcher at Duke University, North Carolina, US. “We therefore named this population of persistent and potentially non-apoptotic NiA as Necrosis-induced Caspase Positive (NiCP) cells and investigated their role in promoting proliferation.”
Their studies revealed an essential role for the caspase, Dronc, in NiCP cells to promote this late proliferation and subsequent regeneration of the disc following necrosis. Dronc refers to Drosophila initiator caspase, a protein involved in apoptosis and other developmental processes, and which has previously been documented as promoting proliferation in a process called Apoptosis-induced Proliferation (AiP).
As AiP depends on the enzyme JNK and reactive oxygen species, which are not associated with NiA/NiCP, the team said it is possible that Dronc’s function in response to necrosis occurs via a distinct mechanism. Indeed, while Dronc’s activity in AiP is influenced by its regulator DIAP1, their analyses demonstrated a key role for Dronc in promoting regenerative proliferation following necrosis which is not affected by this regulator. Its role in regeneration is therefore likely separate from its identified contribution to apoptosis and the AiP mechanism.
“Altogether, our latest findings suggest a model in which necrotic injuries induce caspase activity in cells at a distance from an injury,” says co-author Chloe Van Hazel, Technician at the Harris Lab, Arizona State University. “Some of these cells undergo JNK-independent apoptosis (NiA), while others survive and promote proliferation and regeneration through a novel non-apoptotic function of Dronc in NiCP cells,”.
The team says that future research is now needed to understand how the phenomena they have identified lead to tissue regeneration following necrosis. “Our findings reinforce the idea that there is much more to be understood about the role of caspases in tissue repair,” Harris concludes. “For now, they reveal an important genetic response to cell death that could potentially be leveraged to augment the regeneration of necrotic wounds.”
Harris et al.’s previous study, ‘Necrosis-induced apoptosis promotes regeneration in Drosophila wing imaginal discs’ is available to access here.
Media contacts
Emily Packer
eLife
e.packer@elifesciences.org
+441223855373George Litchfield
eLife
g.litchfield@elifesciences.org
About
eLife transforms research communication to create a future where a diverse, global community of scientists and researchers produces open and trusted results for the benefit of all. Independent, not-for-profit and supported by funders, we improve the way science is practised and shared. In support of our goal, we introduced the eLife Model that ends the accept–reject decision after peer review. Instead, papers invited for review are published as Reviewed Preprints that contain public peer reviews and an eLife Assessment. We also continue to publish research that was accepted after peer review as part of our traditional process. eLife is supported by the Howard Hughes Medical Institute, Knut and Alice Wallenberg Foundation, the Max Planck Society and Wellcome. Learn more at https://elifesciences.org/about.
To read the latest Stem Cells and Regenerative Medicine research in eLife, visit https://elifesciences.org/subjects/stem-cells-regenerative-medicine.




