Teeth and hard structures called dermal odontodes are evolutionarily related, arising from the same developmental system, a new study published today in eLife shows.
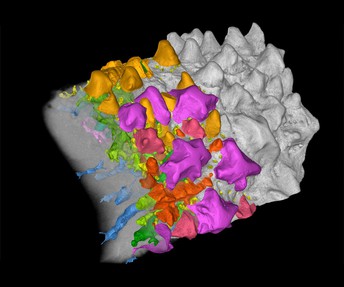
Part of a jawbone of the 422-million-year-old fossil bony fish Lophosteus, visualised with a high-resolution X-ray technique. On the right, the surface of the jawbone is shown in grey. In the middle, exposed teeth are highlighted in gold and dermal odontodes in shades of purple, pink and red. On the left, the bone itself is made transparent, revealing internal blood vessels and pulp cavities, shown in blue and green, as well as the embedded teeth and dermal odontodes. Image credit: Chen et al. (CC BY 4.0)
These findings in ancient fish fossils contradict established claims about the difference between the two structures based on modern sharks, and provide potential new insights into the origins and development of teeth.
Odontodes are hard structures made of dentine, the main substance in ivory, and are found on the outside surfaces of animals with backbones (vertebrates). Teeth are an example of odontodes but some animals also have them on their skin, such as the tooth-like ‘scales’ of sharks. These are known as dermal odontodes.
“Teeth and dermal odontodes are thought to have evolved separately because they seem to develop in different ways,” says lead author Donglei Chen, a researcher at the Department of Organismal Biology, Uppsala University, Sweden. “However, most of what we know is limited to modern sharks in which the difference between these structures has become very distinct. To understand the relationship between the two more clearly, we needed to turn to the fossil record.”
The team looked at fossils of one of the earliest bony fishes called Lophosteus which lived more than 400 million years ago. They chose this fish because it represents an early stage of tooth evolution, bringing them closer to the time when teeth and dermal odontodes could have separated in the hopes that any developmental similarities between the two would be more obvious.
The researchers used high-resolution X-ray imaging to look at the three-dimensional structure of odontodes in Lophosteus at different stages of development. They found that the appearance of odontodes were similar at the early stages of development but would change depending on whether they grew into the mouth or the face. This suggests there were different chemical signals in each area directing their development. At the later stages, some dermal odontodes would move from the face to the mouth and begin to look like teeth.
These findings suggest that both types of odontodes are able to respond to the same signals controlling each other’s development and are made by the same developmental system – not separate systems as previously thought.
“In addition to casting light on the early evolution of our own teeth, our results point to a previously unrecognised evolutionary-developmental relationship between teeth and dermal odontodes,” says senior author Per Ahlberg, PhD, Professor at the Department of Organismal Biology, Uppsala University. “This has potential implications for understanding the signalling that occurs during development and could inspire new lines of developmental research in other organisms.”
Media contacts
Emily Packer
eLife
e.packer@elifesciences.org
+441223855373
About
eLife is a non-profit organisation created by funders and led by researchers. Our mission is to accelerate discovery by operating a platform for research communication that encourages and recognises the most responsible behaviours. We work across three major areas: publishing, technology and research culture. We aim to publish work of the highest standards and importance in all areas of biology and medicine, including Evolutionary Biology, while exploring creative new ways to improve how research is assessed and published. We also invest in open-source technology innovation to modernise the infrastructure for science publishing and improve online tools for sharing, using and interacting with new results. eLife receives financial support and strategic guidance from the Howard Hughes Medical Institute, the Knut and Alice Wallenberg Foundation, the Max Planck Society and Wellcome. Learn more at https://elifesciences.org/about.
To read the latest Evolutionary Biology research published in eLife, visit https://elifesciences.org/subjects/evolutionary-biology.




