| Peer-reviewed | Experimental study | Animals |
Scientists have identified a mechanism that explains how fine air pollution particles might cause lung cancer, according to a study published today in eLife.
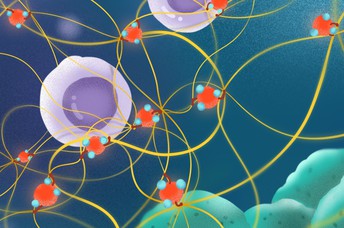
Inhaled fine particulate matter (shown here in red) pulls strings of collagen to disturb the immune defence in mice with lung cancer cells. This activity delays the movement of cytotoxic T-cells (purple) as they migrate towards the cancer cells (green) to destroy them. Image credit: Wang et al. (CC BY 4.0)
The findings could lead to new approaches for preventing or treating the initial lung changes that lead to the disease.
Tiny, inhalable fine particulate matter (FPM) found in air pollutants has been recognised as a Group 1 carcinogen and a substantial threat to global health. However, the cancer-causing mechanism of FPM remains unclear.
“Despite its potential to cause mutations, recent research suggests that FPM does not directly promote – and may even inhibit – the growth of lung cancer cells,” explains first author Zhenzhen Wang, an associate researcher at Nanjing University (NJU), Nanjing, China, who carried out the study between labs at NJU and the University of Macau where she was sponsored by a University of Macau Fellowship. “This suggests that FPM might lead to cancer through indirect means that support tumour growth. For example, some studies suggest FPM can prevent immune cells from moving to where they are needed.”
To explore this possibility, Wang and the team collected FPM from seven locations in China and studied its effects on the main immune cells that defend against tumour growth – called cytotoxic T-cells (CTLs). In mice administered with lung cancer cells that were not exposed to FPM, CTLs were recruited to the lung to destroy the tumour cells. By contrast, in the mice whose lungs were exposed to FPM, the infiltration of CTLs was delayed – potentially allowing the tumour cells to establish in lung tissue.
To investigate why the CTLs did not enter the lung as quickly in the FPM-exposed lungs, the team studied both the CTLs themselves and the lung tissue structure. They found that CTLs exposed to FPM still retained their migratory ability, but that FPM exposure dramatically compressed the lung tissue structure and the spaces that immune cells move between. There were also much higher levels of collagen – a protein that provides biomechanical support for cells and tissues. When the team studied the movement of CTLs in the mice, in lung tissue exposed to FPM, CTLs struggled to move, whereas those in the untreated tissue were able to move freely.
Further analysis of the tissue showed that the structural changes were caused by increases in a collagen subtype called collagen IV, but the team still did not know how FPM triggered this. They found the answer when they looked more closely at the structural changes to collagen IV and the enzyme responsible for making them – called peroxidasin. This enzyme drives a specific type of cross-linking that exposure to FPM was found to cause and aggravate in the lung tissue.
“The most surprising find was the mechanism by which this process occurred,” Wang says. “The peroxidasin enzyme stuck to the FPM in the lung, which increased its activity. Taken together, this means that wherever FPM lands in the lung, increased peroxidasin activity leads to structural changes in the lung tissue that can keep immune cells out and away from growing tumour cells.”
“Our study reveals a completely new mechanism by which inhaled fine particles promote lung tumour development,” concludes senior author Lei Dong, Professor at the School of Life Sciences, Nanjing University. “We provide direct evidence that proteins that stick to fine particulate matter can cause a significant and adverse effect, giving rise to pathogenic activity. Our discovery that peroxidasin is the mediator of this effect in lung tissue identifies it as a specific and unexpected target for preventing lung disease caused by air pollution.”
Media contacts
Emily Packer
eLife
e.packer@elifesciences.org
+441223855373
About
eLife transforms research communication to create a future where a diverse, global community of scientists and researchers produces open and trusted results for the benefit of all. Independent, not-for-profit and supported by funders, we improve the way science is practised and shared. From the research we publish, to the tools we build, to the people we work with, we’ve earned a reputation for quality, integrity and the flexibility to bring about real change. eLife receives financial support and strategic guidance from the Howard Hughes Medical Institute, Knut and Alice Wallenberg Foundation, the Max Planck Society and Wellcome. Learn more at https://elifesciences.org/about.
To read the latest Cancer Biology research published in eLife, visit https://elifesciences.org/subjects/cancer-biology.




