Generalizable knowledge outweighs incidental details in prefrontal ensemble code over time
Figures
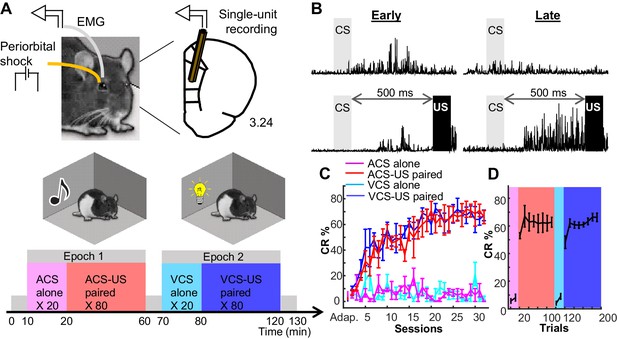
Rats formed two associative memories that differed in incidental, physical features.
(A) Rats were implanted with two eyelid wires to deliver mild electrical current (US) and record anticipatory blinking activity (CRs). Single neural activity was collected from the prelimbic region of the mPFC from the first day of conditioning. Conditioning took place in two chambers, in which either a tone (ACS) or light (VCS) was presented alone (CS alone, 20 trials) or preceding the US by 500 ms (CS-US, 80 trials). (B) Averaged EMG amplitude during Early (left) and Late learning (right) during CS-alone trials (top) and CS-US paired trials (bottom). (C) CR expression (CR%; ±SEM) increased over days for both ACS-US (red) and VCS-US (blue) conditions, but not during the respective CS-alone conditions (magenta, turquoise). (D) Within sessions in which rats reached asymptotic responding, CR% showed an abrupt transition upon the shift from the block of CS-alone trials (magenta; turquoise) to the block of CS-US paired trials (red; blue).
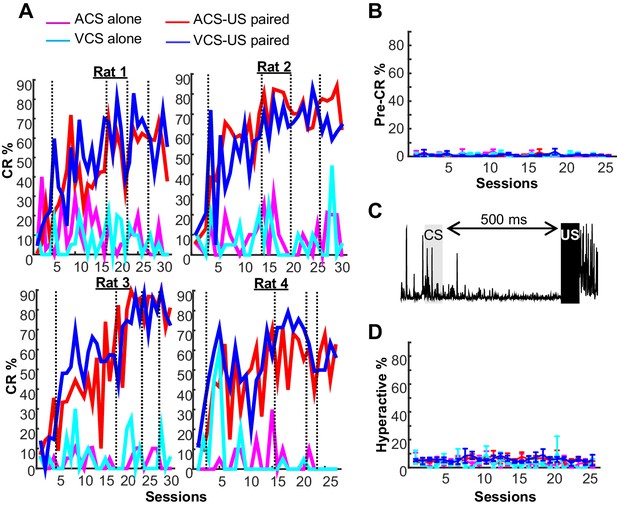
Behavioral performance in two blocks of trace eyeblink conditioning.
(A) In all four rats, trials with a conditioned response (CR%) gradually increased during two conditions with CS-US pairings (ACS-US paired, red; VCS-US paired, blue), but not during two conditions with the CS alone presentation (ACS alone, magenta; VCS alone, turquois). Based on CR% and days since asymptotic responding, sessions were divided into five successive stages (vertical lines). (B) In a few trials, EMG amplitude during a period before CS onset significantly changed from baseline. The proportion of these trials (mean ± SEM, n = 4 rats) did not increase across sessions or differ across four conditions. See (A) for the definition of line color. (C) In some trials, the rats engaged in an activity that greatly increased EMG amplitude before CS onset. (D) The frequency of these ‘hyperactive’ trials did not change across sessions or differ between four conditions (mean ± SEM, n = 4 rats). See (A) for the definition of line color.
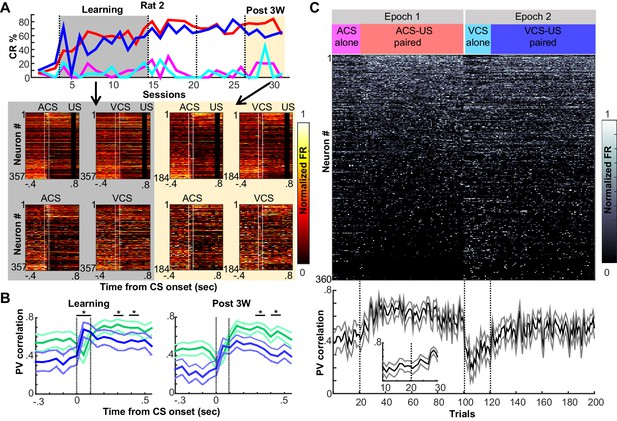
Prefrontal ensemble codes in well-trained rats were selective for relational features.
(A) Example behavior from a single rat. Based on the percentage of trials exhibiting a conditioned response (CR%) and days since asymptotic responding, sessions were divided into five successive stages (vertical lines). Representative pseudocolor plots show normalized firing rate of neurons recorded from the same rat during the learning period (gray) and during the third week after learning (yellow). Top left, ACS-US paired; top right, VCS-US paired; bottom left, ACS alone; bottom right, VCS alone. Neurons were sorted based on the ACS-induced firing rate change during ACS-US pairings from the largest increase (Neuron #1) to the largest decrease (Neuron #357 or 184). During learning, ensemble activity during ACS-US pairings was similar to that during ACS alone presentations and VCS-US pairings; however, during the third post-learning week, it became more similar to that during VCS-US pairings than ACS alone presentations. White lines indicate CS onset and offset, black bars mask US artifacts. (B) During learning (left), binned firing rates of neuron ensembles (PV) during the CS (two vertical lines) were more similar for two conditions with the same modality of CS (ACS-US paired and ACS alone, blue, r ± 95% confidence intervals) than two conditions with the same stimulus relationships (ACS-US paired and VCS-US paired, green). Their similarity became comparable between two condition pairs during subsequent CS-US intervals. During the third post-learning week (right), PV became more similar for two conditions with the shared stimulus relationship than those with the shared CS modality. * indicates p<0.05/10 in random permutation tests. (C) Trial-by-trial display of PVs during CS-US intervals in the third post-learning week. Neurons from four rats were sorted based on the firing rate during ACS-US paired trials from the highest (Neuron #1) to the lowest (Neuron #360)]. A ‘template’ PV was constructed as averaged PVs across 10–80th ACS-US paired trials. The correlation coefficient between the template and PV in each trial (±95% confidence interval) rapidly increased within the first ten ACS-US paired trials (inset). Upon the transition from the epoch with the ACS to the next epoch with the VCS, it abruptly decreased but increased again when the VCS-US pairings began.
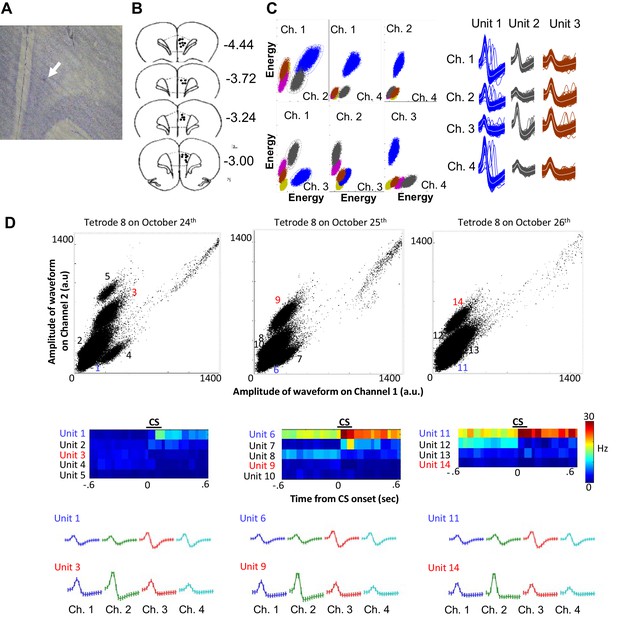
Histology and single unit isolation.
(A) Representative image of a tetrode tip (white arrow) located within the prelimbic region of medial prefrontal cortex. (B) Final locations of all usable tetrodes targeted at the prelimbic cortex (black dots). Coordinates adapted from Paxinos and Watson (2007, sixth edition). (C) During a software-assisted spike sorting process (KlustaKwik and MClust), several parameters were extracted from each spike waveform recorded from four wires of a tetrode (Ch. 1–4), and two of all possible parameter combinations (e.g., amplitude recorded in two of four wires) were plotted as a scatter plot. Spikes were separated into several units corresponding to spikes of each neuron based on the similarity of waveform parameters. Three examples that were isolated from one tetrode during a recording session were depicted as a set of averaged spike waveform on four wires (Ch. 1–4). The color of waveforms corresponds to the color used in the scatter plot. (D) Scatter plots show the amplitude of spike waveforms recorded on the channels 1 and 2 of a tetrode across three consecutive days (a.u., arbitrary unit). A few units were isolated from the recording in each day. Pseudocolor plots of binned firing rate (middle) show that some of these units increased (hotter color) or decreased (cooler color) their firing rate upon CS presentations. Based on the shape of spike waveforms recorded on four wires of the tetrode (Ch. 1–4, bottom), the unit 1, 6, and 11 appeared to be recorded from the same neuron which enhanced firing responses to the CS across three days. The unit 3 and 9 appeared to belong to the same neuron, but this neuron was not present in the recording on the third day. Other units, such as the unit 4, 5, and seven were recorded only in one day.
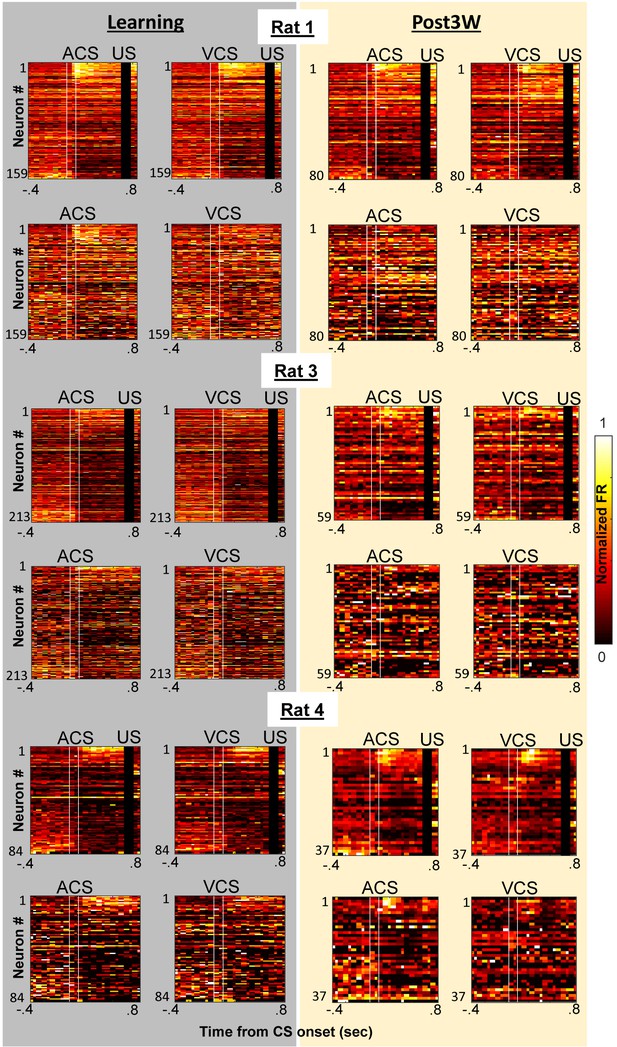
Prefrontal ensemble activity during four conditions with different relational and physical stimulus features.
Pseudocolor plots show normalized firing rates of neurons recorded from each of remaining three rats during learning (left, grey) and the third week after CR% reached asymptote (right, yellow) [top left, ACS-US paired; top right, VCS-US paired; bottom left, ACS alone; bottom right, VCS alone]. In each rat, neurons were sorted based on the ACS-induced firing rate during ACS-US pairings from the largest increase (top) to the largest decrease (bottom). In all three rats, during learning ensemble firing patterns differentiated four conditions while during the third post-learning week, ensemble activity became more similar for two conditions with the same stimulus relationship (i.e. CS-US pairings) than those with the same CS (i.e., auditory or visual CS).
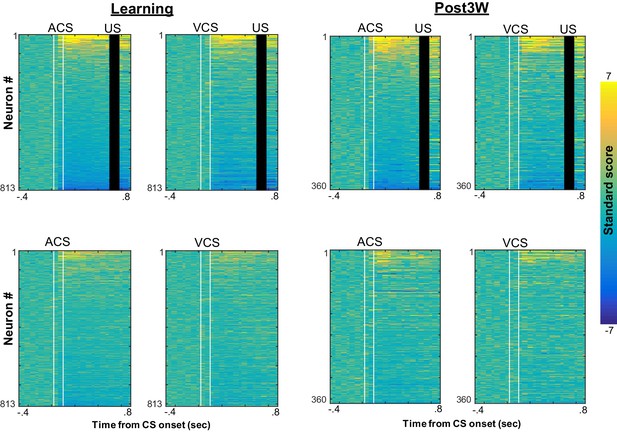
CS-evoked firing patterns during four conditions with different relational and physical stimulus features.
Pseudocolor plots show standard scores of CS-evoked firing rates of neurons recorded from four rats during learning (left) and the third week after CR% reached an asymptote (right; top left, ACS-US paired; top right, VCS-US paired; bottom left, ACS alone; bottom right, VCS alone). Neurons were sorted based on the standard score of firing rates after CS presentations during ACS-US pairings from the largest increase (top) to the largest decrease (bottom). CS-evoked firing patterns were similar between ACS-US paired and ACS alone conditions during learning; however, during the third post-learning week, they became similar between ACS-US paired and VCS-US paired conditions.
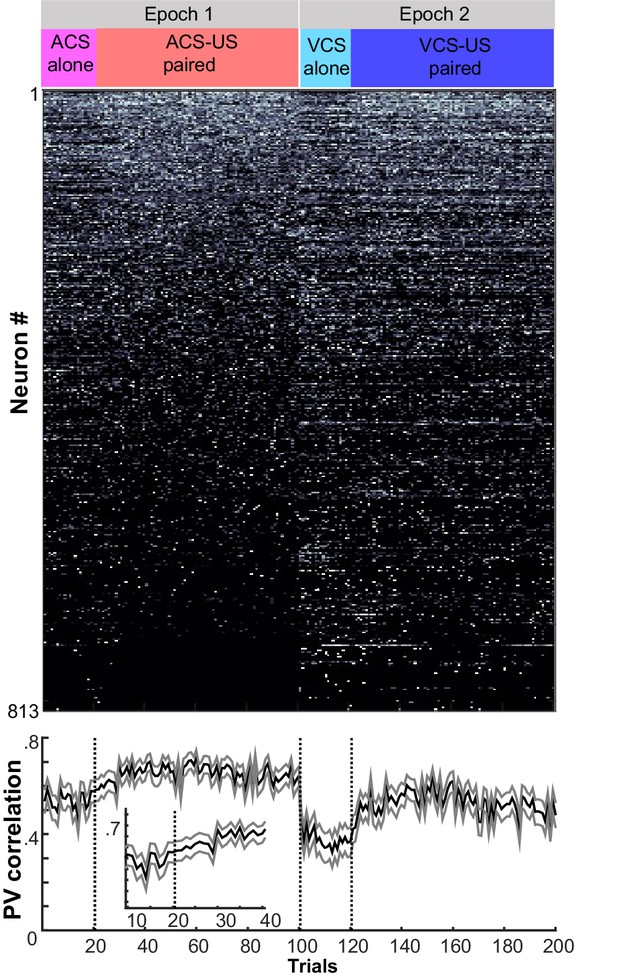
Trial-by-trial changes in the similarity of ensemble activity during learning.
Trial-by-trial display of population firing rate vectors (PVs) during CS-US intervals in the learning stage. Neurons from four rats were sorted based on the firing rate during ACS-US paired trials from the highest (Neuron #1) to the lowest (Neuron #813)]. A ‘template’ PV was constructed as averaged PVs across 10–80th ACS-US paired trials. The correlation coefficient between the template and a PV in each trial (±95% confidence interval) gradually increased within the first twenty ACS-US paired trials (inset). It abruptly decreased upon the transition from the epoch with the ACS to the next epoch with the VCS but increased again when the VCS-US pairings began. Note that the difference in the correlation coefficient between the conditions appeared to be smaller than that observed in the third post-learning week shown in Figure 2C.
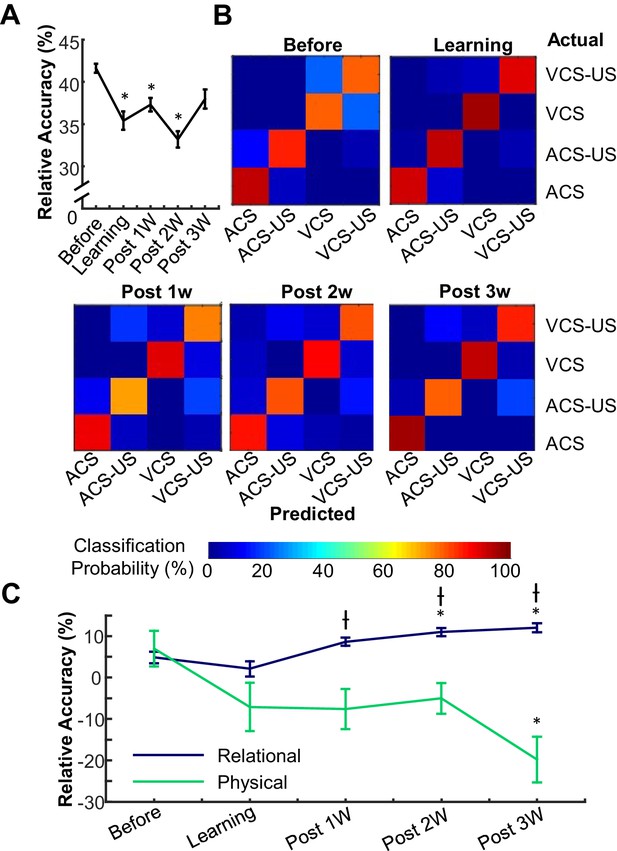
Prefrontal neuron ensembles became more selective for relational and less selective for physical features across learning stages.
(A) The relative accuracy of Support Vector Machine (SVM), trial type decoding (raw accuracy minus accuracy at chance) was separately calculated in each of five successive stages of learning. Decoding accuracy greatly fluctuated across stages. (B) Representative confusion matrices from SVM showing decoding accuracy between trial types. Each square in each 4 × 4 matrix shows the probability (color) that a trial of one type (rows) was classified as another type (columns). Hotter colors along diagonal illustrate the high accuracy of the classifier. During the Before learning stage, most classification errors were between CS-alone and CS-US trials, illustrated by lighter blue squares within the upper left and lower right quadrants. In the later stages, errors became more common between ACS-US and VCS-US trials. (C) Average SVM accuracy for binary discrimination along relational (navy) and physical (green) dimensions. Curves emphasize increased information about stimulus relationships and decreased perceptional information in the ensemble. (* indicates p<0.05 in comparison with Before Learning, † shows p<0.05 in comparison with Learning).
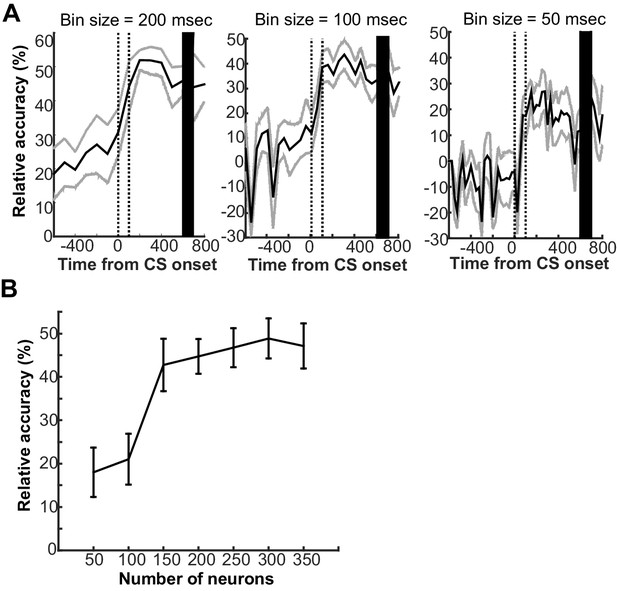
Parameters that affected the ability of support vector machine classifier to decode conditions based on prefrontal ensemble activity.
(A) The classifier was trained with the averaged firing rate of neuron ensembles in a series of 200 ms bins around the onset of the conditioned stimulus (CS). The mean decoding accuracy (black line) and two standard deviations (gray lines) were calculated over 20 decoding runs with 150 randomly sampled neurons from 340 neurons across four rats. Regardless of bin sizes (left, 200 ms; middle, 100 ms; right 50 ms), the decoding accuracy relative to chance level (estimated by a random permutation test, p<0.05) improved upon the CS presentation (two vertical lines indicate the onset and offset) and remained high toward the onset of unconditioned stimulus (black bar). The higher decoding accuracy, however, was achieved with the larger bin size. (B) With population firing vectors during the first 200-ms bin after CS offset as inputs, the decoding accuracy was higher when a larger number of neurons were included into the population firing vectors. Error bars show standard error of the mean across 20 decoding runs with randomly sampled neurons.
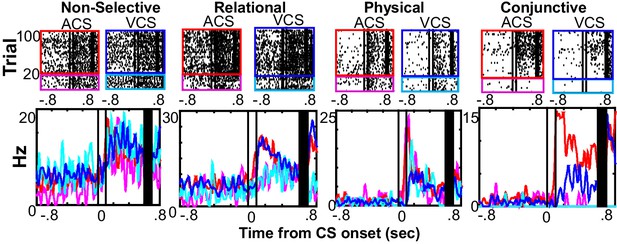
Single prefrontal neurons showed selectivity for the different features of the memory.
Representative raster plots and peri-stimulus time histograms (1 ms bins, smoothed with a 50 ms Hanning window) for each of four conditions (the presentation of auditory CS alone, magenta; pairings of the auditory CS and US, red; the presentation of visual CS alone, turquois; pairings of the visual CS and US, blue). Although some neurons showed the same CS-evoked firing patterns across four conditions (Non-selective), others were found with firing rate changes dependent on a relational feature (Relational, rates in CS-US paired trials differed from rates in CS-alone trials), a physical feature (Physical, rates in trials with the ACS differed from those with the VCS), or their conjunction (Conjunction, rates in one condition differed from the other conditions). Two black lines indicate CS onset and offset, and black bars mask the artifact induced by the US.
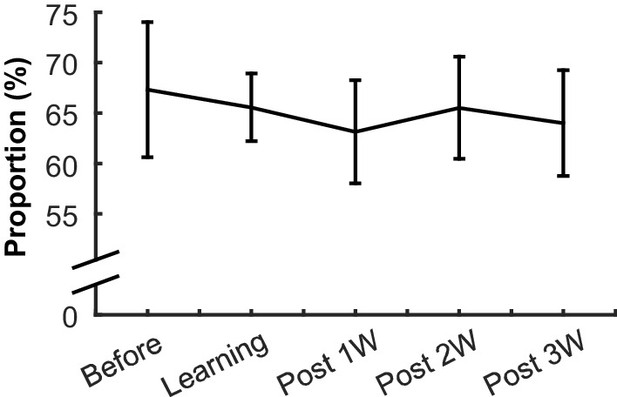
The proportion of neurons responding to the CS in each learning stage.
The proportion of neurons (±95% confidence interval) that significantly changed their firing rate during the CS or CS-US interval in at least one of four conditions did not change during learning and over-training.
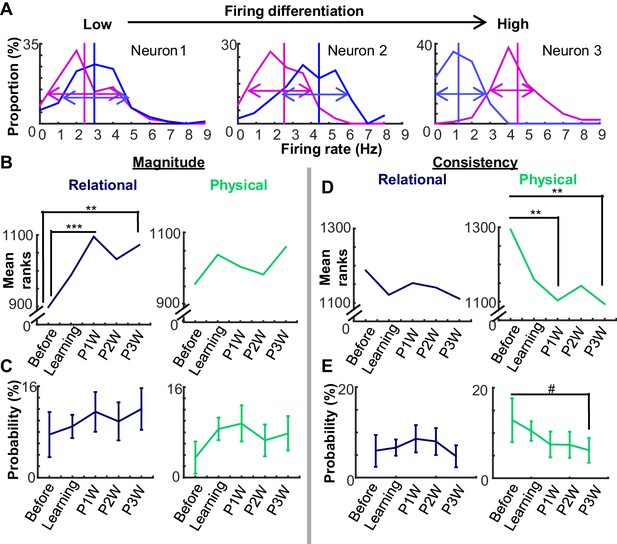
Changes in neural coding over time could be decomposed into changes in differential firing magnitude vs. consistency.
(A) Three examples of firing differentiation of a neuron between trials with the auditory conditioned stimulus (CS, magenta) and those with the visual CS (blue). A neuron is more ‘selective’ for the physical feature, if it has a greater difference in the mean firing rate (vertical lines) between two conditions (Magnitude) and a smaller variance in trial-by-trial firing rates in each condition (arrows; Consistency). (B) Mean ranks of the differential index between CS-alone trials and CS-US paired trials (Relational, left) increased during weeks after learning, whereas that between the auditory and visual CS trials (Physical, right) did not. (C) The proportion of neurons (±95% confidence intervals) with high differentiation index for relational or physical feature did not change across the stages. (D) Across five stages, mean ranks of mutual information for the relational feature did not change. In contrast, mean ranks of mutual information for the physical feature decreased over the stages. (E) The proportion of neurons (±95% confidence intervals) with significantly high mutual information for the physical feature showed a trend toward decreasing across stages. (Symbols: #, **, and *** indicate p<0.01, 0.01, and 0.001, respectively).
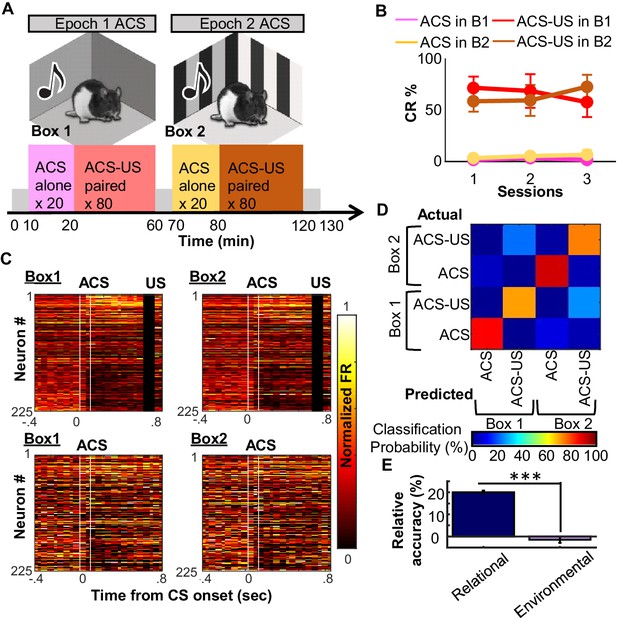
Use of existing ensemble code for CS-US relationship in a new context.
(A) After ~30 days of conditioning three rats underwent three recording sessions in a familiar (Epoch1/Box1) and novel (Epoch2/Box2) environment. (B) Conditioned responses (CRs) were absent during CS-alone trials (Box1 pink, Box2 beige) and high during CS-US trials (Box1 red; Box2 brown), with no apparent differences between familiar and novel environment. (C) Pseudocolor plots show normalized firing rate of 225 neurons (sampled across three rats) during four conditions (top left, ACS-US in Box1; top right, ACS-US in Box2; bottom left, ACS-alone in Box1; bottom right, ACS-alone in Box 2). Neurons were sorted based on ACS-evoked firing rate change during the Box1, ACS-US pairings (top: largest increase in neuron #1, bottom: largest decrease in neuron # 225). Ensemble activity during ACS-US pairings in Box 2 was similar to that during ACS-US in Box 1, but not to ACS alone trials. (D) The confusion matrix from an SVM classifier of trial type. Most errors were misclassifications of Box1 versus Box2 ACS-US trials. (E) Relative decoding accuracy (raw minus accuracy at chance ±SEM across 20 runs) between ACS-US versus ACS alone trials and Box1 versus Box2 trials. *** indicates p<0.001.
Tables
The number of neurons recorded from each rat during each learning stage.
Before | During | Post 1W | Post 2W | Post 3W | Total | |
|---|---|---|---|---|---|---|
Rat 1 | 42 | 159 | 45 | 56 | 80 | 382 |
Rat 2 | 64 | 357 | 174 | 162 | 184 | 941 |
Rat 3 | 78 | 213 | 96 | 77 | 59 | 523 |
Rat 4 | 42 | 84 | 46 | 46 | 37 | 255 |
Total | 226 | 813 | 361 | 341 | 360 | 2101 |





