Photoreceptor avascular privilege is shielded by soluble VEGF receptor-1
Figures
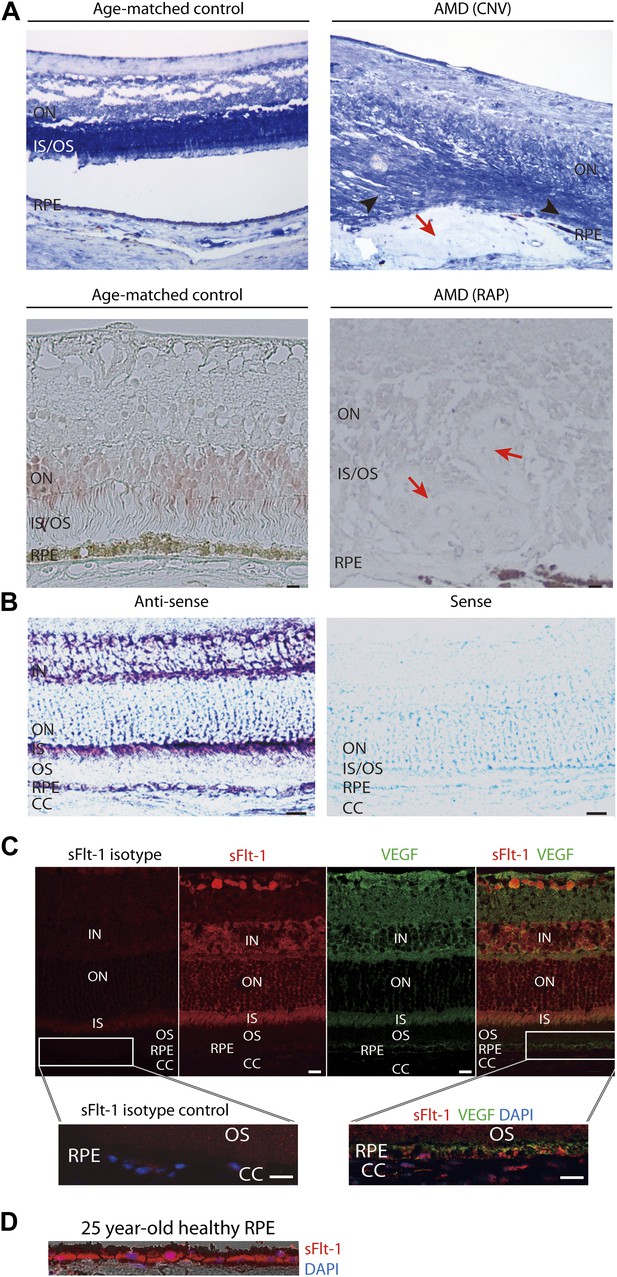
Soluble FLT-1 expression is reduced in the photoreceptors and RPE from AMD eyes.
(A) Representative immunostaining images show soluble FLT-1 expression was significantly decreased in an age-related macular degeneration (AMD) eye with choroidal neovascularization (CNV) (top, 80 years old, female) and retinal angiomatous proliferation (RAP) (bottom, 67 years old, female) compared with aged-matched controls (80 years old and 71 years old, respectively, females; black arrow heads point to the photoreceptors; red arrows point to the CNV or RAP lesions). (B) In situ hybridizations show the localization of sFlt-1 (purple/blue) in the photoreceptors and retinal pigment epithelium (RPE) layers in Balb/c mice. (C) Immunohistochemistry (IHC) staining shows sFLT-1 and VEGF expression in wild type mice. Higher relative expression of sFLT-1 to VEGF is observed in the photoreceptors. The magnified images (bottom) from the framed area showed that soluble FLT-1 is expressed in the basal side of the RPE layer. (D) Representative IHC image shows sFLT-1 is expressed in the basal side of the RPE layer in a young adult healthy human eye (25 years old, male). CC: choriocapillaris; IN: inner nuclear layer; IS: inner segment layer; ON: outer nuclear layer; OS: outer segment layer. Arrows point to the RPE layer. Scale bar: 10 µm.
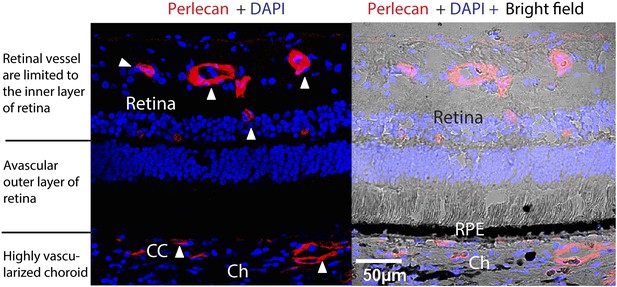
Avascularity of the outer retina (photoreceptors and RPE and BrM) surrounded by the inner retina with abundant vessels and the highly vascularized choroid in a normal human eye.
Red: perlecan staining of vessels; blue: DAPI. Arrow heads point to the vessels. BrM: Bruch's membrane; Ch: choroid; RPE: retinal pigment epithelium; CC: choriocapillaris.
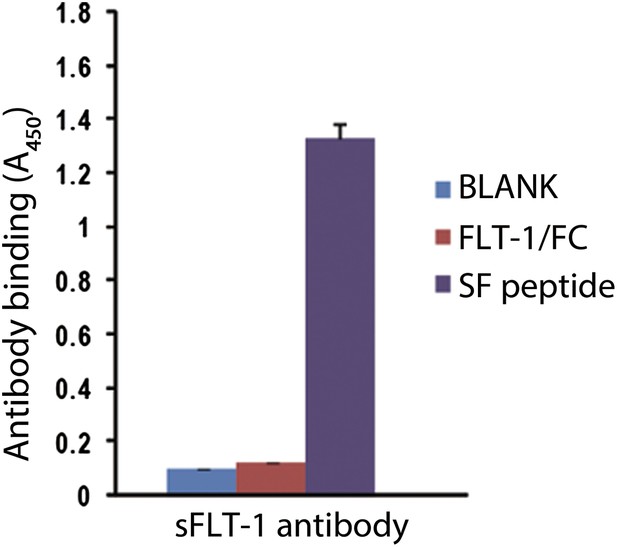
sFLT-1 antibody specifically binds to the unique motif of sFLT-1.
Results correspond to an ELISA in which wells are coated with: BLANK, 1% BSA; FLT/FC, a chimeric protein containing the extracellular region of FLT-1 and the human Fc fragment (R&D Systems, Minneapolis, MN); SF, the peptide utilized to immunize the rabbits to obtain sFLT-1 antibody (the peptide corresponding to a fragment of the unique C-terminal tail of sFLT-1).
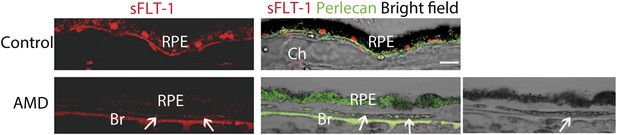
sFLT-1 expression is significantly decreased in RPE from an AMD eye with CNV (88 years old, female) compared with the age-matched control (89 years old, female).
The right bottom bright field images shows autofluorescence from degenerated RPE (green fluorescence blocks the dark pigment of RPE overlying CNV; arrows point to CNV). AMD: age-related macular degeneration; Br: Bruch's membrane; Ch: choroid; CNV: choroidal neovascularization; RPE: retinal pigment epithelium.
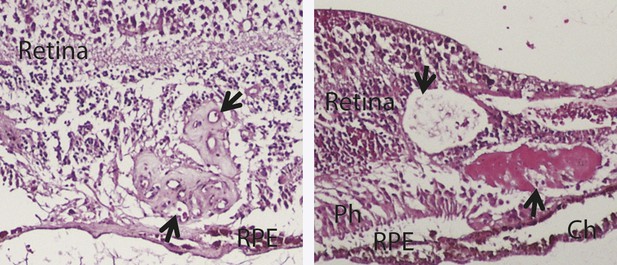
H&E staining images show the histology of two human RAP eyes (arrows point to the RAP lesion).
Ch: choroid; RAP: retinal angiomatous proliferation; RPE: retinal pigment epithelium; Ph: Photoreceptor layer.
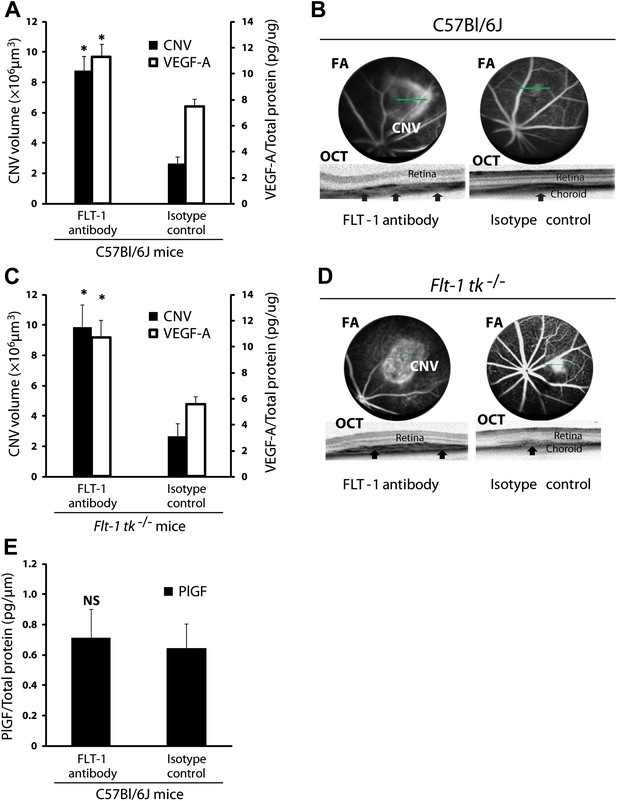
Suppression of sFLT-1 by neutralizing antibodies induces CNV while elevating levels of VEGF.
VEGF-A levels were tested by ELISA of retinal pigment epithelium (RPE)/choroid tissue lysates harvested immediately after CNV images were taken in vivo. (A and C) Subretinal injections of FLT-1 antibody induced CNV and increased VEGF-A levels in both wild type and Flt-1 tk−/− mice. (B and D) Representative FA (at a phase of 3 min after fluorescein injection) and OCT images. Arrows point to CNV. (E) ELISA for PlGF showed no significant difference 3 days after subretinal injections of FLT-1 neutralizing antibody and isotype control. CNV: choroidal neovascularization; FA: fluorescein angiography; OCT: optical coherence tomography.
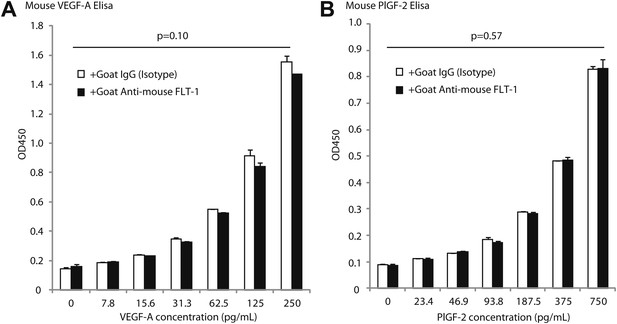
Goat anti-mouse FLT-1 neutralizing antibody does not affect ELISA assessment of mouse VEGF-A and mouse PlGF-2.
Goat anti-mouse FLT-1 neutralizing antibody or control goat IgG was added to the standard at 1 ng/ml. (A) Mouse VEGF-A ELISA. (B) Mouse PlGF-2 ELISA. p Values were calculated by paired Student t test (each average was paired). The presence of anti-FLT-1 antibody did not significantly affect ELISA readings of either cytokine.
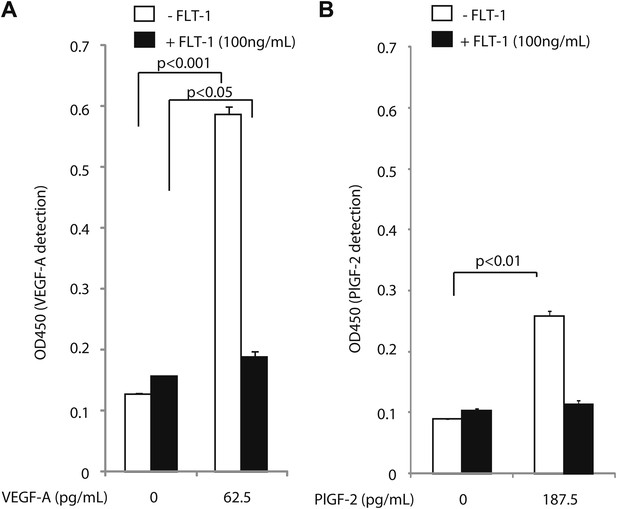
Excess recombinant FLT-1 inhibits VEGF-A and PlGF-2 detection.
Recombinant FLT-1 was added to each concentration of VEGF-A (A) or PlGF-2 (B) and abolished detection of VEGF-A and PlGF-2 over background levels, indicating that VEGF-A or PlGF-2 that is bound to FLT-1 is not detected by this assay.
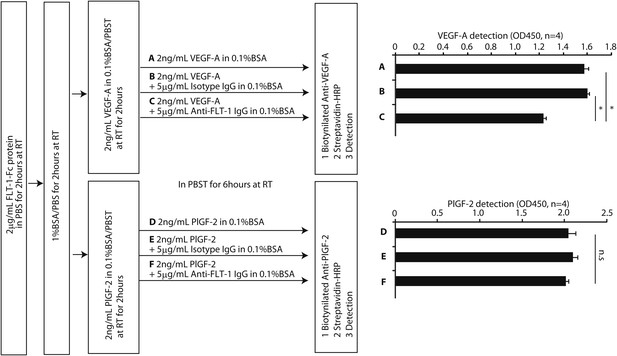
FLT-1 neutralizing antibody incubation resulted in release of VEGF-A from recombinant FLT-1 in vitro.
The design schema is shown on the left. A 2 mg/ml sample of mouse FLT-1-Fc protein (471-F1; R&D Systems, Minneapolis, MN) was immobilized on an ELISA plate. After blocking with 1% BSA, the plate was incubated in 2 ng/ml mouse VEGF-A (493-MV-005; R&D) or mouse PlGF-2 (465-PL-010; R&D) at room temperature (RT) for 2 hr. Then, each well was incubated in (A) 2 ng/ml mouse VEGF-A, (B) 2 ng/ml mouse VEGF-A + 5 mg/ml isotype IgG, (C) 2 ng/ml mouse VEGF-A + 5 mg/ml anti-FLT-1 IgG (AF471; R&D), (D) 2 ng/ml mouse PlGF-2, (E) 2 ng/ml mouse PlGF-2 + 5 mg/ml isotype IgG, or (F) 2 ng/ml mouse PlGF-2 + 5 mg/ml anti-FLT-1 IgG at RT for 6 hr. Using biotinylated anti-VEGF-A (BAF493; R&D) or biotinylated anti-PlGF-2 (BAF465; R&D) and streptavidin-HRP (DY998; R&D), VEGF-A or PlGF-2 was detected. The results (row C vs row F) show that FLT-1 neutralizing antibody desequestered VEGF-A but not PlGF-2 from FLT-1. *p<0.0001 by two-tailed Student t test.
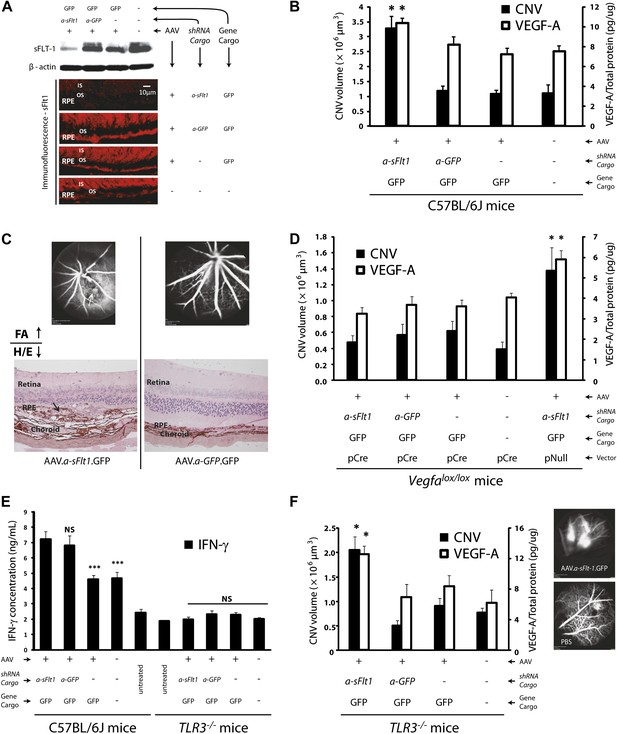
Subretinal adeno-associated viral delivery of short hairpin RNA targeting sFLT-1 induces VEGF-A and CNV.
VEGF-A levels from retinal pigment epithelium (RPE)/choroid tissues were tested by ELISA from tissue lysates harvested immediately after choroidal neovascularization (CNV) images were taken in vivo. (A) Western blot (top) and immunohistochemistry (IHC) staining of sFLT-1 results confirmed that sFLT-1 was knocked down by AAV.shRNA.sFlt-1.Gfp but not in controls at 4 weeks. (B) Subretinal AAV.shRNA.sFlt-1.Gfp induced CNV and VEGF-A compared to controls in wild type mice (4 weeks). CNV was observed in controls due to disruption of Bruch’s membrane by subretinal injection. (C) Representative fluorescein angiography (FA) and H&E staining images (40×) (arrows point to leakage or neovessels) show CNV is more extensive after AAV.shRNA.sFlt-1.Gfp treatment compared to controls. (D) CNV was not induced by subretinal AAV.shRNA.sFlt-1 when VEGF-A release was suppressed (4 weeks) by pCre in Vegfalox/lox mice compared with pNull control in which VEGF levels rose and CNV was induced. (E) IFN-γ levels increased in AAV.shRNA.sFlt-1.Gfp and AAV.shRNA.Gfp (4 weeks) compared with AAV.Gfp and phosphate buffered saline (PBS) in C57BL/6J mice. No significant difference was found among groups in the Tlr3−/− mice (4 weeks). (F) Subretinal AAV.shRNA.sFlt-1.Gfp induced VEGF-A and CNV (4 weeks) in Tlr3−/− mice. Representative FA images show the leakage in each group. VEGF-A levels were tested by ELISA using RPE/choroid complex samples. *p<0.05; **p<0.005; ***p<0.0005. NS: no significant difference. Scale bar: 10 µm.
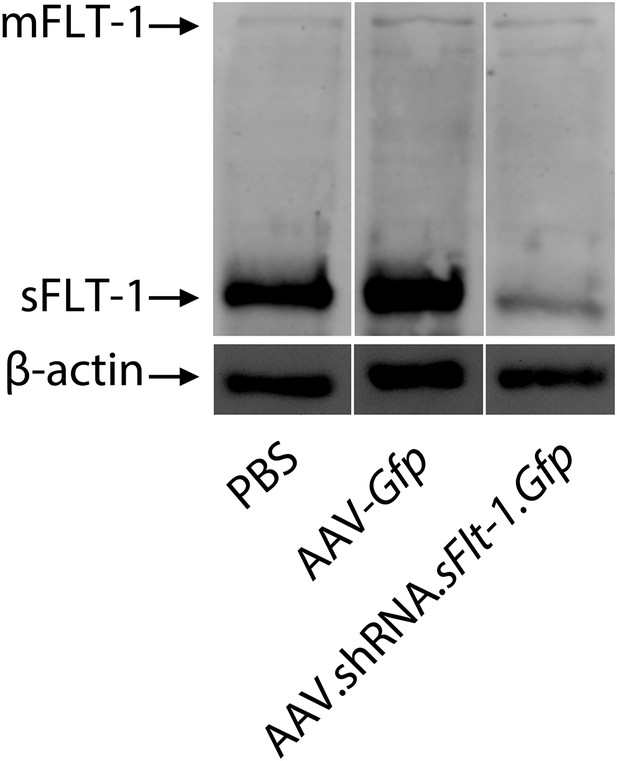
Western blot shows that AAV.shRNA.sFlt-1.Gfp specifically affects sFLT-1 but not mFLT-1.
https://doi.org/10.7554/eLife.00324.014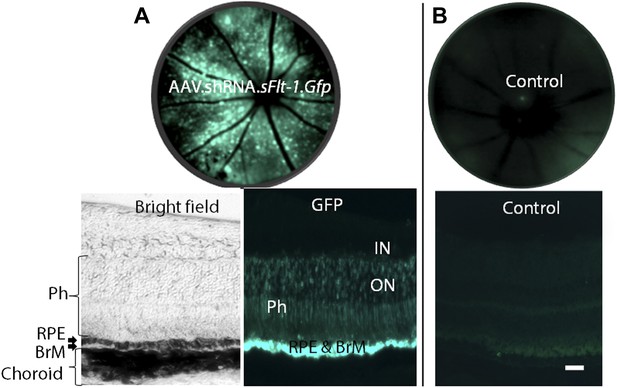
AAV.shRNA.sFlt-1.Gfp (A) transfected more than 3 out of 4 quadrants and targeted the photoreceptors, RPE, and BrM by subretinal injection (2 weeks). (B) Sham control.
The fundi are imaged by a Spectralis imager in vivo (top). Retinal cross-sections (bottom) are imaged by confocal microscopy. Green is GFP, blue is DAPI. Scale bar: 10 µm. BrM: Bruch's membrane; GFP: green fluorescent protein; IN: inner nuclear layer; ON: outer nuclear layer; Ph: photoreceptors; RPE: retinal pigment epithelium.
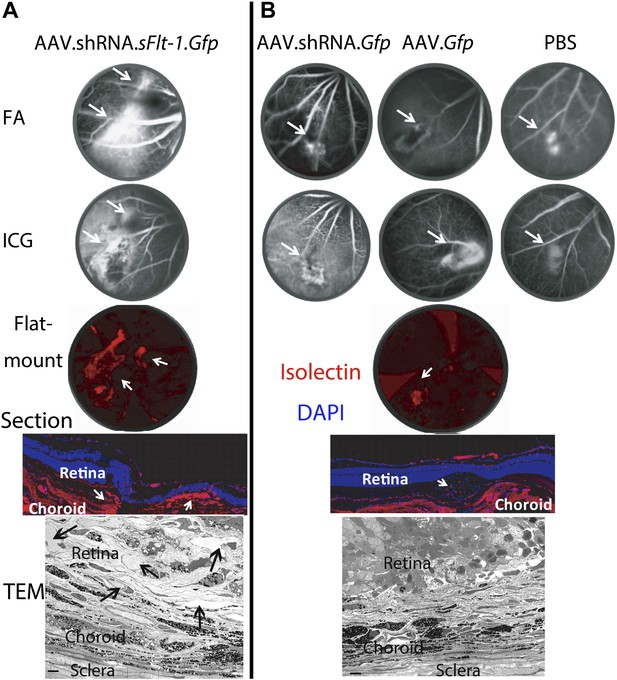
Representative images show different sizes of CNV in AAV.shRNA.sFlt-1.Gfp (A) and controls (B) at 4 weeks after subretinal injection.
FA and ICG images were taken in vivo. Representative immunohistochemistry staining on flat-mounts (4×) and cryosections (20×) and transmission electron microscopy images showed abundant neovessels (arrows) in AAV.shRNA.sFlt-1.Gfp but not control injection. Scale bar: 10 μm. CNV: choroidal neovascularization; FA: fluorescein angiography; ICG: indocyanine green.
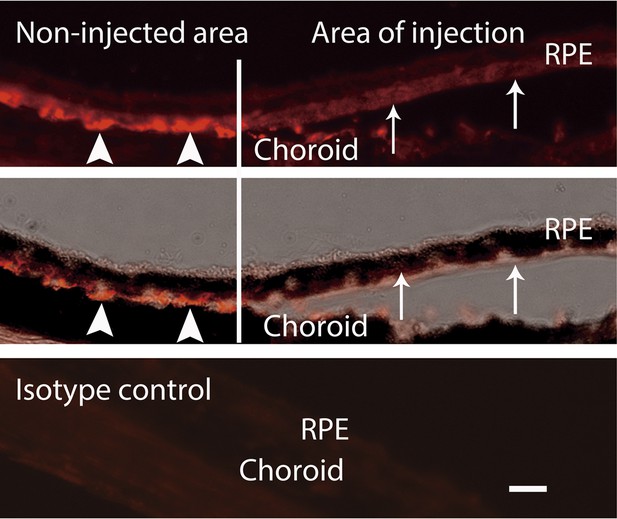
IHC staining of VEGF indicates that the VEGF decreased in the area of injection (arrows) but not in the non-injected area (arrow heads).
Top: VEGF; middle: VEGF + bright field; bottom: isotype control of VEGF. Scale bar: 10 µm. IHC: immunohistochemistry; RPE: retinal pigment epithelium.
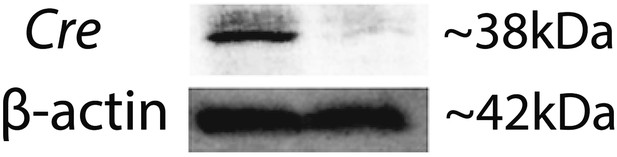
Western blot confirms Cre expression in Vegfalox/lox mice 10 days after pCre subretinal delivery (lane 1: pCre, lane 2: pNull).
https://doi.org/10.7554/eLife.00324.018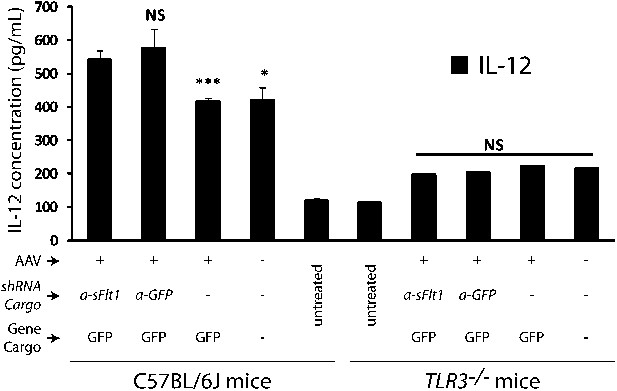
IL-12 levels increased in both AAV.shRNA.sFlt-1.Gfp and AAV.shRNA.Gfp treated mice (4 weeks) compared to AAV.Gfp and PBS control in the C57BL/6J mice.
No significant difference was found between each group of Tlr3−/− mice (4 weeks).
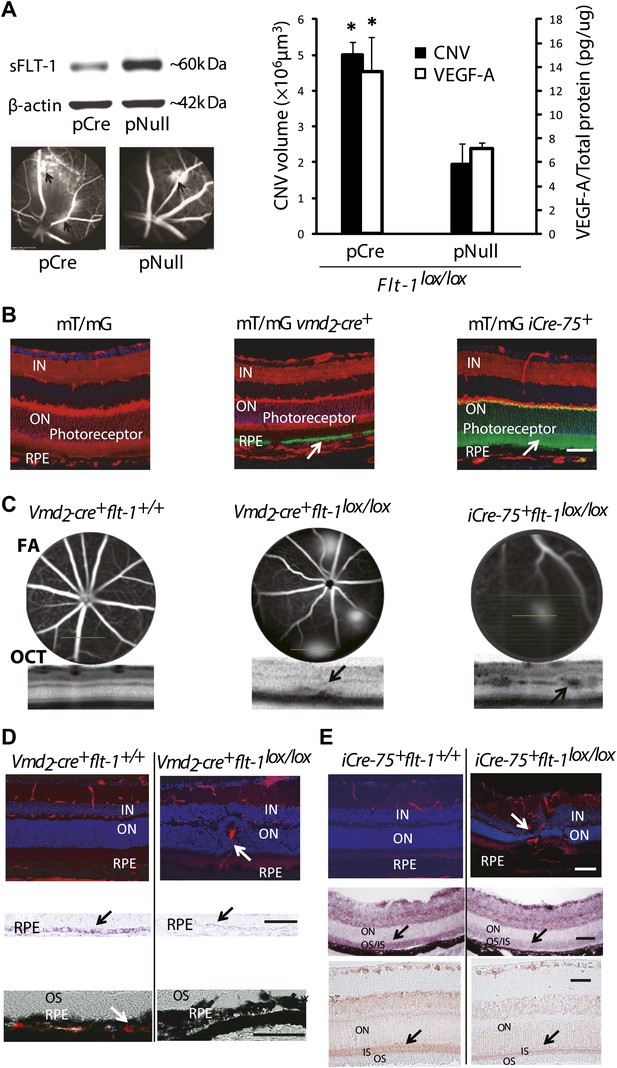
Suppression of soluble Flt-1 by conditional Cre/lox-mediated Flt-1 gene ablation induced CNV.
(A) Western blot from the retinal pigment epithelium (RPE)/choroid tissue confirmed sFLT-1 knockdown by pCre. Subretinal injection of pCre induced CNV and increased VEGF-A levels in Flt-1lox/lox mice (2 weeks; arrows point to CNV). Representative FA images showed CNV lesions in both the pCre and pNull groups (left bottom). (B) Deletion of tomato fluorescence showed that Cre expression (mosaic pattern) was restricted to RPE in transgenic mT/mg vmd2-cre+ mice (at 4 weeks of age) and was restricted to photoreceptors in transgenic mT/mG iCre-75+ mice (at 4 weeks of age), respectively (arrows point to the location of Cre expression). (C) Representative FA and OCT (bottom) images showed in vivo that a homozygous RPE-specific Flt-1 knockout (Vmd2-cre+flt-1lox/lox) mouse (48 days old) developed CNV spontaneously without surgical injury, and a homozygous photoreceptor-specific Flt-1 knockout mouse (iCre-75+flt-1lox/lox) (30 days old) developed retinal angiomatous proliferation (RAP) spontaneously without surgical injury. The littermate controls without loxp were normal. (D) Representative IHC images (top panels) showed the retinal neovessels in the above Vmd2-cre+flt-1lox/lox (CNV) mouse at 3 months of age. The littermate controls without loxp were normal (red stained vessels). In situ hybridization (middle panels, blue stained sFlt-1) and IHC images (bottom panels, red stained sFLT-1) showed decreased expression of sFLT-1 RNA and protein in the RPE (Vmd2-cre+flt-1lox/lox) (arrows). (E) Representative IHC images (top panels) showed the retinal neovessels in the above iCre-75+flt-1lox/lox mouse (RAP) at 3 months of age. The littermate controls without loxp were normal (red stained vessels). In situ hybridization (middle panels, blue stained sFlt-1) and IHC images (bottom panels, red stained sFLT-1) showed decreased expression of sFLT-1 RNA and protein in the photoreceptors (iCre-75+flt-1lox/lox) (arrows). Scale bar: 100 µm. CNV: choroidal neovascularization; FA: fluorescein angiography; IHC: immunohistochemistry; IN: inner nuclear layer; OCT: optical coherence tomography; ON: outer nuclear layer; OS: outer segment layer.
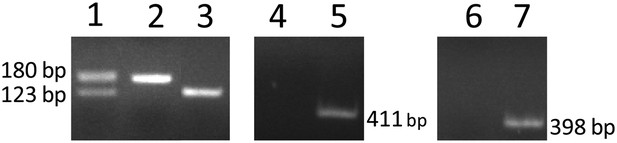
Genotyping of RPE-specific Flt-1 knockout mice (obtained by cross-breeding with Vmd2-cre+ mice and Flt-1lox/lox mice) with PCR.
Lanes 1–3: heterozygous (Flt-1lox/+), homozygous (Flt-1lox/lox), and wild type (Flt-1+/+), respectively. Lanes 4 and 5: cre− and cre+, respectively. Lanes 6 and 7: rtTA− and rtTA+, respectively. RPE: retinal pigment epithelium.
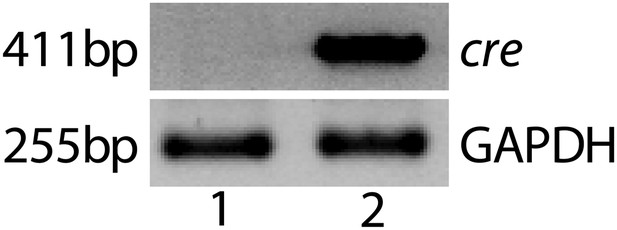
Representative RT-PCR shows Cre expression in a Vmd2-cre+ flt-1+/+ mouse (lane 2).
No Cre expression was observed in wild type mice (lane 1).
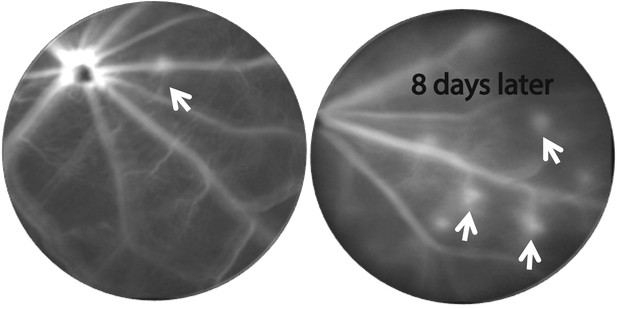
CNV progression in homozygous RPE-specific Flt-1 knockout (Vmd2-cre+flt-1lox/lox) mice.
Representative FA images (at same time after fluorescence injection) from a 38 day old mouse demonstrate that CNV lesions appear 8 days after initial imaging in previously non-fluorescent areas, indicating progression of CNV. CNV: choroidal neovascularization; FA: fluorescein angiography; RPE: retinal pigment epithelium.
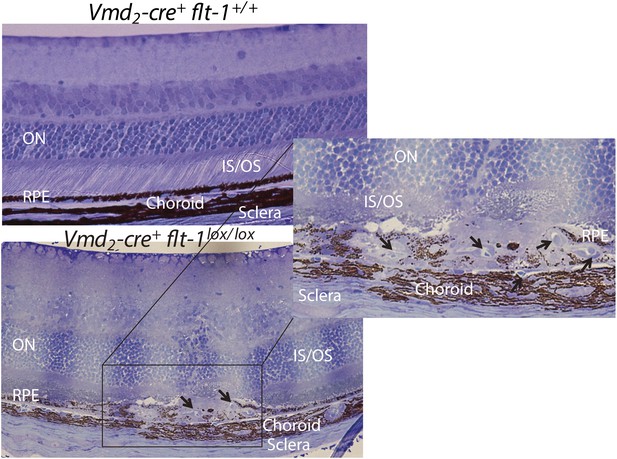
Representative toluidine blue staining images show CNV in a homozygous conditional Flt-1 knockout mouse (Vmd2-cre+flt-1lox/lox, 3 months old) compared to normal retinal morphology in a littermate control.
CNV: choroidal neovascularization; IS: inner segment layer; ON: outer nuclear layer; OS: outer segment layer; RPE: retinal pigment epithelium.
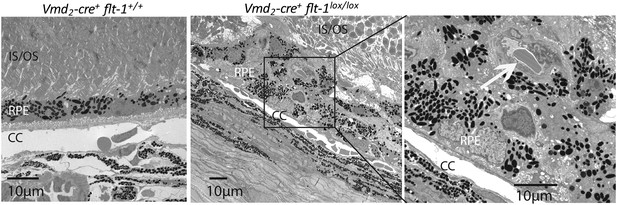
Representative TEM images show CNV in a homozygous conditional Flt-1 knockout mouse (Vmd2-cre+flt-1lox/lox) compared to normal retinal architecture in a littermate control.
CC: choriocapillaris; CNV: choroidal neovascularization; TEM: transmission electron microscopy; RPE: retinal pigment epithelium.
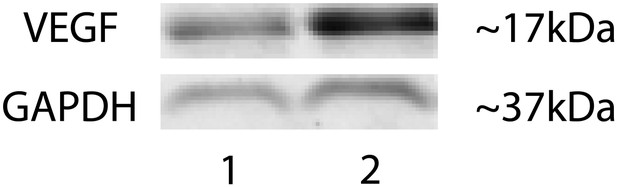
Representative western blot shows increased VEGF-A levels in the RPE/choroid of a Vmd2-cre+flt-1lox/+ mouse with CNV (lane 2) compared to a littermate control (Vmd2-cre+flt-1+/+) without CNV (lane 1).
CNV: choroidal neovascularization; RPE; retinal pigment epithelium.
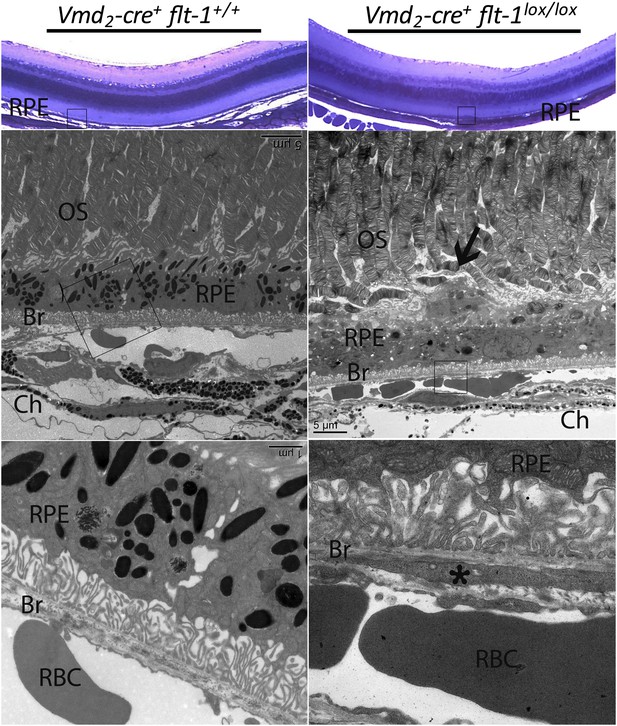
Early vascular changes with normal retinal structures occur in homozygous conditional Flt-1 knockout mice (Vmd2-cre+flt-1lox/lox).
Top panels: Toluidine blue staining images show the morphology of the retina. There are no significant changes in the retina structure. There is a bump (frame) in the RPE layer in a homozygous conditional Flt-1 knockout mouse (Vmd2-cre+flt-1lox/lox) compared to littermate control. Middle and bottom panels (magnified transmission electron microscopy images of the corresponding frames): There is an elongated profile (asterisk) within Bruch's membrane that is consistent with a tongue of endothelial cell cytoplasm by its electron density. There is a nucleus on the external side of the choriocapillary, and it is separated from the endothelial lining by a basement membrane. Bruch's membrane is intact. This is consistent with nascent choroidal endothelial changes preceding the development of frank CNV breaching Bruch's membrane. Br: Bruch's membrane; Ch: choroid; CNV: choroidal neovascularization; OS: outer segment layer; RBC: red blood cell; RPE: retinal pigment epithelium.
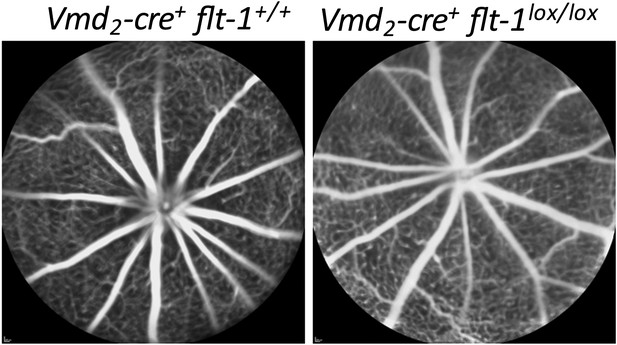
FA images show that no CNV has developed in homozygous conditional Flt-1 knockout mice (Vmd2-cre+flt-1lox/lox) at p21.
CNV: choroidal neovascularization; FA: fluorescein angiography.
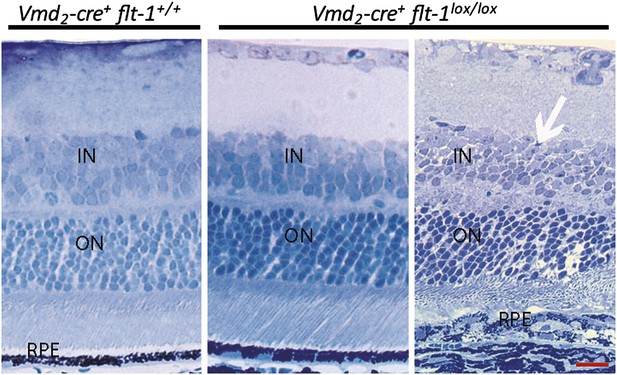
Toluidine blue staining images show the morphology of the retina at p28.
Most areas of retina are normal in Vmd2-cre+flt-1lox/lox mice. In a CNV lesion, the overlying neuronal outer nuclear layer is mainly intact. Scale bar: 10 µm. CNV: choroidal neovascularization.
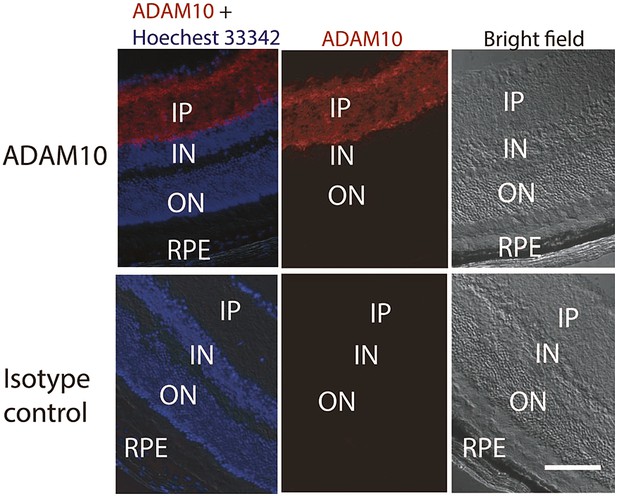
Representative IHC staining images show the ADAM10 expression in the inner layer of the retina.
IHC: immunohistochemistry; IN: inner nuclear layer; IP: inner plexiform layer; ON: outer nuclear layer; RPE: retinal pigment epithelium.
Tables
Demographic information for human globes
| Normal | Normal | Normal | Normal | Normal | AMD (CNV) | AMD (dry) | AMD | RAP | RAP | RAP |
|---|---|---|---|---|---|---|---|---|---|---|
| 25/M | 71/M | 79/F | 80/F | 89/F | 80/F | 86/F | 88/F | 67/M | 86/F | 86/F |
-
All humans are Caucasians.
-
AMD: age-related macular degeneration; CNV: choroidal neovascularization; F: female; M: male; RAP: retinal angiomatous proliferation.
Transgenic mice with Cre/lox-mediated conditional gene ablation of Flt-1 in the retinal pigment epithelium (RPE) developed choroidal neovascularization (CNV) (<3 months old)
| homozygous (Vmd2-cre+ flt-1lox/lox) | heterozygous (Vmd2-cre+ flt-1lox/+) | littermate control (Vmd2-cre+ flt-1+/+) | |
|---|---|---|---|
| Litter 1 | 1/1 mice, 2/2 eyes | NA | NA |
| Litter 2 | 2/2 mice, 3/4 eyes | 5/7 mice, 9/14 eyes | 0/1 mouse 0/2 eyes |
| Litter 3 | 1/1 mouse, 2/2 eyes | 1/2 mice, 1/4 eyes | NA |
| Litter 4 | 1/1 mouse, 2/2 eyes | 2/3 mice, 2/6 eyes | NA |
| Litter 5 | 2/2 mice, 4/4 eyes | 1/2 mice, 1/4 eyes | 1/2 mice, 1/4 eyes |
| Litter 6 | 3/3 mice, 4/6 eyes | 1/2 mice, 1/4 eyes | 1/5 mice, 1/10 eyes |
| Litter 7 | 1/1 mouse, 1/2 eyes | 2/5 mice, 3/10 eyes | 0/3 mice, 1/6 eyes |
| Total | 11/11 mice (100%) | 12/21 mice (57%) | 2/11 mice (18%) |
| 18/22 eyes (82%, p= 1.3E-6) | 17/42 (40%, p=0.009) | 2/22 eyes (9%) |
Transgenic mice with Cre/lox-mediated conditional gene ablation of Flt-1 in the photoreceptors developed retinal angiomatous proliferation (RAP) (<3 months old)
| homozygous (iCre-75+ flt-1lox/lox) | heterozygous (iCre-75+ flt-1lox/+) | littermate control (iCre-75+ flt-1+/+) | |
|---|---|---|---|
| Litter 1 | 2/4 mice, 3/8 eyes | NA | 0/1 mouse 0/2 eyes |
| Litter 2 | 1/1 mice, 1/2 eyes | 3/4 mice, 6/8 eyes | NA |
| Litter 3 | 1/1 mouse, 2/2 eyes | 2/4 mice, 4/8 eyes | 0/1 mouse, 0/2 eyes |
| Litter 4 | 1/1 mice, 2/2 eyes | 1/2 mice, 1/4 eyes | 1/2 mice, 1/4 eyes |
| Litter 5 | NA | 1/1 mouse, 2/2 eyes | NA |
| Litter 6 | NA | 0/1 mouse, 0/2 eyes | 0/1 mouse, 0/2 eyes |
| Total | 6/7 mice (86%) | 7/12 mice (58%) | 1/5 mice (20%) |
| 8 /14 eyes (57%, p<0.05) | 13/24 (54%, p<0.05) | 1/10 eyes (10%) |





