Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors
Figures
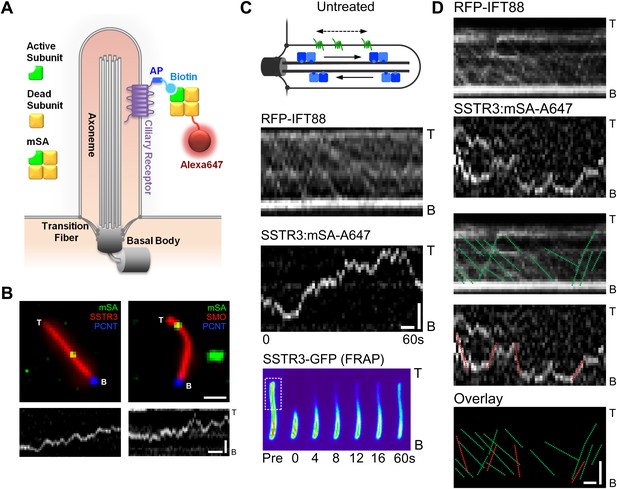
Real-time imaging of single signaling receptors in cilia of live cells.
(A) Schematic of single molecule labeling strategy. SSTR3 or Smo were fused at the extracellular N-terminus to an acceptor peptide (AP) for the biotin ligase BirA. Biotinylated AP-SSTR3 and AP-Smo molecules were sparsely revealed by Alexa647-conjugated monovalent streptavidin (mSA-Alexa647) added at low concentrations (50 pM) to the extracellular medium. (B) IMCD3 cells stably expressing AP-SSTR3-GFP (SSTR3, pseudo-colored red, left panel) or AP-Smo-YFP (SMO, pseudo-colored red, right panel) were transfected with Pericentrin-RFP (PCNT, pseudo-colored blue) to mark the ciliary base and BirA to biotinylate AP-SSTR3-GFP. Biotinylated SSTR3 or Smo were detected with mSA-Alexa647 (mSA, pseudo-colored green). The kymograph represents the movement of a single mSA-Alexa647 labeled AP-SSTR3-GFP or AP-Smo-YFP in live cells. The tip (T) and the base (B) of the cilium are indicated. Scale bars, 2 μm (y), 4 s (x). (C) Kymographs of simultaneous live cell imaging of TagRFP.T-IFT88 (RFP-IFT88, IFT train) and single molecule SSTR3 (SSTR3:mSA-A647) movement in untreated cells. The mobility of ciliary SSTR3 was assessed by half-cilium FRAP (montage of heat-maps, bottom). Scale bars, 2 μm (y), 5 s (x). (D) Comparison of IFT88 foci track with single SSTR3 directional tracks in untreated cells. The processive movement of mSA labeled SSTR3 (SSTR3:mSA-A647, red dashed line) and IFT88 foci tracks (RFP-IFT88, green dashed line) are indicated. Little overlap is observed between IFT88 foci tracks and single SSTR3 tracks in untreated cells.
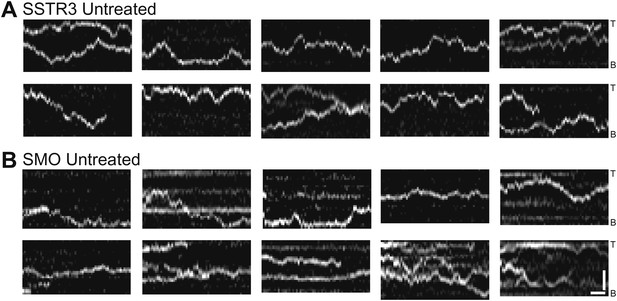
Additional kymographs.
Additional kymographs representing the movement of single mSA-Alexa647 labeled AP-SSTR3-GFP (A) and AP-Smo-YFP (B). The tip (T) and the base (B) of the cilium are indicated. Scale bars, 2 μm (y), 4 s (x).
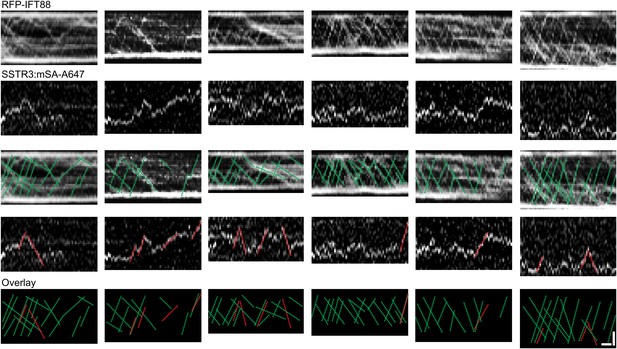
Additional dual channel kymographs.
Additional kymographs of simultaneous live cell imaging of TagRFP.T-IFT88 (RFP-IFT88, IFT complex) and single molecule SSTR3 (SSTR3:mSA-A647). The processive movement of mSA labeled SSTR3 (red dashed line) and IFT88 foci tracks (greed dashed line) are indicated. Scale bars, 2 μm (y), 4 s (x).
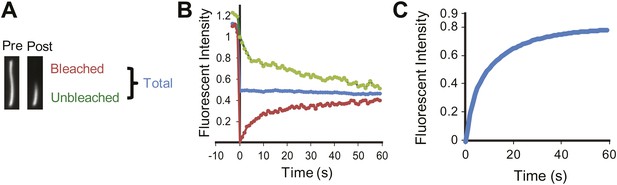
Half-cilium FRAP.
(A) Representative example of a half-cilium FRAP. (B) Kinetics of fluorescence recovery of photobleached region (red curve), unbleached region (green curve), and the total cilium (blue curve). (C) Averaged fitted curves of SSTR3-GFP half-cilium FRAP.
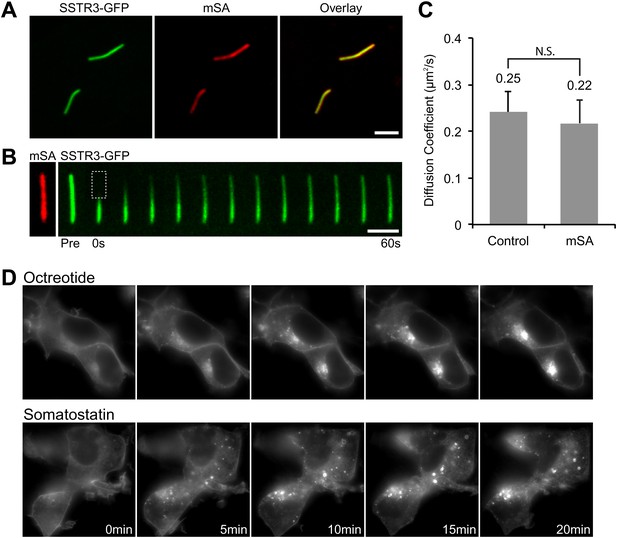
Functionality of the AP-SSTR3-GFP fusion protein.
(A) Saturated labeling of biotinylated AP-SSTR3-GFP (SSTR3-GFP) with 20 nM of mSA-Alexa647 (mSA) for 1 hr. Scale bar, 2 μm. (B) Time series montage represents the mobility of ciliary AP-SSTR3-GFP saturatedly labeled with mSA-Alexa647 after photobleaching. Scale bar, 2 μm. (C) The diffusion coefficients quantified from control cells vs cells with saturated staining of mSA-Alexa647 (mSA). More than 10 cilia were analyzed for each condition. Error bars, SD. p>0.05. (D) Live cell imaging of HEK293T cells expressing AP-SSTR3-GFP. Cells were treated with 10 μM Octreotide or Somatostatin immediately before imaging.
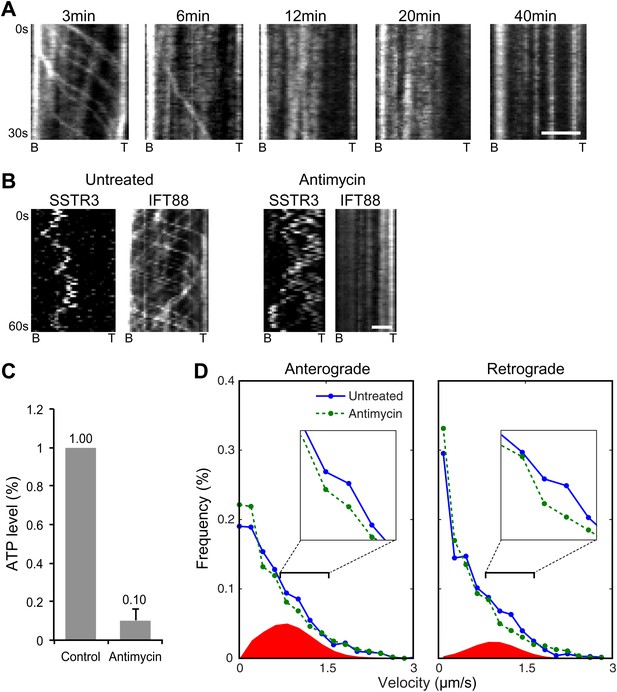
Single molecule imaging in ATP-depleted cells reveals the receptor population undergoing active transport in live cells.
(A) Kymographs of GFP-IFT88 foci movements after Antimycin A and 2-deoxyglucose (2DG) treatment. Scale bar, 2 μm. (B) Kymographs of simultaneous live cell imaging of tagRFP.T-IFT88 (IFT88) and single molecule SSTR3 (SSTR3) movement before and after 40 min of Antimycin A and 2DG treatment. Scale bar, 2 μm. (C) ATP levels quantified by luciferin-luciferase bioluminescence assay normalized to the levels in control-treated cells. To deplete intracellular ATP, IMCD3 cells were treated with 20 μM Antimycin + 10 mM 2DG for 40 min. Each treatment was measured in triplicate. (D) Instant velocity distributions of single SSTR3 movements in untreated vs ATP-depleted cells. Statistical analyses show a significant difference between velocity distributions of untreated and ATP-depleted cells for both the anterograde velocities (p=0.03) and retrograde velocities (p=0.03). The live cell data (blue) was fitted to a mixed model combining the ATP-depleted data (green) and an additional Gaussian distribution (red), with the latter found to contribute a fraction of 26.6 +/− 5.9% (anterograde) and 12.8 +/− 3.1% (retrograde). n>1200.
Additional kymographs.
Additional kymographs representing the movement of single mSA-Alexa647 labeled AP-SSTR3-GFP in cells treated with Antimycin A and deoxyglucose. The tip (T) and the base (B) of the cilium are indicated. Scale bars, 2 μm (y), 4 s (x).
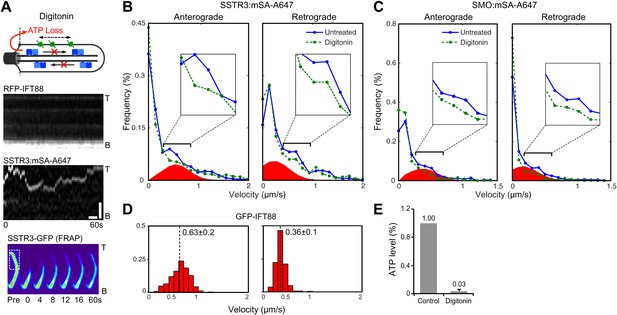
Single molecule imaging in digitonin-permeabilized cells reveals the receptor population undergoing active transport in live cells.
(A) Kymographs of simultaneous live cell imaging of TagRFP.T-IFT88 (RFP-IFT88, IFT trains) and single molecule SSTR3 (SSTR3:mSA-A647) movements in digitonin semi-permeabilized cells. The immobilization of ciliary SSTR3 was confirmed by half-cilium FRAP (montage of heat-maps, bottom). Scale bars, 2 μm (y), 5 s (x). (B) Instant velocity distribution of single SSTR3 movements along cilia in untreated and digitonin semi-permeabilized cells. The live cell data (blue) was fitted to a mixed model combining the permeabilized data (green) and an additional Gaussian distribution (red), with the latter found to contribute a fraction of 21.8 +/− 12.6% (anterograde) and 24.3 +/− 5.5% (retrograde). n>1200. (C) Instant velocity distribution of single Smo movements along cilia in untreated and digitonin semi-permeabilized cells. The live cell data (blue) was fitted to a mixed model combining the permeabilized data (green) and an additional Gaussian distribution (red), with the latter found to contribute a fraction of 32.1 +/− 5.9% (anterograde) and 34.1 +/− 1.9% (retrograde). n>1200. (D) Velocity distribution of GFP-IFT88 foci movements measured from kymographs. The mean velocities are shown in the plot. n>290. (E) ATP levels quantified by luciferin-luciferase bioluminescence assay normalized to the levels in control-treated cells. To deplete intracellular ATP, IMCD3 cells were permeabilized with 60 μg/ml digitonin for 7 min. Each treatment was measured in triplicate.
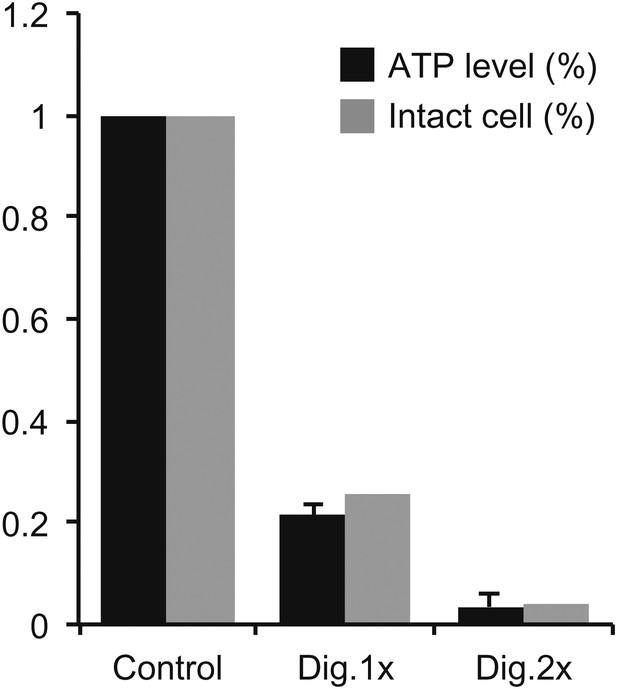
Measurement of ATP levels.
ATP levels quantified by luciferin-luciferase bioluminescence assay normalized to the levels in control-treated cells (ATP level [%]). To deplete intracellular ATP, IMCD3 cells were permeabilized with 30 μg/ml (Dig.1×) or 60 μg/ml (Dig. 2×) digitonin for 7 min. The efficiency of digitonin permeabilization was tested by Mab414 antibody staining. The percentages of unlabeled cells (Intact cell [%]) was similar to the corresponding ATP levels indicating that when a cell is permeabilized with digitonin, it looses all its ATP content.
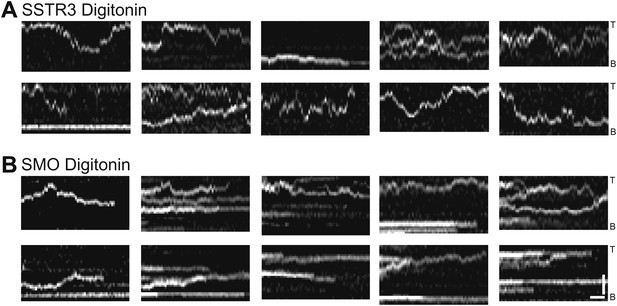
Additional kymographs.
Additional kymographs representing the movement of single mSA-Alexa647 labeled AP-SSTR3-GFP (A) and AP-Smo-YFP (B) in cells permeabilized with digitonin. The tip (T) and the base (B) of the cilium are indicated. Scale bars, 2 μm (y), 4 s (x).
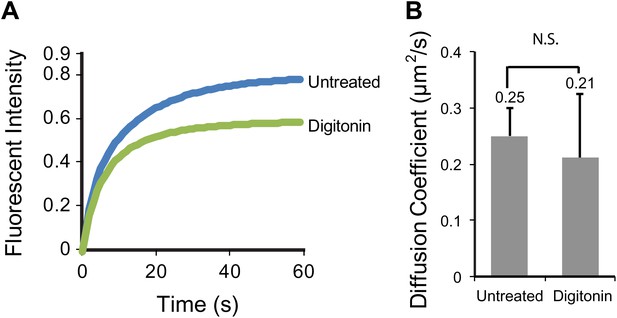
Digitonin permeabilization does not affect the mobility of SSTR3 in cilia.
(A) Averaged fitted curves of SSTR3-GFP half-cilium FRAP in different conditions. (B) The diffusion coefficients quantified from the FRAP recovery curves in (A). More than 10 cilia were analyzed for each condition. Error bars, SEM. p>0.05.
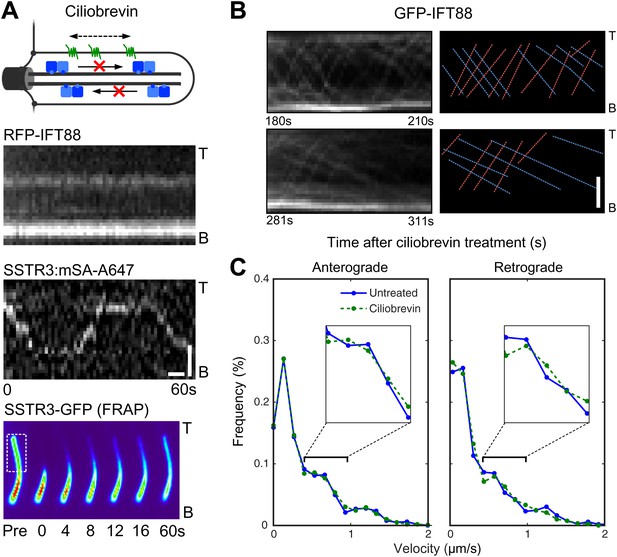
Ciliobrevin treatment abolishes IFT train movement but not active transport of SSTR3.
(A) Kymographs of simultaneous live cell imaging of TagRFP.T-IFT88 (RFP-IFT88, IFT complex) and single molecule SSTR3 (SSTR3:mSA-A647) movements in ciliobrevin treated cells (>30 min). The mobility of ciliary SSTR3 was confirmed by half-cilium FRAP (montage of heat-maps, bottom). Scale bars, 2 μm (y), 5 s (x). (B) Early time course of GFP-IFT88 foci movements after ciliobrevin D treatment. The velocity of retrograde tracks (blue dashed line) progressively decreased after 3.5 min, while anterograde foci (red dashed line) movements were unaffected until 5 min and then progressively reduced. (C) Instant velocity distributions of single SSTR3 movements in untreated vs ciliobrevin-treated cells. Statistical analyses show no significant difference (p>0.9) between the distributions of velocities of untreated and ciliobrevin-treated cells. n>1200.
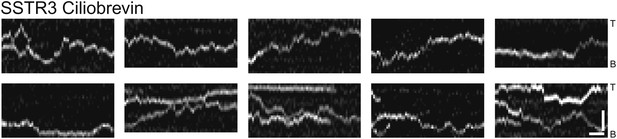
Additional kymographs.
Additional kymographs representing the movement of single mSA-Alexa647 labeled AP-SSTR3-GFP in cells treated with ciliobrevin. The tip (T) and the base (B) of the cilium are indicated. Scale bars, 2 μm (y), 4 s (x).
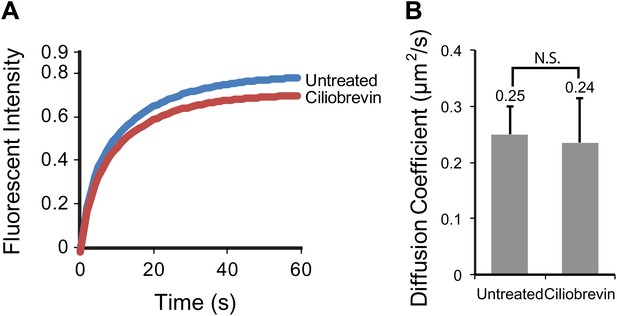
Ciliobrevin treatment does not affect the mobility of SSTR3 in cilia.
(A) Averaged fitted curves of SSTR3-GFP half-cilium FRAP in different conditions. (B) The diffusion coefficients quantified from the FRAP recovery curves in (A). More than 10 cilia were analyzed for each condition. Error bars, SEM. p>0.05.
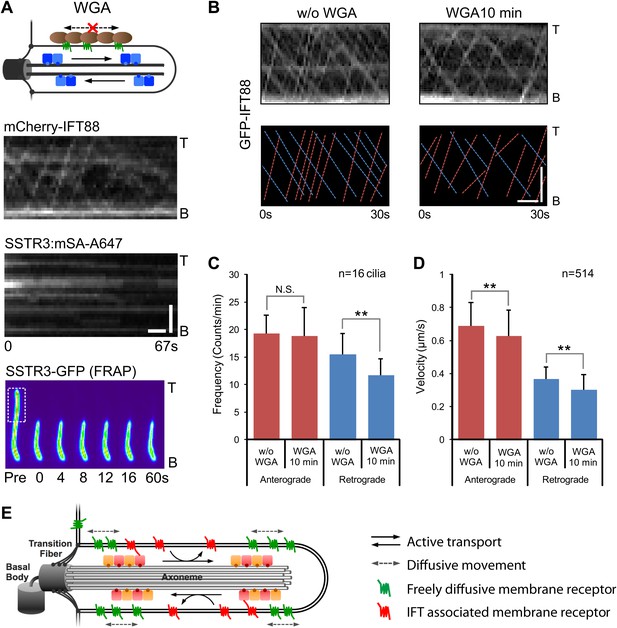
Effect of immobilizing membrane proteins on IFT train movements.
(A) Kymographs of simultaneous live cell imaging of mCherry-IFT88 (IFT complex) and single molecule SSTR3 (SSTR3:mSA-A647) movements in WGA treated cells (>7 min). The immobilization of ciliary SSTR3 was confirmed by half-cilium FRAP (montage of heat-maps, bottom). Scale bars, 2 μm (y), 5 s (x). (B) Representative kymographs of GFP-IFT88 fluorescent foci movements before WGA treatment (left, w/o WGA) and after 10 min of WGA treatment (right, WGA 10 min). The anterograde (red dashed lines) and the retrograde (blue dashed lines) movements are indicated in the bottom panels. Scale bars, 2 μm. (C and D) Bar charts representing the frequency (C) and velocity (D) of GFP-IFT88 fluorescent foci movements before and after 10 min of WGA treatment in IMCD3 cells. While the differences between WGA-treated and control cells are relatively small, with the exception of the frequency of anterograde trains they are statistically significant; N.S.: p>0.05; **p<0.05. (E) Schematics of ciliary membrane protein dynamics. Single molecule imaging reveals that the majority of ciliary SSTR3 (green) undergo free diffusion, which allows them to explore the ciliary surface efficiently. Only a small portion of ciliary SSTR3 (red) movement is related to IFT. The interaction between SSTR3 and IFT appears to be transient and dynamic.

Additional kymographs.
Additional kymographs representing the movement of single mSA-Alexa647 labeled AP-SSTR3-GFP in cells treated with WGA. The tip (T) and the base (B) of the cilium are indicated. Scale bars, 2 μm (y), 4 s (x).
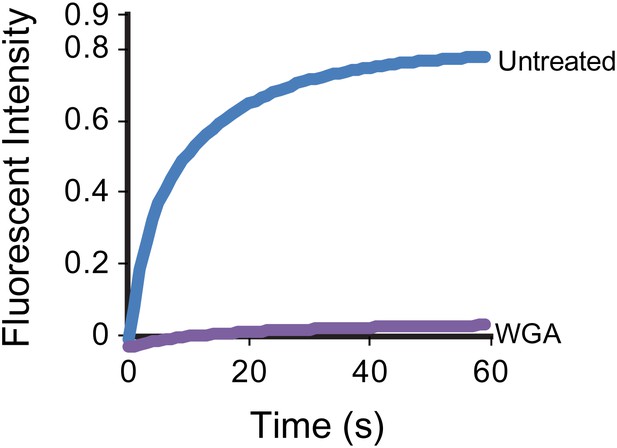
WGA treatment stops the mobility of SSTR3 in cilia.
Averaged fitted curves of SSTR3-GFP half-cilium FRAP in different conditions.
Videos
Live cell imaging of a single ciliary SSTR3 molecule.
IMCD3 cells stably expressing AP-SSTR3-GFP (pseudo-colored red) were transfected with BirA-ER to biotinylate AP-SSTR3-GFP. Biotinylated SSTR3 was detected with mSA-Alexa647 (mSA, pseudo-colored green). The tip (T) and the base (B) of the cilium are indicated. Scale bar, 2 μm.
Live cell imaging of a single ciliary Smo molecule.
IMCD3 cells stably expressing AP-Smo-YFP (Smo, pseudo-colored red) were transfected with BirA-ER to biotinylate AP-Smo-YFP. Biotinylated Smo was detected with mSA-Alexa647 (mSA, pseudo-colored green). The tip (T) and the base (B) of the cilium are indicated. Scale bar, 2 μm.
Representative examples of half-cilium FRAP in different conditions.
The distal half of the cilia were photobleached and the recovery rate of the SSTR3-GFP fluorescent signal in the bleached region was recorded at 1 s interval. Scale bar, 2 μm.
Live cell imaging of GFP-IFT88 fluorescent foci movement before (−WGA) and after 10 min of WGA treatment (+WGA) in IMCD3 cells.
Scale bar, 2 μm.





