Stochastic homeostasis in human airway epithelium is achieved by neutral competition of basal cell progenitors
Figures
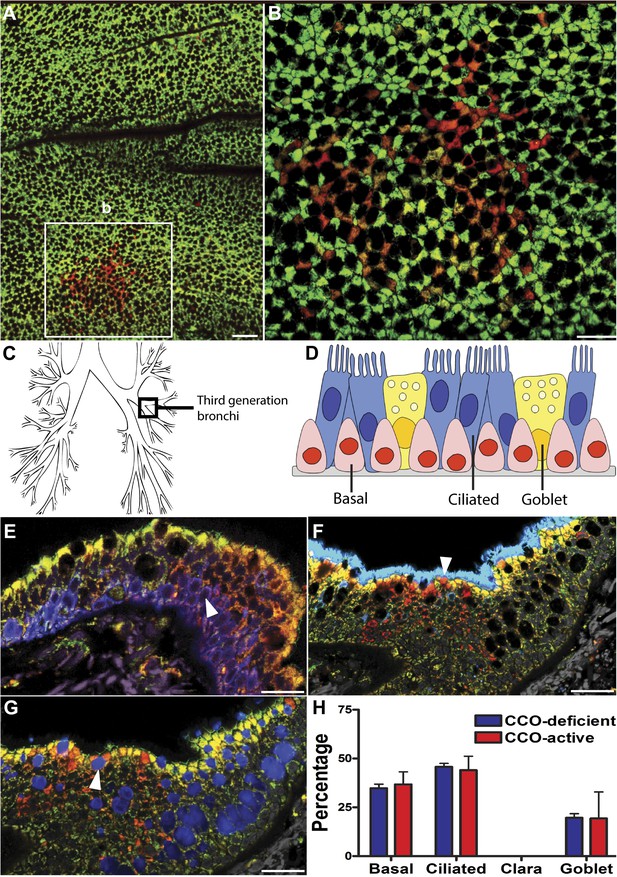
CCO-deficient human epithelial patches in the upper airway demonstrate multipotent differentiation.
(A and B) Third generation bronchi stained for CCO (green) and counterstained with mitochondrial marker porin (red) show CCO-deficient patches. The black spaces are Goblet cells with vesicles containing mucus. (C) Schematic showing the location of the third generation human bronchi. (D) Schematic showing lung epithelial cell types of the upper airways. (E) CCO-deficient clonal patches contain keratin 5 positive basal cells (blue; arrowhead), (F) acetylated tubulin positive ciliated cells (blue; arrowhead) and (G) Mucin 5AC positive goblet cells (blue: arrowhead). (H) There was no difference in lineage-specific differentiation between the CCO-deficient patches and the CCO-active lung epithelial cells. Cell percentages were calculated after counting all cells from 11 CCO-deficient patches and 11 CCO-active patches (basal cells–34.7 ± 2.1 vs 36.7 ± 6.5; ciliated cells–45.7 ± 1.7 vs 44 ± 7.2, Goblet cells–19.6 ± 2.1 vs 19.3 ± 13.5 [mean ± SD]). Scale bars—50 µm.
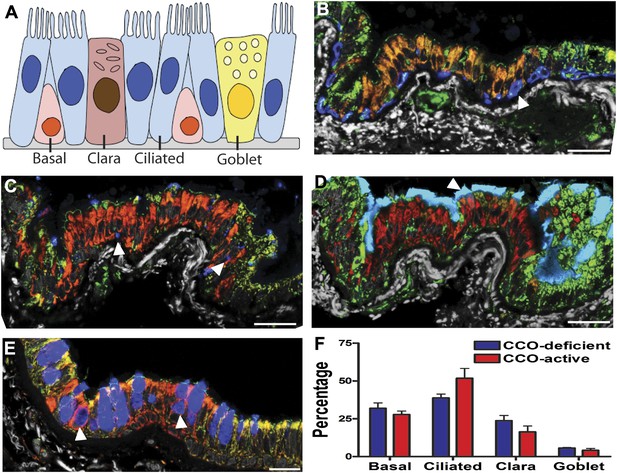
CCO-deficient human epithelial patches in the lower airway demonstrate multipotent differentiation.
(A) Schematic showing lung epithelial cell types of the lower airways. (B) CCO-deficient clonal patches contain keratin 5 positive basal cells (blue; arrowhead), (C) Clara cell secretory protein positive Clara cells (blue; arrowhead), (D) acetylated tubulin positive ciliated cells (blue; arrowhead) and (E) Mucin 5AC positive goblet cells (blue; arrowhead). (F) There was no difference in lineage-specific differentiation between the CCO-deficient patches and the CCO-active lung epithelial cells. Cell percentages were calculated after counting all cells from 11 CCO-deficient patches and 11 CCO-active patches (basal cells–31.9 ± 3.6 vs 27.8 ± 2.3; ciliated cells–38.8 ± 2.6 vs 51.8 ± 6.5, Clara cells–23.7 ± 3.5 vs 16.3 ± 5.6, Goblet cells–5.6 ± 0.4 vs 4.1 ± 1.3 [mean ± SD]). Scale bars—50 µm.
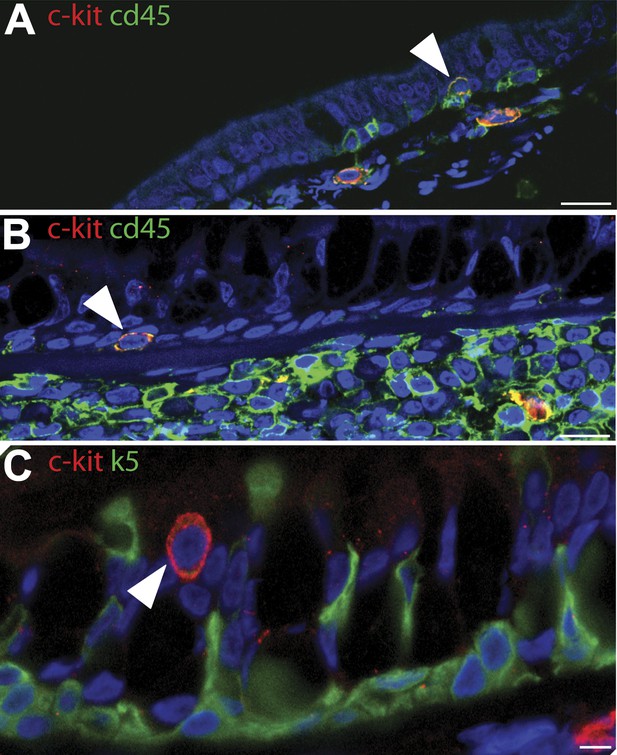
c-kit cells stained (red) did not stain with epithelial markers (green) and were not present in most patches.
(A and B) c-kit positive cells (red) were positive for CD45, a leucocyte common antigen, (green) making cells appear yellow and (C) were not positive for K5 basal cell marker (green) and they were not present in most patches.
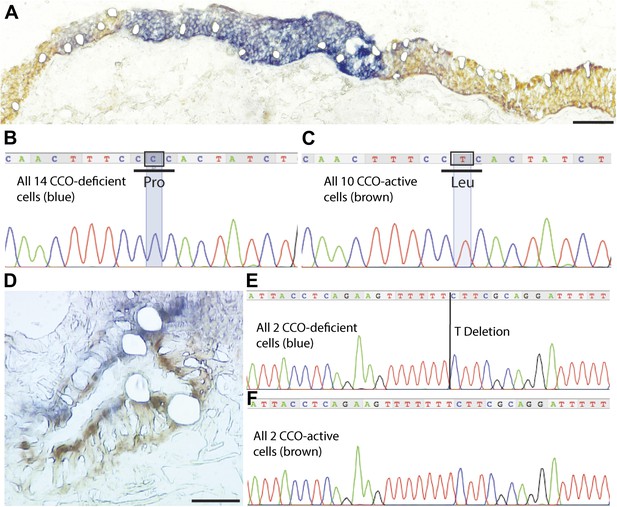
CCO-deficient patches are clonal with distinct mutations from neighbouring patches.
(B and D) Histochemistry demonstrates CCO-deficient patch (dark blue) surrounded by CCO-active adjacent lung epithelial cells (brown) with individual cells laser micro-dissected. (B and E) Homoplasmic mutation shared by all CCO-deficient (blue) lung epithelial cells both within the main patch and blue cells scattered around the edges of the patch. (C and F) CCO-active cells show wild-type genotype. Scale bars—100 µm.
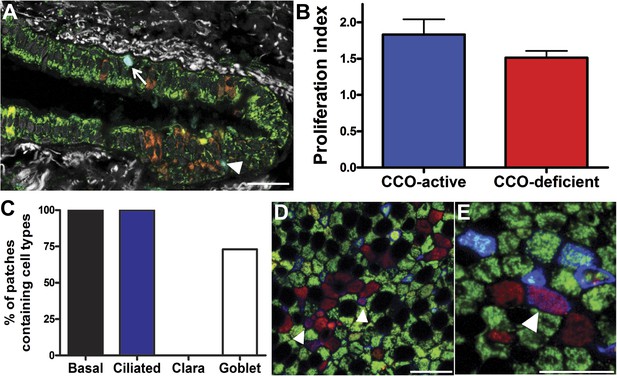
CCO-deficient upper airway patches are representative of the upper airway.
(A) Ki67 immunofluorescence showing a positive CCO-deficient cell (arrowhead) and a positive CCO-active cell (arrow). (B) There was no difference in the proliferation index between CCO-deficient clonal patches and CCO-active lung epithelia. CCO-active patches (n = 11) vs CCO-deficient patches (n = 11) (mean ± SD, 1.8 ± 0.2 vs 1.5 ± 0.1). (C) Cell type examination of patches greater than five cells showed that only ciliated and basal cells were present in all CCO-deficient patches. (D) Shows a clone greater than five cells with basal cells—arrowhead points to violet basal cells due to an expression of Porin (red) and Krt5 (blue) within a CCO-deficient patch. (E) Shows a small clone with a basal cell—arrowhead points to violet basal cells due to an expression of Porin (red) and Krt5 (blue) within a CCO-deficient patch. (CCO, green; Porin, red; Keratin 5, blue). The black spaces in D and E are Goblet cells with vesicles containing mucus. Scale bars—50 µm.
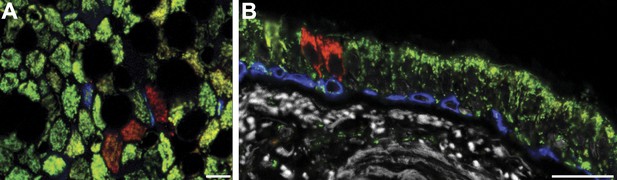
Shows rare small clones with no basal cells.
CCO-deficient cells (red) after porin (red) + CCO (green) staining show occasional small clones with no basal cells (blue). Scale bars—50 µm.
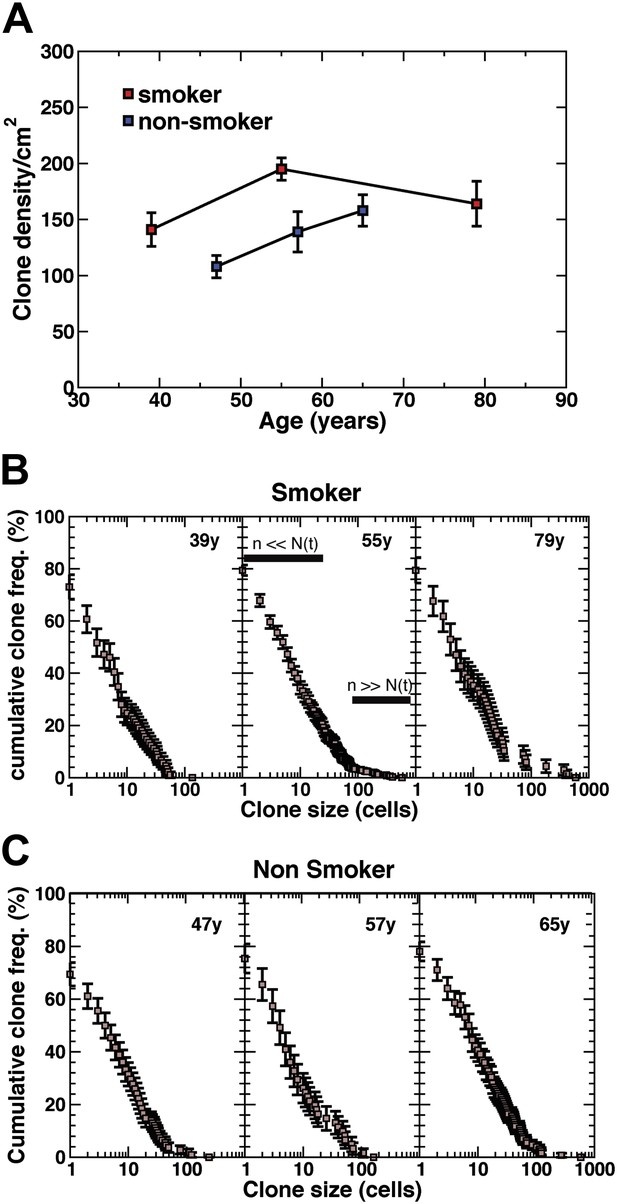
Density of CCO-deficient clones and their size distribution.
(A) Measured clone density (number per unit area) of CCO-deficient clones measured in three smokers and three non-smokers of different ages. Note that, with increasing age of patients, the clone density changes little, while the overall density in the non-smoker is significantly smaller than smokers of a similar age. (B and C) Cumulative clone size distribution of CCO-deficient clones, Cn(t), for the three smokers (B) and three non-smokers (C) showing the probability that a clone has a size larger than n cells in patients of age t = 39 years, 55 years, and 79 years for the smokers and 47 years, 57 years and 65 years for the non-smokers. (Errors denote SEM).
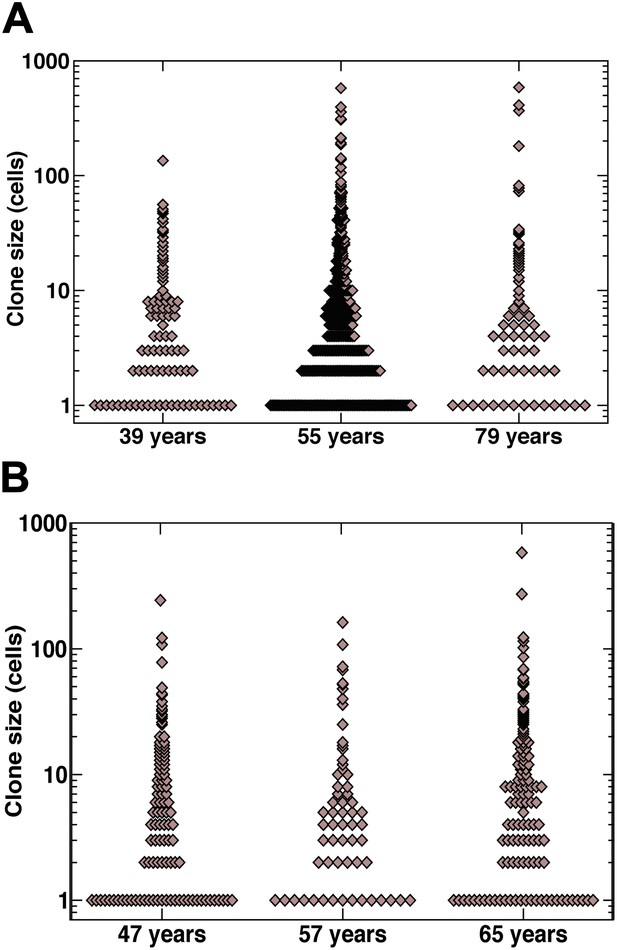
Raw clone size data.
(A) Smokers and (B) non-smokers. Each data point depicts an individual clone.
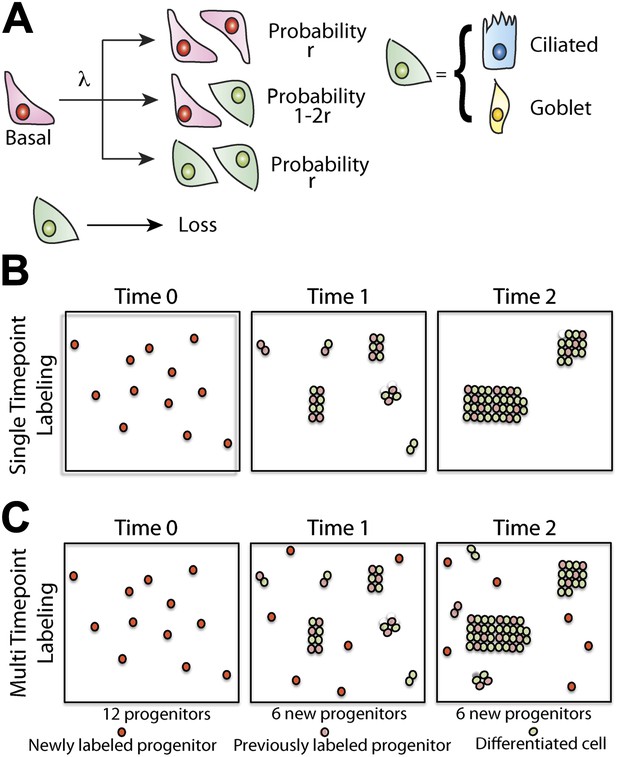
Maintenance of lung epithelium involves neutral competition.
(A) Schematic showing the model hypothesis used to interpret and analyse the clonal fate data. According to this model, maintenance of the human airway epithelium involves the balanced stochastic fate of tissue-maintaining cells in which cell division results in all three fate outcomes: symmetric duplication, asymmetric division, or symmetric differentiation. λ denotes the corresponding cell division rate, and r controls the fraction of divisions that lead to symmetric fate outcome. Note that differentiation can lead to any one of the differentiated cell types, with probabilities commensurate with the tissue composition. Here, for simplicity, we associate tissue-maintaining cells with the basal progenitor population. However, if tissue-maintaining cells constitute only a subpopulation of these basal progenitors, we would obtain the same long-term clone fate behaviour, Equation 1, while the overall fraction of tissue-maintaining cells, ρ, would have to be adjusted accordingly. (B) Schematic depicting the pattern of clonal evolution following pulse-labelling of tissue. As tissue turns over, chance clonal loss is perfectly compensated by the expansion of other clones so that the overall number of labelled progenitor cells remains approximately constant—a process reminiscent of ‘neutral drift’. (C) Through the spontaneous acquisition of somatic mtDNA mutation single cells become clonally marked throughout adulthood. As result, the clones distribution at any given time point represents the amalgamation of clones of different ages from those induced at the earliest times when CCO-deficient cells first become visible, to those marked within the recent past.
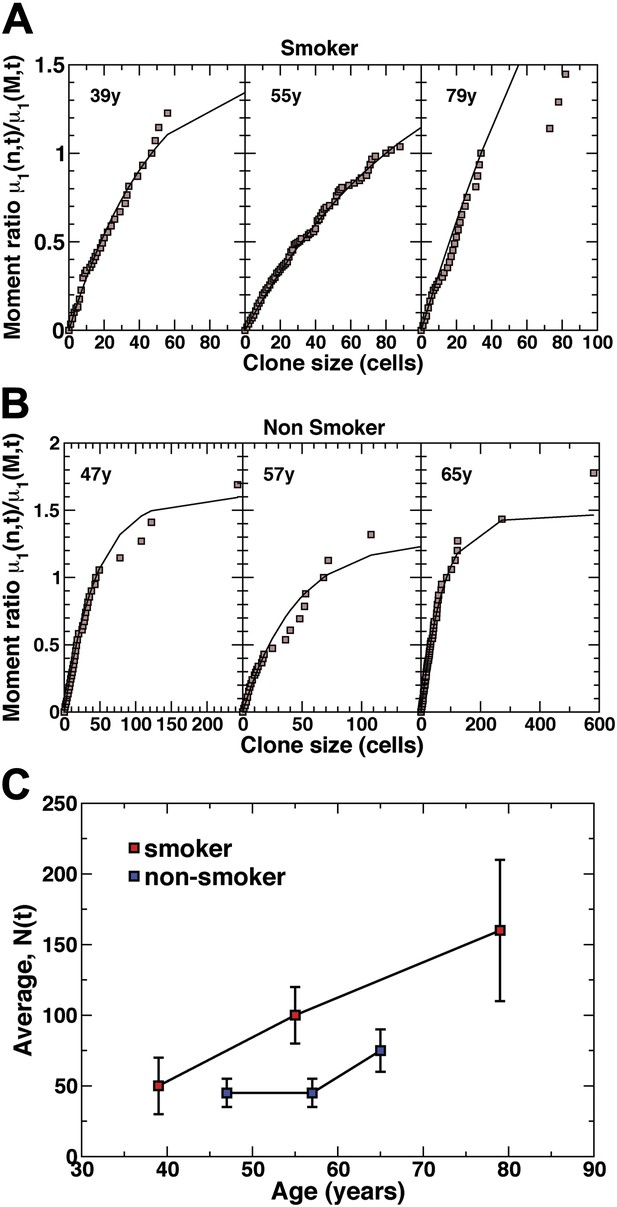
Quantitative analysis of the clonal fate data.
Comparison and fit of the first incomplete moment, a derivative of the cumulative clone size distribution (Figure 4B,C), to the model prediction, Equation 1 (for details, see ‘Mathematical analysis’) for (A) smokers and (B) non-smokers. Points show data and lines show the model prediction. From these comparisons, we obtain the one fitting parameter, N(t), defining the average size of clones created at the earliest time point, as described in the main text. The resulting values of N(t) from these fits are shown in (C) for the three smokers and three non-smokers. Error bars depict the approximate range of N(t) values that provide satisfactory fits to the measured clonal fate data. Note that these inferred values are consistent with the predicted linear dependence of N(t) on age t providing further corroboration of the model dynamics.
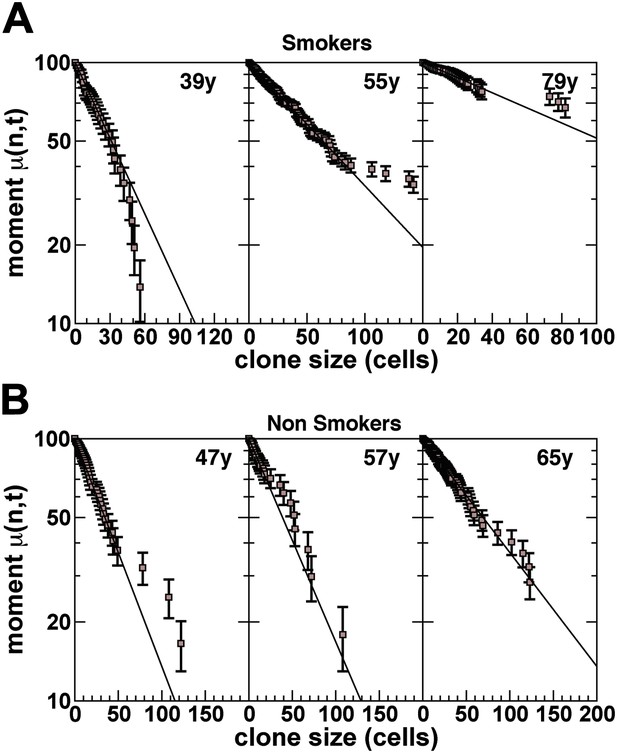
First incomplete moment distribution, derived from the cumulative clone size distribution.
(A) Smokers and (B) non-smokers (for details of the definition of the distribution, see ‘Mathematical analysis’). Points show data and lines show a fit to an exponential size dependence, as predicted by the model dynamics.
Tables
Patient characteristics
https://doi.org/10.7554/eLife.00966.006| Patient | Age (Surgery) | Sex | Smoking (pack years) |
|---|---|---|---|
| 1 | 39 | M | 30 |
| 2 | 55 | F | 40 |
| 3 | 79 | M | 40 |
| 4 | 25 | F | Non smoker |
| 5 | 47 | M | Non smoker |
| 6 | 57 | F | Non smoker |
| 7 | 65 | M | Non smoker |
Analysis of mtDNA mutations of seven CCO-deficient patches, from three patients
https://doi.org/10.7554/eLife.00966.008| Patient | Patch | CCO-deficient cells mutation | Gene |
|---|---|---|---|
| 1 | 1 | 9850 T>C | MT-CO3 |
| 2 | 6708 G>A | MT-CO1 | |
| 3 | 6838 T>C | MT-CO1 | |
| 4 | 6690 G>A | MT-CO1 | |
| 5 | 6692 A deletion | MT-CO1 | |
| 2 | 6 | 9478 T deletion | MT-CO3 |
| 3 | 7 | 6087 G>A | MT-CO1 |
-
Multiple single cells laser captured from each patch confirmed the same sporadic mutation and hence patch clonality.





