Charcot-Marie-Tooth 2B mutations in rab7 cause dosage-dependent neurodegeneration due to partial loss of function
Figures
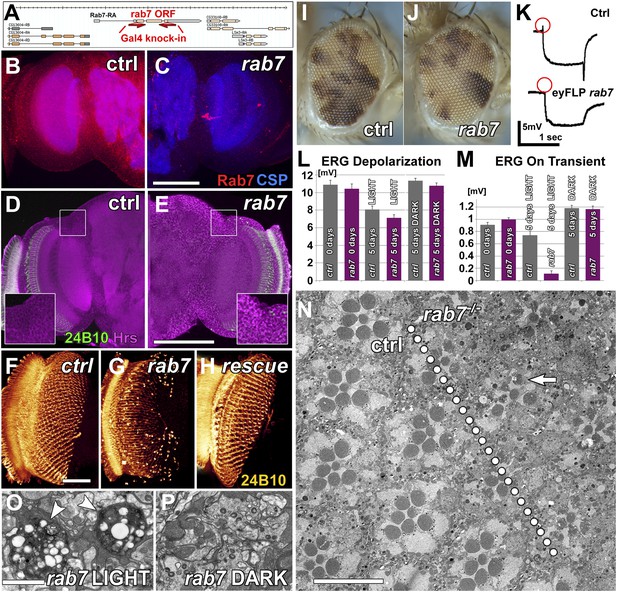
Loss of rab7 in neurons causes adult-onset degeneration that begins with a loss of synaptic function.
(A) Knock-out strategy: replacement of the complete rab7 open reading frame with a Gal4 knock-in cassette (Chan et al., 2011, 2012). (B and C) Pupal brains at P+35% for wild-type (B) and the rab7 mutant (C). Red: Rab7, Blue: synaptic vesicle marker CSP. Note that the red labeling in the center of (C) stems from 3xP3-RFP expression that marks the knock-in cassette. (D and E) Pupal brain at P+50% from wild-type (D) and the rab7 mutant (E). Green: photoreceptor-specific mAb 24B10; magenta: the endosome marker HRS. (F–H) 3D visualization of photoreceptor axon projections in ctrl, rab7 homozygous mutant, and a rab7 homozygous mutant expressing UAS-YFP-Rab7 (rescue) (Zhang et al., 2007). (I and J) Genetic mosaics with rab7 mutant photoreceptors in otherwise heterozygous flies exhibit no eye development defects. (K–M) Electroretinogram (ERG) responses from flies with rab7 mutant eyes. Light stimulation for 5 days leads to the almost complete loss of synaptic function (ERG ‘on’ transient, M); despite normal photoreceptor responses to light (ERG Depolarization, L). (K) Sample ERG traces from 5-day old flies. (N–P) Electron microscopy of mutant eyes showing rhabdomere degeneration in rab7 mutant clones (arrow) (N) and synaptic terminals (O and P). Note that the presence of pigment between ommatidia marks patches of wild-type ommatidia (compare I and J and arrowheads in Figure 1—figure supplement 1C). (O) Light stimulation leads to vacuolarization and degeneration of rab7 synaptic terminals (arrowheads). Scale bar in (C) for (B and C) and (E) for (D and E): 50 µm; in (F) for (F–H): 20 µm; in (N): 10 µm; in (O) for (O and P): 1 µm.
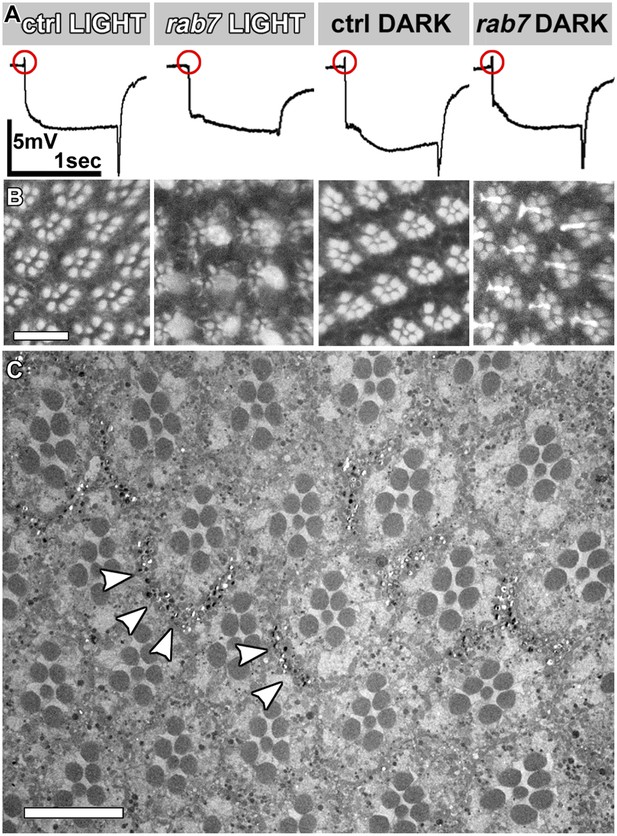
Functional and morphological degeneration in rab7 mutant photoreceptors in 5-day light or 5-day dark-raised flies.
(A) Representative ERG traces for indicated conditions and quantification in Figure 1L–M. (B) Phalloidin labeling of rhadomeres in the eye reveals structural degeneration in rab7 mutants as a function of light stimulation. (C) Electron micrograph of an eye cross-section in 5-day dark-raised flies. Arrowheads indicate pigment, which mark heterozygous or wild type control patches in the eye. Scale bars (B) and (C): 10 µm.
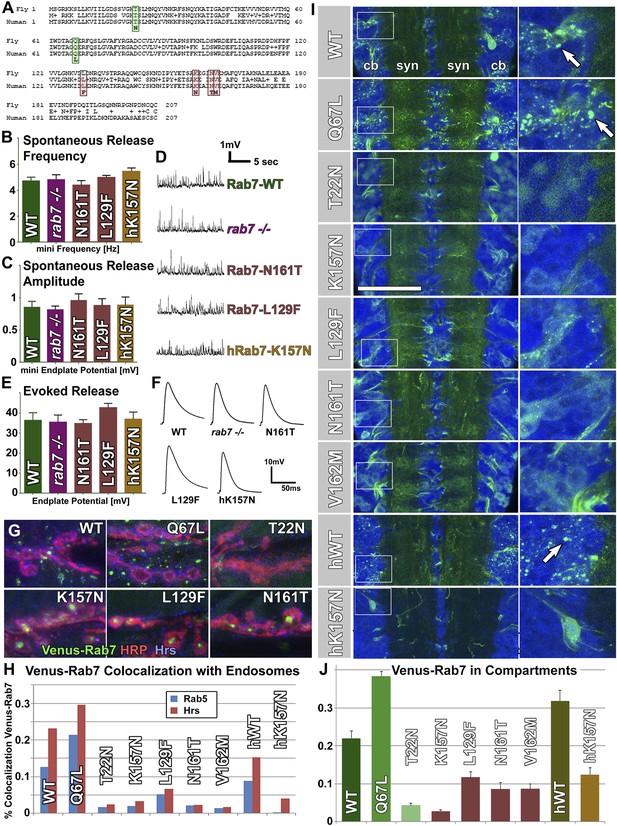
Overexpression of Venus-tagged Rab7 variants in motor neurons.
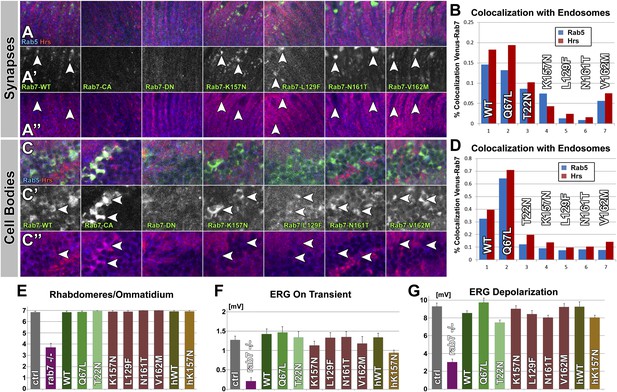
(A) Protein alignment of fly and human Rab7 reveals 100% conservation of protein length and the precise locations of all CMT2B mutations (red) and the classically designated ‘dominant negative’ and ‘constitutively active’ mutations (green). (B–F) Electrophysiological recordings from the larval neuromuscular junction for ctrl, the rab7 null mutant and overexpression of rab7N161T, rab7L129F and the human disease gene hrab7K157N. Spontaneous vesicle release (minis) exhibit normal frequency (B) and amplitude (C). Similarly, evoked neurotransmission is indistinguishable for all genotypes (E and F). (G) Synaptic boutons at the larval neuromuscular junction, immunolabeled for the presynaptic membrane (HRP, red), the endosomal marker Hrs (blue) and six different Venus-Rab7 proteins. (H) Colocalization quantification for all nine Venus-Rab7 proteins at the neuromuscular junction with the endosomal markers Rab5 and Hrs. (I and J) Analysis of the subcellular protein localizations of all Venus-tagged Rab7 variants (green) in the larval ventral ganglion, as previously performed for all Rab GTPases (Chan et al., 2011). Blue (DNA labeled with Toto-3) indicates areas of cell bodies (cb) and synapses (syn) in two center stripes. (J) Quantification of Venus fluorescence signal in 3D datasets inside clearly discernable compartments (arrows in I) as ratio of total fluorescence signal. See ‘Materials and methods’ for details. Scale bar in (I): 50 µm.
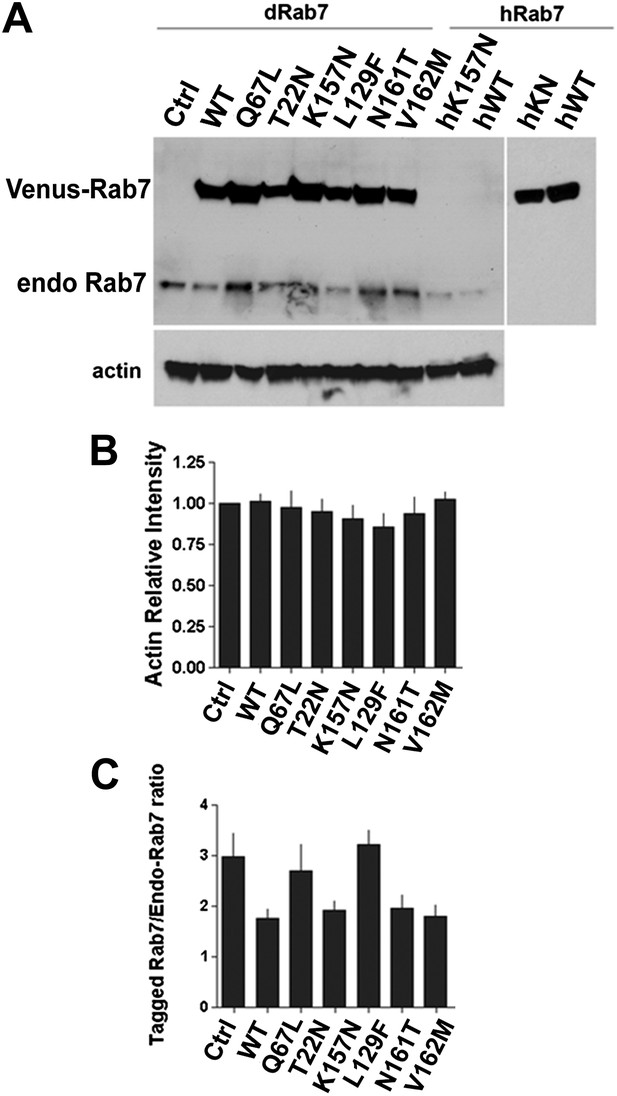
Western blot analysis of total protein extract from fly eyes.
Single copies of Venus-Rab7 transgenes of the indicated mutant were driven by rab7Gal4-knock-in. (A) An antibody against Drosophila Rab7 recognizes both endogenous Rab7 and the Venus-tagged Drosophila Rab7 variants, but not human Rab7 protein. An antibody again human Rab7 only recognizes the Venus-tagged human protein variants, but not th endogenous Drosophila Rab7. (B) Actin Loading Control. (C) Quantification of relative band intensities over three separate experiments reveals that the Rab7-Gal4 expressed UAS-rab7 transgenes are expressed at 1.8–3-fold higher amounts than the endogenous Rab7 protein in heterozygosity.
Overexpression of Venus-tagged Rab7 variants in photoreceptor sensory neurons.
(A–D) Immuno-histochemical analyses of Venus-Rab7 protein localization and colocalization with the endosomal markers Rab5 (blue) and Hrs (red). (A) Longitudinal sections through the adult lamina, where photoreceptor neurons R1-R6 terminate. (C) Cell bodies of neurons in the medulla cortex. Green: Venus-Rab7 proteins, red: Hrs, blue: Rab5. (B and D) Quantification of Venus-Rab7 colocalization with Rab5 and Hrs for the indicated genotypes at synapses (B) and in cell bodies (D). (E and F) Quantification of morphological and functional analyses of Rab7 overexpression for all nine variants reveals a loss of endosomal colocalization for all CMT2B mutants. (genotype: UAS-rab7-X/+; rab7Gal4-knock-in/+) (E) The numbers of rhabdomeres per ommatidial cross section reveal that overexpression of none of the rab7 mutants leads to morphological disruption similar to the mutant. (F and G) Overexpression of none of the mutant rab7 variants causes defects in ERG depolarization or synaptic transmission (‘on’ transient). Control and overexpression experiments exhibit no statistically significant variance (ANOVA). Picture and ERG traces for all genotypes are shown in Figure 3—figure supplement 1.
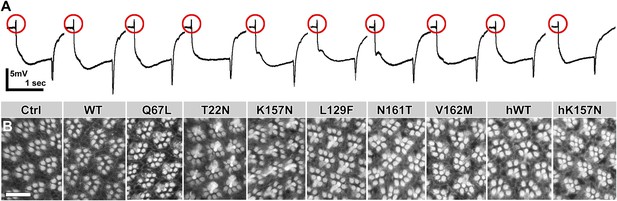
ERG recordings from overexpression experiments of the indicated rab7 mutant variants at identical levels using rab7Gal4-knock-in in heterozygosity.
(A) Representative ERG traces. Quantification in Figure 2C,D. (B) Representative rhabdomere labelings in eye cross-sections. Quantification in Figure 2B. Scale bar in (B): 10 µm.
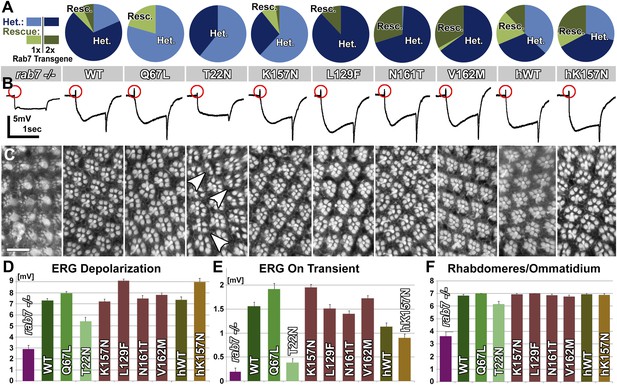
Rescue experiments using rab7Gal4-knock-in driven UAS-venus-rab7 variants.
(A) A population experiment over four generations reveals the fittest and unhealthy genotypes. Light Blue shows the fraction of flies heterozygous for the null mutant and expressing 1 copy (2–3-fold overexpression) for each of the nine transgenes (genotype: UAS-rab7-X/+; rab7Gal4-knock-in/+). Dark Blue shows the fraction of heterozygous rab7 flies expressing two copies of the respective transgenes (genotype: UAS-rab7-X/UAS-rab7-X; rab7Gal4-knock-in/+). Light green shows the fraction of homozygous null mutant flies rescued through expression of one copy (2–3-fold overexpression) of the respective transgene (genotype: UAS-rab7-X/+; rab7Gal4-knock-in/rab7Gal4-knock-in). Dark green shows the fraction of null mutant flies rescued by two copies (4–6-fold) overexpression of one of the transgenes (genotype: UAS-rab7-X/UAS-rab7-X; rab7Gal4-knock-in/rab7Gal4-knock-in). (B–F) Rescue experiments of synaptic and neuronal degeneration using each of the nine rab7 transgenes in null mutant photoreceptors after 10 days of constant light stimulation. (B) Representative ERG traces with measured components in (D) and (E). (C) Representative eye cross sections showing the array and number of rhabdomeres per ommatidium, and corresponding counts in (F). Variance for measurements in (D–F) is significantly different for rab7 null mutant and rab7T22N rescue (ANOVA). Scale bar in (C): 10 µm.
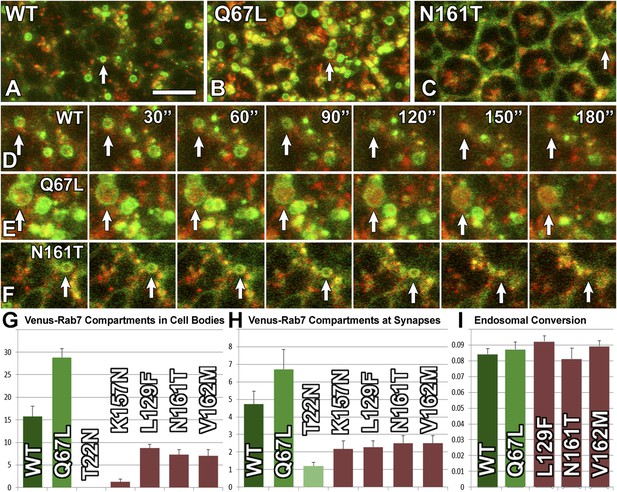
Live imaging reveals that CMT2B Rab7 proteins exhibit defective endosomal recruitment but do not affect endosomal maturation.
(A–F) Snapshots from live imaging datasets for three genotypes with a time lapse of 30 s (A and D) Venus-Rab7-WT and spin-RFP; (B and E) Venus-Rab7-Q67L and spin-RFP; (C and F) Venus-Rab7-N161T and spin-RFP. Arrows mark individual Rab7-positive compartments >=0.5 µm diameter. The same compartments marked in (A–C) are followed over time in (D–F). (G) Quantification of compartments as in (A–F) for all genotypes per 500 μm2 area. (H) Quantification of Venus-Rab7 compartments (individual distinguishable green punctae) per photoreceptor axon terminal in the brain for the indicated genotypes. (I) Quantification of the fraction of compartments that underwent green-to-red conversion over a 5 min period in same data as (G). Note that Rab7-T22N and Rab7-K157N did not mark sufficient compartments for this analysis. See also: Videos 1–10. Scale bar in (A): 10 µm.
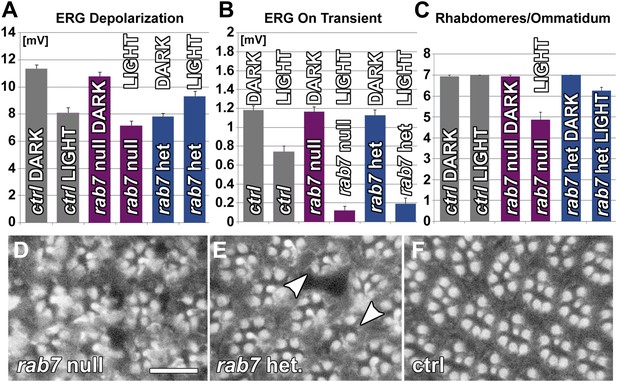
Photoreceptor neurons are haploinsufficient for rab7 function as revealed by functional and morphological measurements 10 days after constant light stimulation.
(A and B). Quantification of ERG depolarization and synaptic transmission (‘on’ transients) in null mutants and heterozygotes for rab7. (C–F) Ommatidial rhabdomere composition from eye cross sections exposed to various experimental conditions. Scale bar in (D) for (D–F): 10 µm.
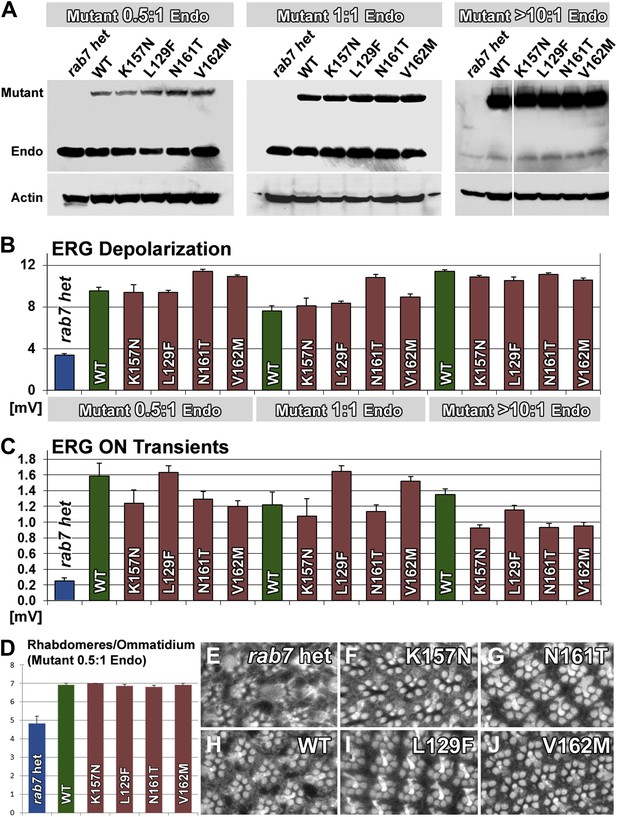
A wide range of CMT2B expression levels rescues partial loss of rab7 function without dominant toxic effects.
(A) Western blots for Rab7 of adult fly eyes expressing different levels of the Venus-tagged mutant transgenes. (B and C) ERG depolarization and On transient measurements after a 5-day light stimulation protocol. WT and CMT2B mutant values are not statistically significantly different in an ANOVA test. (D) Morphological analyses of rhabdomere structure in fly eyes with a ratio of 0.5:1 expression of the mutant transgenes vs endogenous heterozygous protein amounts. (E–J) Representative images of the rhabdomer structure for the indicated genotypes.
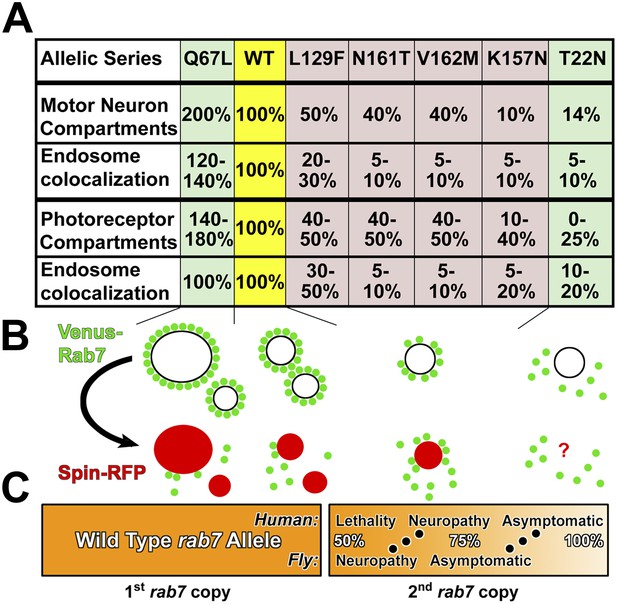
Summary of rab7 allele function and partial loss-of-function model.
(A) The seven rab7 alleles analyzed here form an allelic series based on endosome recruitment and Rab5/Hrs colocalization in motor neurons and photoreceptor sensory neurons. First row summarizes data in Figure 2J; second row summarize data in Figure 2H; third row summarizes data in Figure 5G,H and the fourth row summarizes data in Figure 3B,D. Note that only the constitutively active Rab7-Q67L exhibits clearly reduced fitness at higher expression levels, but none of the CMT2B mutants (Figure 4A). (B) The allelic series is characterized by a gradual loss of the ability of Rab7 to be recruited to endosomal compartments. In contrast, conversion of Rab7-positive compartments is normal. (C) Partial loss-of-function model. A few day-old flies exhibit neuropathy-like phenotypes at 50% rab7 function, which can be rescued by mildly increasing rab7 function by expressing partial loss-of-function alleles. Humans exhibit neuropathy symptoms only after ≥12 years at levels that are, based on our analysis of the CMT2B alleles plus one wild type copy, between 60% and 90% of total rab7 function.





