Inter-Golgi transport mediated by COPI-containing vesicles carrying small cargoes
Figures
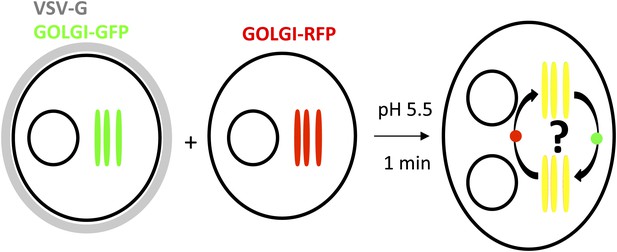
General procedure.
HeLa cells co-expressing a GFP-labeled Golgi localized protein and VSV-G are mixed with HeLa cells expressing a RFP-labeled Golgi localized protein. Cell fusion is triggered by acidic exposure of cell surface targeted VSV-G. Cycloheximide was added 1 hr prior to fusing the cells and during the imaging procedure, to prevent de novo protein synthesis. Golgi-content mixing is assessed by live imaging confocal imaging, visualization and characterization of the putative transport intermediates are assessed by STED microscopy.
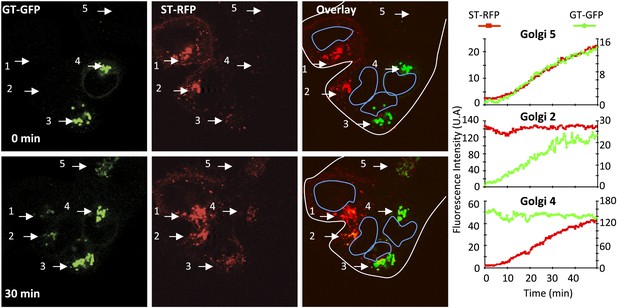
Inter-Golgi transport of Golgi resident glycosyl-transferase proteins.
HeLa cells expressing either GT-GFP and VSV-G, or ST-RFP were mixed and fused by acidic exposure (1 min, pH 5) and then monitored by confocal video-microscopy at 20°C. Cells were treated with CHX (100 μg/ml) 2 hr prior to fusion and during the imaging. Graphs show fluorescence intensity of markers within Golgi 2, 4, and 5 over time. Results are representative of three independent experiments.
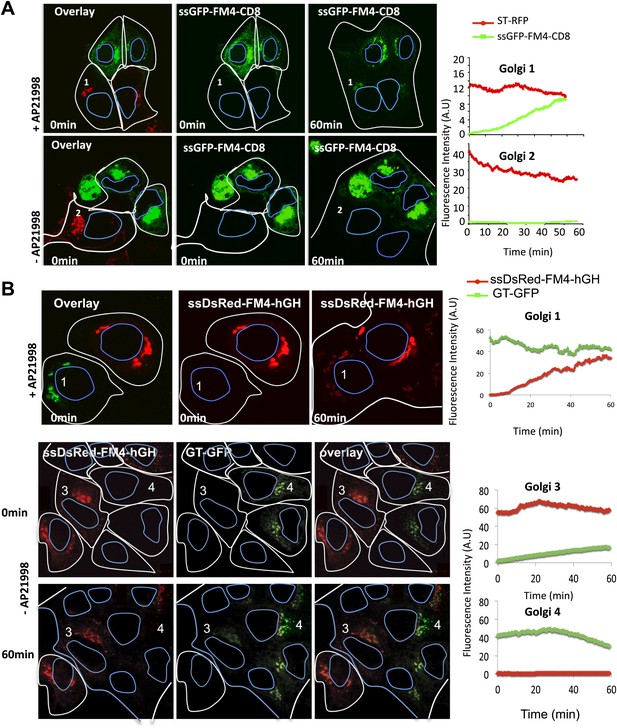
Inter-Golgi transport of small anterograde cargo.
(A) HeLa cells expressing either ssGFP-FM4-CD8 or ST-RFP (+VSV-G) were mixed and fused. Before fusion, cells were incubated at 20°C for 2 hr in the presence of CHX (100 μg/ml) and AP21998 (500 nM) to trigger the release of the cargo from the ER and its accumulation in the Golgi. Both drugs were maintained during the imaging. For the study of the aggregated cargo (staples), AP21988 was removed 30 min before fusion, and cells were imaged in an AP21988-depleted medium at 20°C. Graphs show quantification of both markers over time for Golgi 1 and 2. Results are representative of three independent experiments. (B) HeLa cells expressing either ssDsred-FM4-hGH or GT-GFP (+VSV-G) were mixed and fused. As for (A) AP21988 was removed to trigger the formation of soluble aggregates within the Golgi. Graphs show quantification of both markers over time for Golgi 1 to 4. Results are representative of two independent experiments.
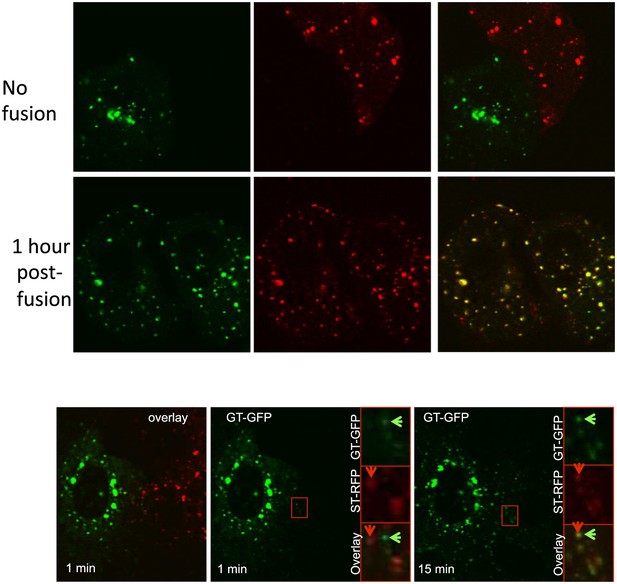
Inter-Golgi transport is microtubule independent.
HeLa cell expressing GT-GFP (+VSV-G) and ST-RFP were mixed and fused. When required, nocodazole (2.10−3 μg/ml) was added 2 hr before the fusion and was maintained during the time course of the experiment. Upper panel, fixed cell fixed 1 hr post-fusion. Lower panel, live imaging of inter-Golgi exchange in nocodazole-treated poly-karyon.
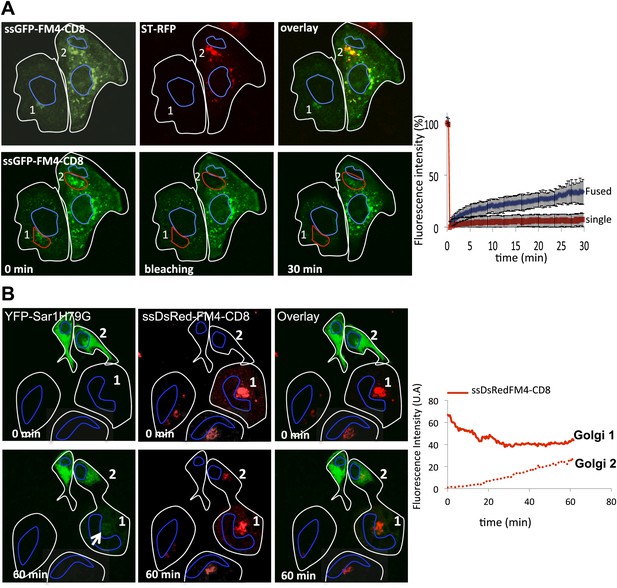
ER as a minor source of anterograde cargo during inter-Golgi transport.
(A) FRAP performed on Golgi within fused or single cells treated with H89 (50 μM). Top panel, Golgi 1 (green) from a single cell and Golgi 2 (green and red) from fused cells 30 min post-fusion in presence of CHX and AP21988 at 20°C. Lower panel, 15 min after the H89 treatment (t = 0 min), Golgi 1 and 2 were photobleached and the fluorescence recovery was monitored by confocal video-microscopy. The graph shows the fluorescence intensity over time of the GFP marker within fused cells (di- or polykaryons) or single cells treated with H89. The values are the mean of three to five independent experiments. (B) Sar1H79G does not inhibit inter-Golgi transport of anterograde cargo. HeLa cells expressing either ssDsRed-FM4-CD8 (+VSVG) or YFP-SarH79 G were mixed and fused. Before fusion, cells were incubated at 20°C for 2 hr in the presence of CHX (100 μg/ml) and AP21998 (500 nM) to trigger the release of the cargo from the ER and its accumulation in the Golgi. Graphs show quantification of ssDsRed-FM4-CD8 over time at both donor (Golgi 1) and acceptor (Golgi 2) compartments.
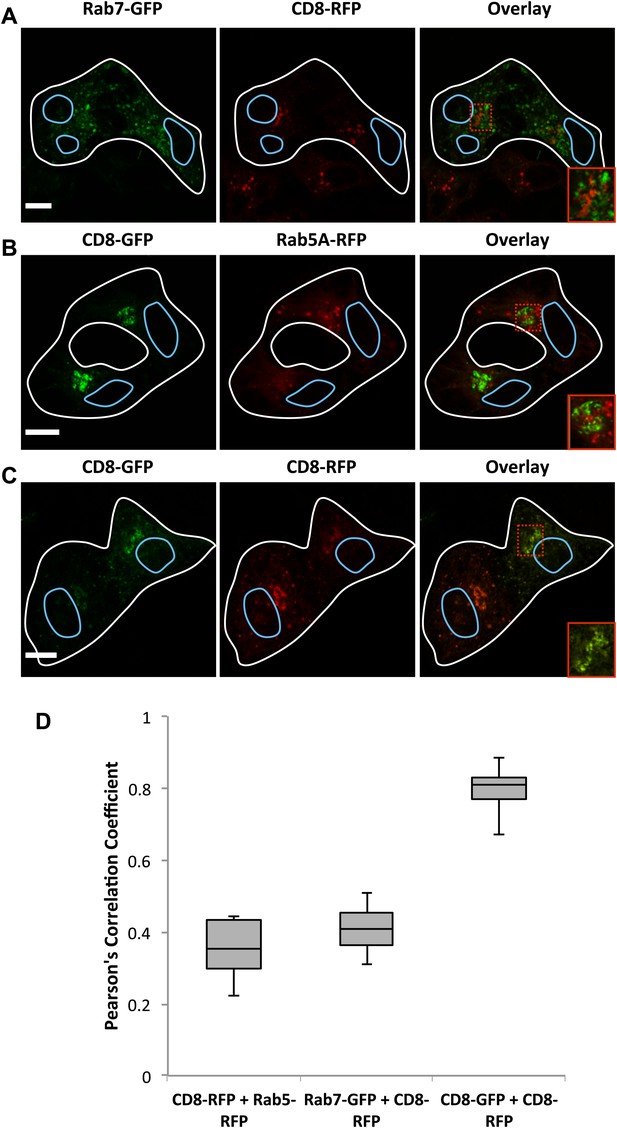
Inter-Golgi transport does not involved transit through endosomes.
HeLa cells expressing (A) Rab7-GFP (+VSV-G) or ssDsRed-FM4-CD8 (+VSV-G), (B) ssGFP-FM4-CD8 (+VSV-G) or Rab5A-RFP (+VSV-G), (C) ssGFP-FM4-CD8 (+VSV-G) or ssDsRed-FM4-CD8 (+VSV-G), were incubated at 20°C in the presence of AP21998 and CHX for 2 hr, fused and fixed after 15 min. Cells were imaged and the Pearson's correlation was measured for each fused cell using Volocity. (D) Box plots showing the distributions of the calculated Pearson's coefficient for experimental conditions described in A–D. Rab7-GFP and ssDsRed-Fm4-CD8 = 12, ssGFP-FM4-CD8 and Rab5A-RFP n+10, ssGFP-FM4-GFP and ssDsRed-FM4-CD8 n = 36, ssGFP-FM4-CD8 and COPI n = 42. Fused outlined in white, nuclei in blue. Red box shows zoom area in lower right of overlay. Scale bar = 10 μm, zoom scale bar = 2 μm.
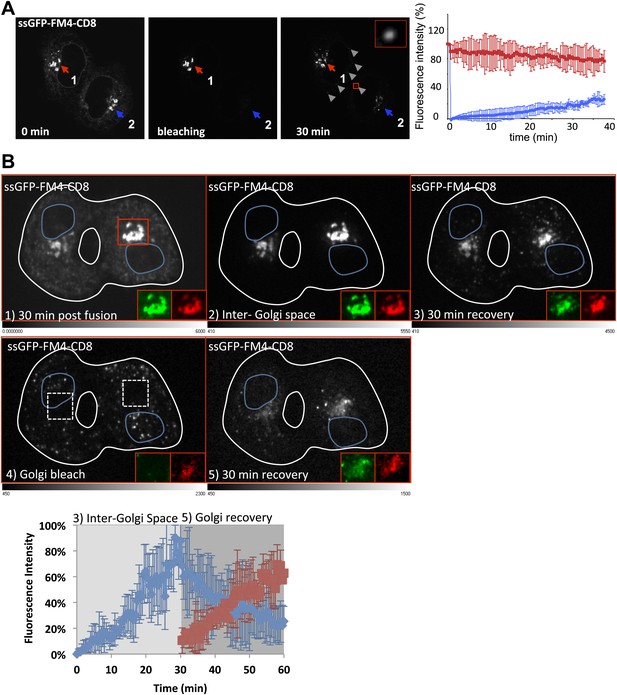
Diffusible inter-Golgi transport intermediates.
(A) 30 min post fusion, the entire volume a dikaryon (ER and acceptor Golgi 2) was photobleached except for the Golgi 1 that remains the only source of fluorescence. Small diffusing fluorescent dots (gray arrow heads) were observed throughout the cytoplasm of fused cells. The graph shows the intensity of both Golgi (acceptor in blue and donor in red). Values are the mean of three independent experiments. (B) Cells expressing ssGFP-FM4-CD8 and ST-RFP (+VSV-G) were incubated at 20°C for 2 hr in the presence of CHX (100 µg/ml) and AP21998 (500 nM) to trigger the release of the cargo from the ER and its accumulation in the Golgi. Both drugs were maintained during the imaging. (1) Cells were fused and imaged 30 min post fusion. (2) Entire cell volume (Inter-Golgi space) was bleached, except the two Golgi and was allowed to (3) recover for 30 min. (4) Donor and acceptor Golgis were bleached (dashed white boxes) and allowed to (5) recover for 30 min. Red box indicates zoom area (ssGFP-FM4-CD8 in green and ST-RFP in red). Images are representative of three independent experiments. Images displayed are single slices of z-stacks (21 one µm slices) acquired. LUTs displayed below each grayscale image. Graph, quantification of total fluorescence intensity in region outside of the Golgis (blue line) and in Golgi areas (red line). These values represent the mean of three independent experiments. Fluorescence intensity for each timepoint was calculated over entire z-stack.
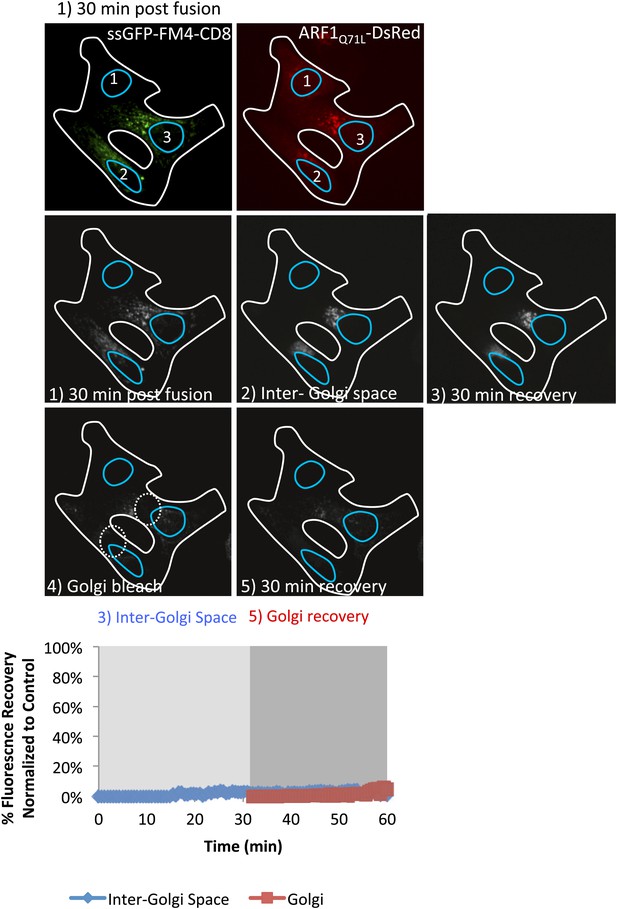
ARF1Q71L abolished fluorescence recovery in the inter-Golgi zone and at the Golgi.
Cells expressing either ssGFP-FM4-CD8 or ARF1Q71L–DsRed (+VSV-G) were incubated at 20°C for 2 hr in the presence of CHX (100 µg/ml) and AP21998 (500 nM) to trigger the release of the cargo from the ER and its accumulation in the Golgi. Both drugs were maintained during the imaging. (1) Cells were fused and imaged 30 min post fusion. CD8-GFP remained at the Golgi 2 and 3 (donor Golgi) whereas ARF1 bound each Golgi within the polykaryon (including Golgi 1, the theoretical acceptor Golgi for the cargo). (2) Entire cell volume (Inter-Golgi space) was bleached, except the two Golgi and was allowed to (3) recover for 30 min. (4) Donor and acceptor Golgis were bleached (dashed white boxes) and allowed to (5) recover for 30 min. Graph shows quantification of total fluorescence intensity in region outside of the Golgis (blue line) and in Golgi areas (red line) compare to control (when ARF1 mutant is absent). Fluorescence intensity for each timepoint was calculated over entire z-stack.
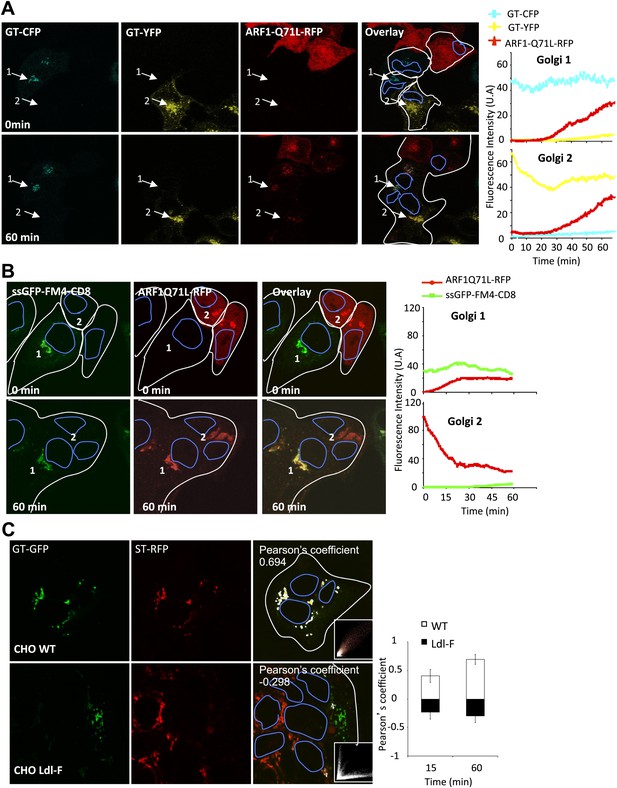
Role of ARF1 and ε−COP on inter-Golgi transport.
(A) Mixed and fused HeLa cells expressing GT-CFP (+VSV-G), GT-YFP (+VSV-G) or ARF1Q71L–DsRed, in presence of CHX. Graphs show the fluorescence intensity over time for each fluorescent marker within Golgi 1 and 2. Results are representative of two independent experiments. (B) Mixed and fused HeLa cells expressing either ssGFP-FM4-CD8 (+VSV-G) or ARF1Q71L–DsRed in the presence of AP21988 and CHX at 20°C. The graphs show the fluorescence intensity overtime of each marker for each Golgi. Results are representative of three different experiments. (C) Mixed and fused WT-CHO cells or LdlF-CHO cells expressing GT-GFP or ST-RFP (+VSV-G), in presence of CHX. After fusion, cells were incubated at 39°C for 15 or 60 min, fixed, and monitored by confocal microscopy. Graph shows the relative Pearson’s correlation coefficients for each cells type at each time point. Values are the mean of three independent experiments, with 10–15 polykaryons being analyzed for each condition. Pictures are representative of three experiments and illustrate cells at 1-hr post fusion.
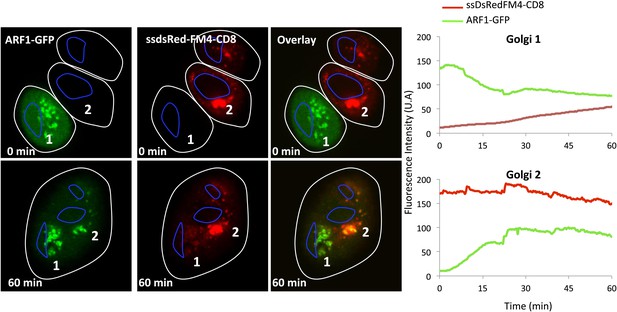
ARF1 WT does not prevent inter Golgi exchange of small anterograde cargo.
Mixed and fused HeLa cells expressing either ssDsRed-FM4-CD8 (+VSV-G) or ARF1WT-GFP in the presence of AP21988 and CHX at 20°C. The graphs show the fluorescence intensity overtime of each marker for each Golgi.
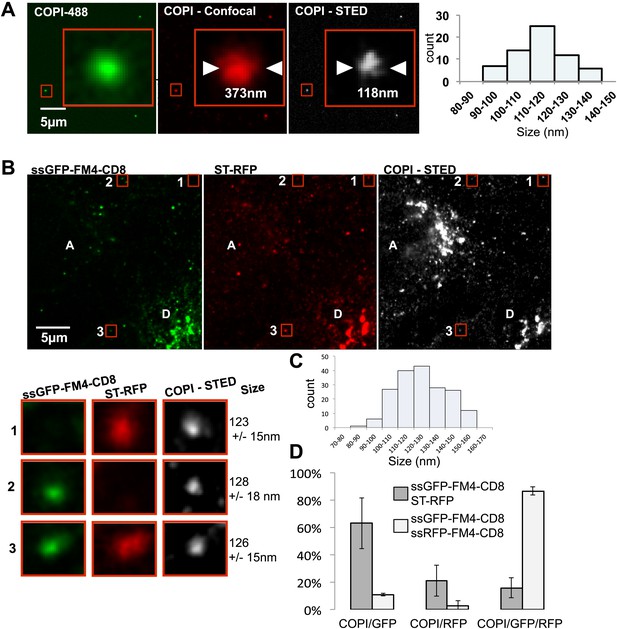
Inter-Golgi transport intermediates are compatible with COPI vesicles.
(A) In vitro prepared COPI vesicles labeled with Alexa488 were attached to glass coverslips. Immunofluorescence against COPI was performed, and samples were imaged by both confocal and STED microscopy. The size of COPI vesicles was determined by STED microscopy using a custom Matlab routine by fitting to a 2D Lorenztian function and confocal images were fit to a 2D Gaussian function. Graph, size distribution of 64 in vitro COPI vesicles fit with a 2D Lorentzian function. The mean is 114 nm. (B) HeLa cells expressing ssGFP-FM4-CD8, ST-RFP and VSV-G were incubated at 20°C in the presence of AP21998 and CHX for 2 hr, fused, and fixed after 30 min. Immunofluorescence was performed against β-COP to label all the Golgi related structures. COPI like transport intermediates imaged by STED microscopy were identified based on size and the contents were evaluated. Numbered boxes illustrating types of cargo containing COPI like intermediates. Red squares, different objects at low and high magnification. The sizes of the intermediates were determined using a 2D Lorentzian function. Graph (C) shows the size distribution of 179 carriers fit with a 2D Lorentzian function, The mean is 128 nm. Graph (D) shows the distribution of COPI spots positive for either ssGFP-FM4-CD8 cargo or ST-RFP, or both (gray columns). As a positive control we used cells co-expressing ssRFP-FM4-CD8 instead of ST-RFP (white columns). Control: 48 cells, 138 spots analyzed; Experimental: 31 cells, 179 spots analyzed. Results are representative of two independent experiments, each one being duplicated.
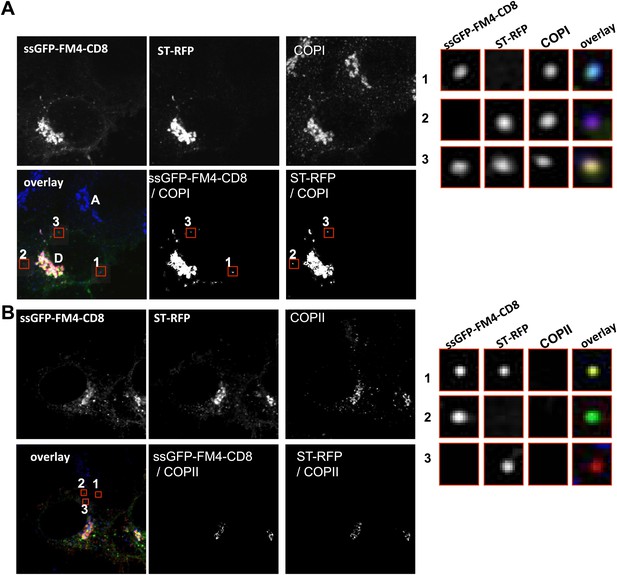
Inter-Golgi carriers are COPI positive and COPII negative.
(A) Cells expressing CD8-GFP-FM4, ST-RFP and VSV-G were incubated at 20°C in the presence of AP21998 and CHX for 2 hr, fused, and fixed after 30 min. Immunofluorescence was performed against β-COP to label all the Golgi related structures. The CD8-GFP-FM4/β-COP and ST-RFP/β-COP co-localizations are highlighted with a white mask generated with ImageJ. Red squares, different objects at low and high magnification. Results are representative of three different experiments. (B) Cells expressing CD8-GFP-FM4, ST RFP and VSV-G were incubated at 20°C in presence of AP21998 for 2 hr, fused and fixed 30 min after. Immunofluorescence was performed against sec31 to label COPII related structures (COPII vesicle and ER exit sites). The CD8-GFP-FM4/sec31 and ST-RFP/sec31 co-localizations are highlighted with a white mask generated with ImageJ. Due to the resolution limit, the ER exit sites that are at the vicinity of the Golgi appear as co-localizing with the Golgi. However, virtually none of the small particles carrying the cargoes, which are distant from the donor Golgi, were positive for sec31. The red squares illustrated the different objects at low and high magnification. Results are representative of two different experiments.
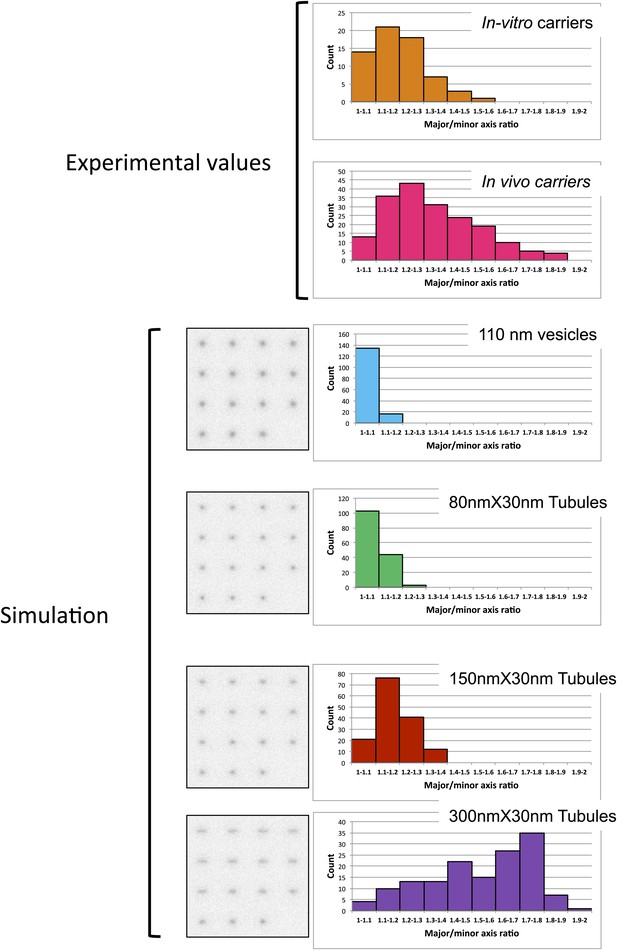
Discriminating tubules from vesicles with STED.
Vesicles and tubules with different dimensions simulated in a set of different 3D orientations to represent an isotropic distribution (supplementary method section). Pictures illustrate the object according to their different 3D orientation. For both in vitro COPI vesicles and intracellular COPI objects (imaged in Figure 7), the size was determined by STED microscopy using a custom Matlab routine by fitting to a 2D Lorenztian function and the radial symmetry (major/minus axis ratio) was calculated to assess the degree of symmetry of the objects. The simulated objects were analyzed with the same custom-written MATLAB routine used for the experimental data.
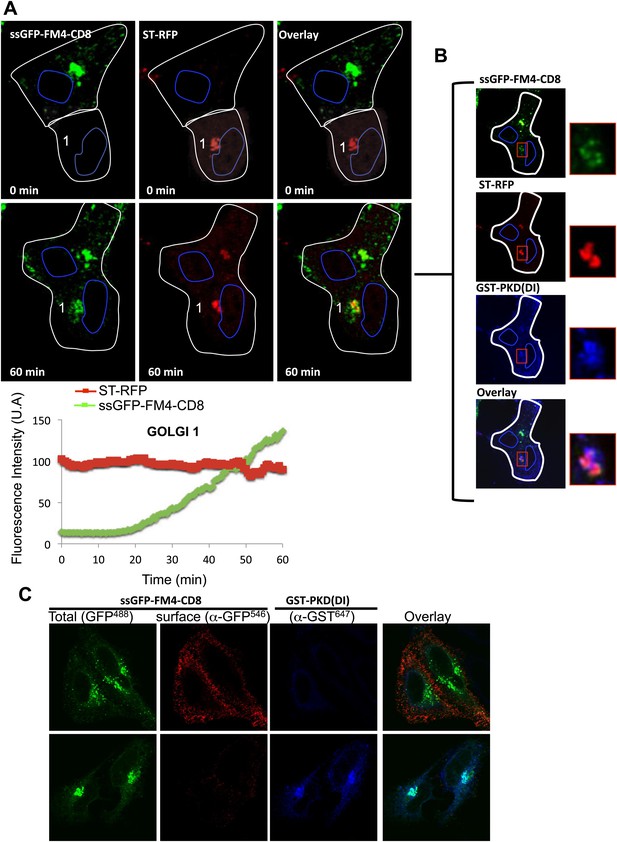
Inter-Golgi exchange of anterograde cargo at 37°C.
(A) HeLa cells expressing ss-GFP-FM4-CD8 and GST-PKD(DI) were mixed and fused with cells expressing ST-RFP, GST-PKD(DI) and VSV-G. Prior to fusion, cells were incubated at 37°C for 15 min in the presence of the disaggregating drug and CHX. Cells were monitored by video confocal microscopy at 32°C in the presence of the disaggregating drug and CHX. Graphs show quantification of both markers over time for Golgi 1. Results are representative of two independent experiments. (B) 1 hr post-fusion, cells were fixed and prepared for immunofluorescence against GST to assess the presence of the PKD(DI). Note that the confocal micrograph showed the very same field of cells (shown in A) after fixation. (C) PKD(DI) inhibits plasma membrane targeting of ss-GFP-FM4-CD8. HeLa cells expressing ss-GFP-FM4-CD8 alone (upper panel) or with GST-PKD(DI) (lower panel) were incubated for 1 hr at 37°C in the presence of the disaggregating drug. Non-permeabilized cells were incubated on ice and incubated with an anti-GFP antibody (detected with a secondary antibody labeled with Alexa-546) to assess for cell surface exposure of ss-GFP-FM4-CD8. Then, cells were fixed, permeabilized and processed for immunofluorescecne against GST (using a secondary antibody labeled with Atto-647).





