Intestinal epithelium-derived BMP controls stem cell self-renewal in Drosophila adult midgut
Figures
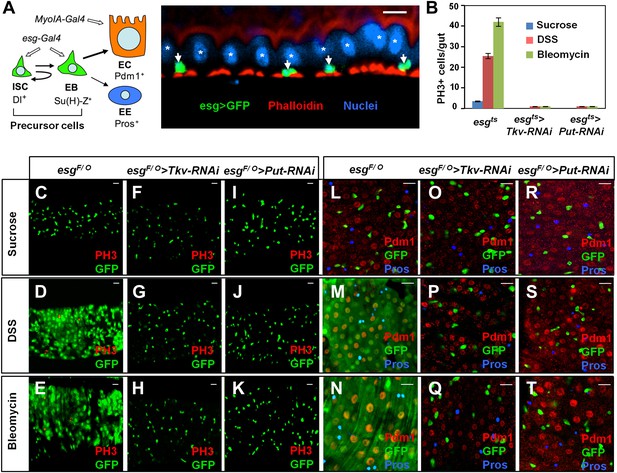
BMP signaling is required for midgut regeneration.
(A) Left: an ISC lineage in Drosophila adult midguts. ISC: intestinal stem cell; EB: enteroblast; EC: enterocyte; EE: enteroendocrine cell. ISC and EB are collectively called precursor cells. Dl and Su(H)-lacZ mark ISC and EB, respectively, whereas Pdm1 and Pros are the markers for EC and EE, respectively. esg-Gal4 and Myo1A-Gal4 are precursor and EC-specific Gal4 drivers, respectively. Right: sagittal view of Drosophila midgut epithelium immunostained with an anti-GFP antibody (green), Phalloidin (red) and a nuclear dye (DRAQ5, blue). Arrows and asterisks indicate precursor cells and ECs, respectively. (B) Quantification of PH3+ cells in midguts from adults of the indicated genotypes (mean ± SD, n = 20 for each genotype). Tkv and Put RNAi in precursor cells blocked damage-induced mitotic index. (C–T) 3- to 5-day-old adult females of esgF/O without (C–E and L–N) or with UAS-Tkv-RNAi105834 (F–H and O–Q) or UAS-Put-RNAi (I–K and R–T) were shifted to 29°C for 8 days and treated with sucrose, DSS and bleomycin for 2 days, followed by immunostaining for GFP and PH3 (C–K), or GFP, Pdm1 and Pros (L–T). Top views of midguts are shown in these panels and in panels of all other figures unless indicated otherwise. Scale bars in this and other figures (except for Figure 6A–C) are 10 μm. esgts: esg-Gal4 tub-Gal80ts. esgF/O: esg-Gal4 tub-Gal80ts UAS-GFP; UAS-flp Act>CD2>Gal4.
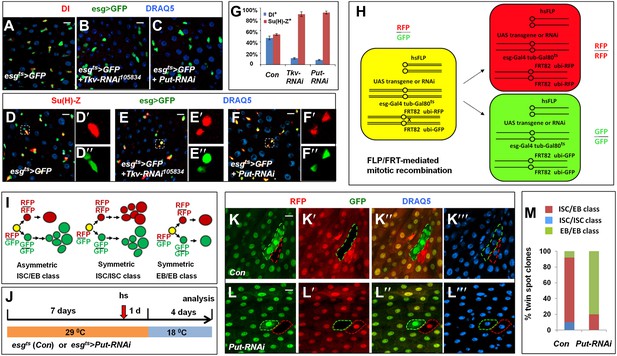
BMP signaling is required for ISC self-renewal.
(A–F″) 3- to 5-day-old adult females expressing esgts>GFP (A, D–D″) or expressing esgts>GFP together with UAS-Tkv-RNAi (B, E–E″) or UAS-Put-RNAi (C, F–F″) were shifted to 29°C for 8 days, followed by immunostaining for DI (red in A–C) or Su(H)-lacZ (red in D–F″), GFP and DRAQ5 (a nuclear marker). In the control guts, most pairs of precursor cells contain one Dl+ ISC and one Su(H)-lacZ+ EB (A, D–D″); however, in Tkv or Put RNAi guts, most pairs of precursor cells contain two Su(H)-lacZ+ cells without Dl staining (B–C, E–F″). (G) Percentage of Dl+ or Su(H)-Z+ cells out of GFP+ precursor cells (mean ± SD, n = 10 for each genotype). (H) Schematic drawing of an ISC division that produces differentially labeled twin-spot (RFP+ GFP− and RFP− GFP+) through FRT-mediated mitotic recombination. The expression of GFP and RFP is under the control of the ubiquitin (ubi) promoter. Transgenic overexpression or RNAi through esgts allows determining the effect of gain- or loss-of-function of genes of interest on the outcome of an ISC division. (I) Schematic drawings of differentially labeled twin-spot clones generated by FLP/FRT-mediated mitotic recombination of dividing ISCs. (J) Scheme for twin-spot experiments involving Put RNAi. 3–5-day-old control or esgts>Put-RNAi adult flies were grown at 29°C for 7 days before heat shock (hs) to induce clones. After one-day recovery at 29°C, the flies were raised at 18°C for 4 days prior to analysis. (K–L‴) Representative twin-spot clones from control and Put RNAi guts. (M) Quantification of twin spots of different classes from control and Put-RNAi guts: Con (n = 110, ISC/EB: 82%, ISC/ISC: 10%, EB/EB: 8%), Put-RNAi (n = 110, ISC/EB: 20%, ISC/ISC: 0%, EB/EB: 80%).
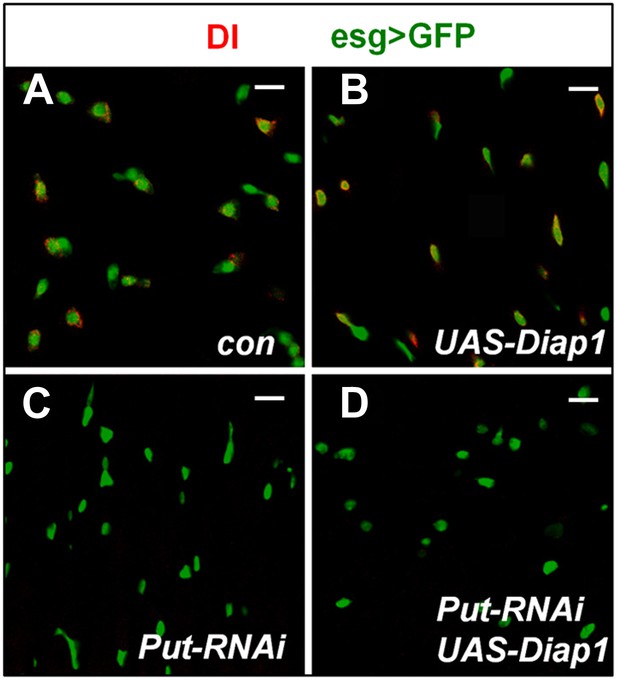
Blocking apoptosis does not rescue ISC loss caused by inactivation of BMP signaling.
(A–D) 3–5-day-old adult females expressing esgts>GFP (Con; A), esgts>Diap1 (B), esgts>Put-RNAi (C), or esgts>Put-RNAi + Diap1 (D) were shifted to 29°C for 8 days, followed by immunostaining for DI and GFP. Overexpression of the apoptosis inhibitor Diap1 did not rescue the loss of Dl+ cells caused by Put RNAi.
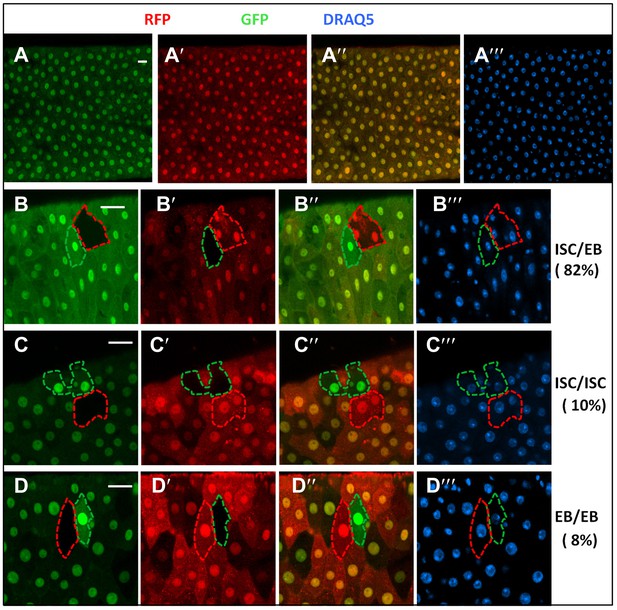
RFP/GFP two-color twin spot clonal analysis.
(A) Confocal images of a posterior midgut containing FRT82 ubi-GFP (red) and FRT82 ubi-RFP (red) and immunostained with the nuclear marker DRAQ5 (blue) prior to clonal induction. Both ubi-GFP and ubi-RFP were expressed quite uniformly in the posterior region of adult midguts. ubi: ubiquitin promoter. B–D‴, Examples of three indicated classes of twin spots generated by heat-shock induced FRT/FLP-mediated mitotic recombination in control midguts under normal homeostasis. 82% (90/110) of twin spots contained one multi-cellular clone and one single-cell clone derived from ISC/EB pairs whereas 10% (11/110) and 8% (9/110) contained two multicellular clones (ISC/ISC class) or two single-cell clones (EB/EB class), respectively.
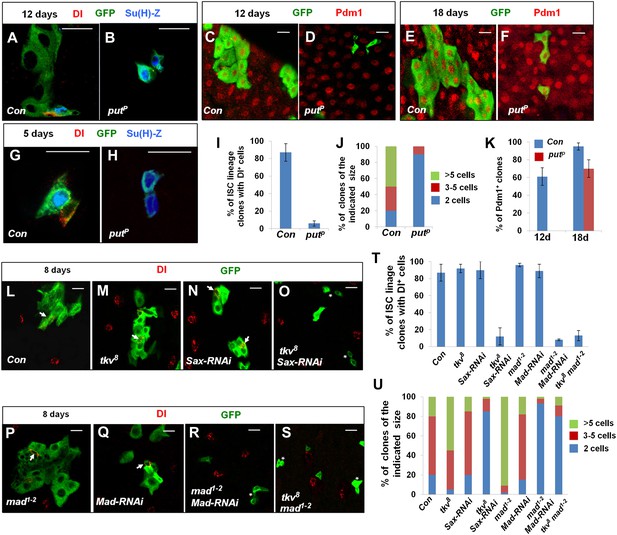
Characterization of midgut phenotypes caused by differential inactivation of BMP pathway components.
(A–H) Midguts containing the control clones (A, C, E, G) or putP clones (B, D, F, H) were immunostained for DI (red in A, B, G, H) or Pdm1 (red in C–F), GFP (green), and Su(H)-lacZ (blue in A, B, G, H) at 5 (G, H), 12 (A–D), or 18 (E, F) days after clone induction (ACI). Control and mutant clones are marked by GFP expression. Control ISC lineage clones usually contain one Dl+ cell, one or more Su(H)-lacZ+ cells, and many cells with large nuclei at 12 days ACI. By contrast, most put mutant ISC lineage clones contain two cells that are Dl− but Su(H)-lacZ+. At 5 days ACI, 91% of control ISC lineage clones contained one Dl+ cell and one Su(H)-lacZ+ cell while 82% of put mutant ISC lineage clones contained two Su(H)-lacZ+ cells. (I) Quantification of ISC lineage clones containing Dl+ cells 12 days ACI (mean ± SD, n = 125 for each genotype). (J) Quantification of clone size for control (Con) or putP ISC lineage clones 12 days ACI (n = 150 for each genotype). (K) Quantification of Pdm1+ clone frequency for control (Con) and putP ISC lineage clones at 12 or 18 days ACI (mean ± SD, n = 150 for each genotype). (L–S) Adult midguts carrying MARCM clones of the indicated genotype were immunostained for Dl and GFP at 8 days ACI. Arrows indicate Dl+ cells and asterisks in H indicate clones without Dl+ cells. (T) Quantification of Dl+ clone frequency for the indicated genotypes at 8 days ACI (mean ± SD, n = 130 for each genotype). (U) Quantification of clone size for the indicated genotypes at 8 days ACI (n = 170 for each genotype).
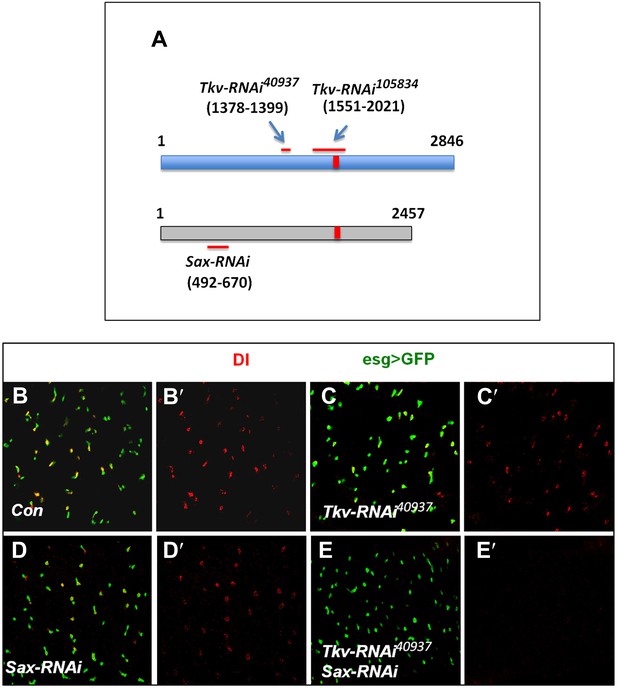
Tkv and Sax act redundantly in the regulation of ISC self-renewal.
(A) Schematic drawing of Tkv and Sax coding regions with numbers indicating the nucleotide positions. The red lines indicate the regions targeted by individual RNAi lines. The red bars indicate the conserved region between Tkv and Sax coding sequences. (B–E′) Control midguts (esgts>GFP) or midguts expressing the indicated RNAi lines with esgts>GFP at 29°C for 12 days were immunostained to show the expression of Dl (red) and esg>GFP (green).
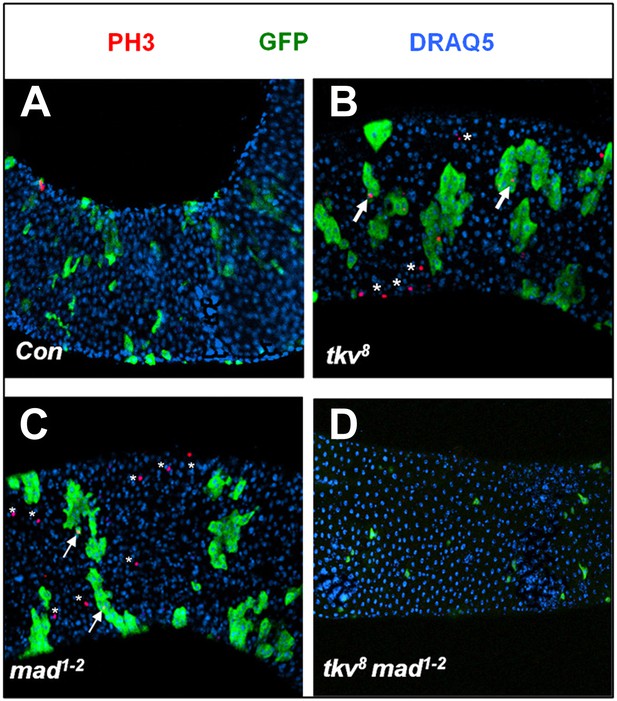
Both tkv8 and mad1–2 mutant clones caused non-cell autonomous ISC overproliferation.
Posterior midguts carrying control (A), tkv8 (B), mad1–2 (C), or tkv8 mad1–2 (D) clones and immunostained for PH3, GFP, and DRAQ5 at 8 days ACI. Arrows and asterisks indicate PH3+ cells inside and outside the clones, respectively.
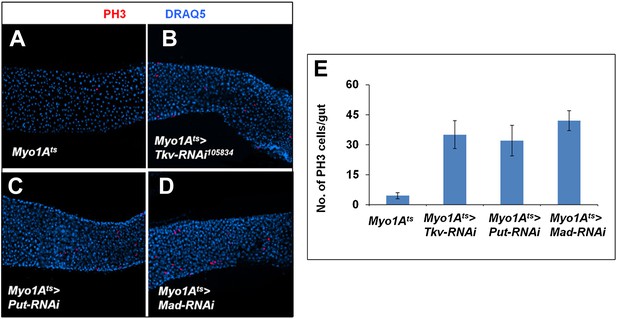
Inactivation of BMP signaling in ECs caused ISC proliferation.
(A–D) Control midguts (Myo1Ats) or midguts expressing the indicated RNAi lines at 29°C for 8 days were immunostained for PH3 and DRAQ5. Inactivation of BMP signaling in ECs resulted in elevated ISC proliferation. (E) Quantification of PH3+ cells for the indicated genotypes (n = 10 for each genotype).
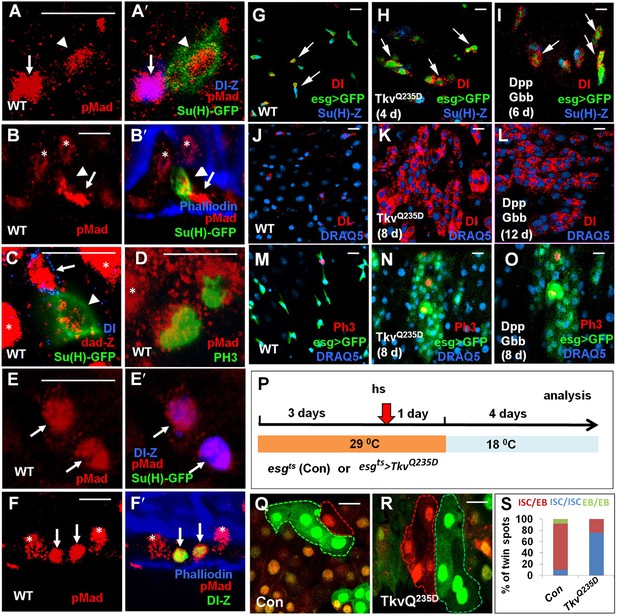
Asymmetric BMP signaling regulates ISC self-renewal.
(A–A′, C–E′) High magnification views of wild type adult midguts immunostained for pMad (red in A–A′, D–E′), dad-lacZ (red in C), Dl-lacZ (blue in A′, E′), Su(H)-GFP (green in A′, C, E′) or PH3 (green in D). (B–B′, F–F′) Sagittal views of wild type adult midguts immunostained for pMad (red), Su(H)-GFP (green in B′), Dl-lacZ (green in F′), and Phalliodin (blue). Arrows and arrowheads indicate ISCs and EBs, respectively. Asterisks indicate the pMad signals in ECs. (G–O) Adult midguts expressing esgts>GFP (G, J, M), esgts>GFP + TkvQ235D (H, K, N), or esgts>GFP + Dpp + Gbb (I, L, O) at 29°C for the indicated time periods were immunostained for Dl (red in G–L), PH3 (red in M–O), GFP (green), and Su(H)-lacZ or DRAQ5 (blue). (P) Scheme for twin-spot experiments involving TkvQ235D overexpression. 3–5-day-old control or esgts>TkvQ235D adult flies were grown at 29°C for 3 days before heat shock (hs) to induce clones. After 1-day recovery at 29°C, the flies were raised at 18°C for 4 days prior to analysis. (Q–R) Representative twin-spot clones from control and esgts>TkvQ235D guts. (S) Quantification of twin spots of different classes from control and esgts>TkvQ235D guts: Con (n = 160, ISC/EB: 83%, ISC/ISC: 9%, EB/EB: 8%), TkvQ235D (n = 190, ISC/EB: 23%, ISC/ISC: 77%, EB/EB: 0%).
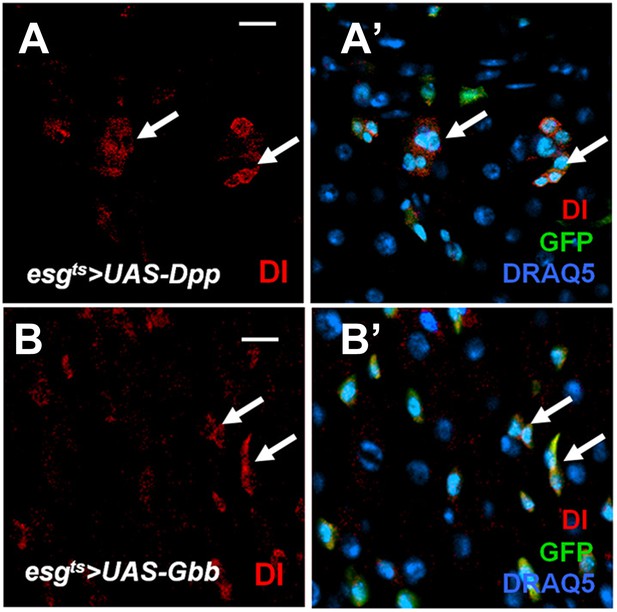
Effect of misexpressing Dpp or Gbb alone in precursor cells on ISC self-renewal.
(A–B′) Adult midguts expressing esgts>Dpp + GFP (A, A′) or esgts>Gbb + GFP (B, B′) at 29°C for 12 days were immunostained for Dl (red), GFP (green) and DRAQ5 (blue). Misexpression of Dpp or Gbb alone resulted in ectopic Dl+ cells that formed small clusters (arrows).
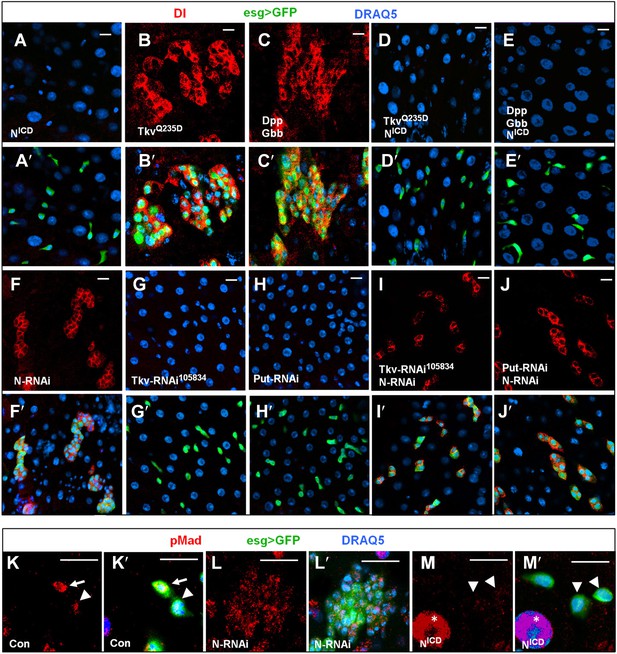
BMP signaling promotes ISC self-renewal by antagonizing N.
(A–E′) Adult midguts expressing NICD (8d) (A–A′), TkvQ235D (8d) (B–B′), Dpp + Gbb (12d) (C–C′), or the indicated combinations of transgenes (D–E′) under the control of esgts were immunostained for Dl (red), GFP (green) and DRAQ5 (blue). Coexpression of NICD suppressed the excessive Dl+ cells caused by TkvQ235D or Dpp/Gbb misexpression, leading to loss of Dl+ cells similar to expression of NICD alone. (F–J′) Adult midguts expressing the indicated RNAi lines under the control of esgts for 8 days were immunostained for Dl (red), GFP (green), and DRAQ5 (blue). N RNAi rescued Dl+ cells in midguts expressing Tkv-RNAi105834 or Put-RNAi. (K–M′) High magnification views of adult midguts expressing esgts>GFP (K–K′), esgts>GFP + N-RNAi (L–L′), or esgts>GFP + NICD (M–M′) and immunostained for pMad (red), GFP (green), and DRAQ5 (blue). Arrows and arrowhead indicate ISC and EB, respectively. Asterisks indicate the pMad signals in ECs.
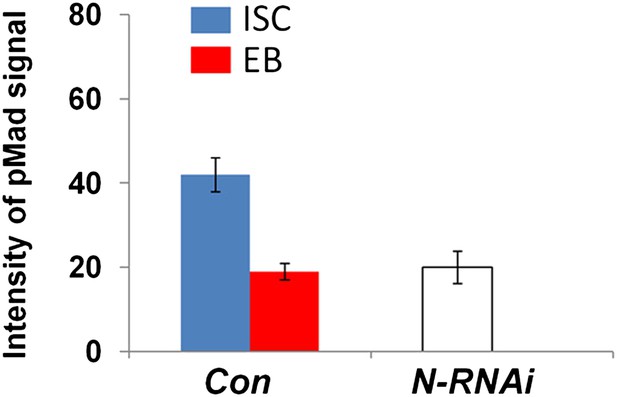
Integrated pMad levels in control or N knock down precursor cells.
Quantification of pMad signals in pairs of ISC/EB in the posterior control midguts (Con; esgts>GFP) or in clusters of precursor cells expressing esgts>N-RNAi (mean ± SD: n = 20 for control ISC/EB pairs; N > 100 for N RNAi precursor cells).
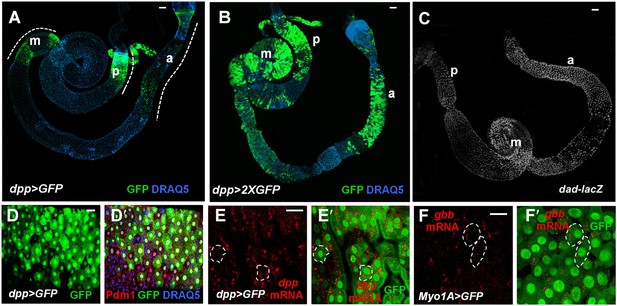
Both Dpp and Gbb are expressed in ECs.
(A and B) Low magnification views of adult midguts expressing one (a) or two (b) copies of UAS-GFP transgene under the control of dpp-Gal4 were immunostained for GFP and DRAQ5. dpp>GFP is expressed in most of the midgut epithelia with strong expression in the posterior (p), middle (m), and anterior (a) regions. (C) Low magnification view of a midgut expressing dad-lacZ. (D–D′) High magnification view of the posterior region of a dpp>GFP expressing midgut immunostained for GFP, Pdm1, and DRAQ5. (E–E′) RNAi in situ hybridization of a dpp>GFP expressing midgut (posterior region) shows the coincidence of dpp mRNA and dpp>GFP signals. dpp mRNA signal is detected in the ECs (outlined by dashed line as examples). (F–F′) RNA in situ hybridization of midguts expressing Myo1A>GFP shows that gbb mRNA is detected in ECs. Two ECs are marked by dashed line as examples.
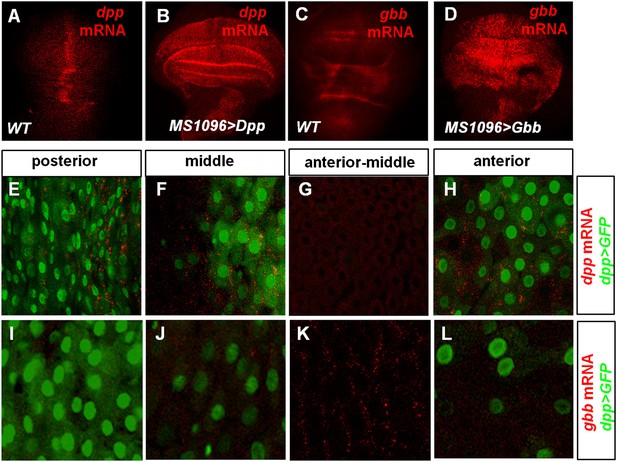
Characterization of dpp and gbb expression in Drosophila by RNA in situ hybridization.
(A–D) RNA in situ hybridization with dpp (A and B) or gbb (C and D) probe for wild type wing discs (A, C) or wing discs expressing UAS-Dpp (B) or UAS-Gbb (D) with wing disc specific Gal4 driver MS1096. (E–L) High magnification views of the indicated regions of midguts expressing dpp>GFP and hybridized with dpp (E–H) or gbb (I–L) probe. dpp mRNA expression correlates with that of Dpp>GFP (E–H) whereas the high gbb mRNA expression domain corresponds to the low expression region of Dpp>GFP (I–L).
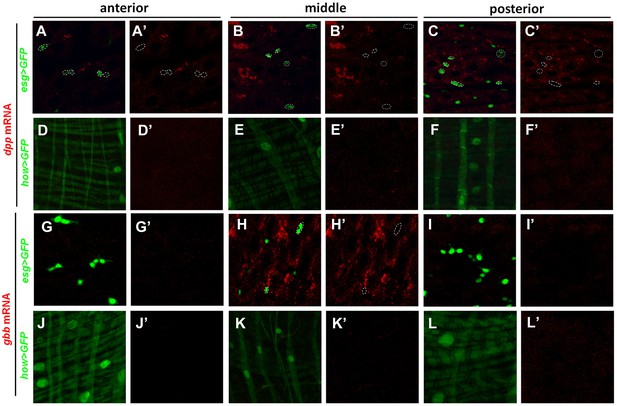
dpp and gbb mRNAs are not detected in precursors or VM.
High magnification views of the indicated regions of adult midguts expressing esg>GFP (A–C′, G–I′) or how>GFP (D–F′’, J–L′) and probed for dpp (A–F′) or gbb (G–L′) expression by RNA in situ hybridization. esg>GFP and how>GFP mark the precursor cells and VM, respectively. Neither dpp mRNA nor gbb mRNA was detected in precursor cells (outlined by dashed circles in A, A′, B, B′, C, C′, H, H′) or VM.
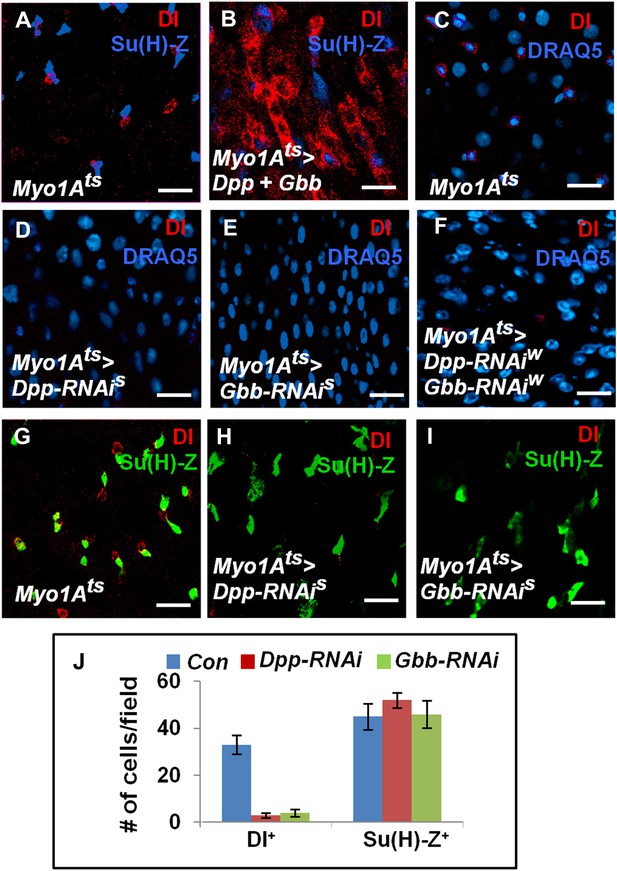
EC-derived Dpp and Gbb regulate ISC self-renewal.
(A and B) Control (A) or midguts coexpressing both Dpp and Gbb with Myo1Ats (B) were immunostained for Dl and Su(H)-lacZ. (C–I) Control guts (C, G), guts expressing strong Dpp-RNAi line (D, H), strong Gbb-RNAi line (E, I), or a combination of weak Dpp- and Gbb-RNAi lines (F) were immunostained for Dl (red), Su(H)-lacZ (green), and DRAQ5 (blue). (J) Quantification of Dl+ or Su(H)-Z+ cell number (mean ± SD, n = 15 for each genotype). Scale bars in A–C are 100 μm. Of note, to ensure sufficient knockdown of Dpp or Gbb, two copies of individual RNAi lines were expressed in midguts for 25 days. See ‘Materials and methods’ the genotypes.
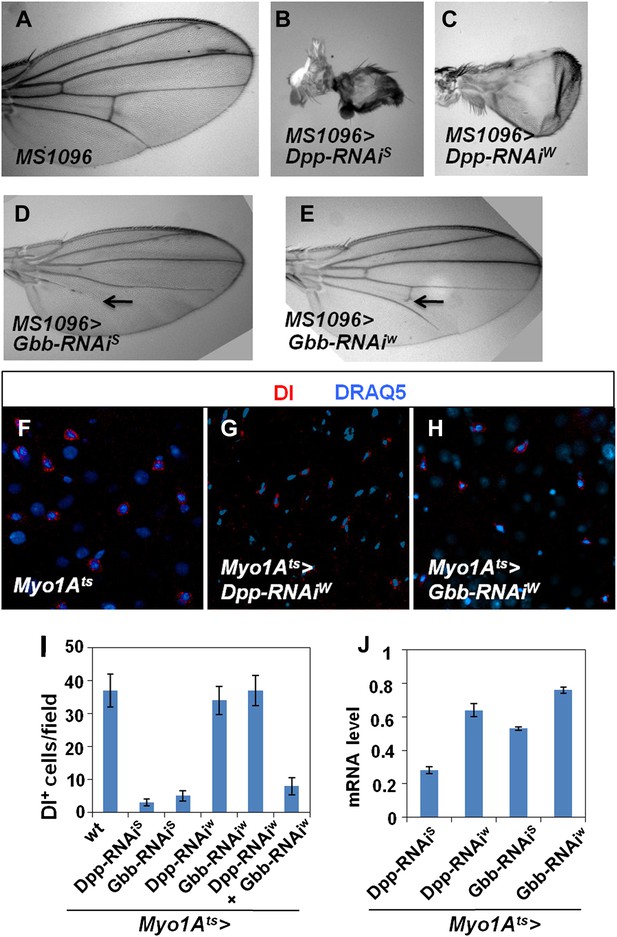
Characterization of Dpp and Gbb RNAi lines.
(A–E) Adult wing phenotypes associated with Dpp (B and C) or Gbb (D and E) knockdown using a wing specific Gal4 driver, MS1096, to express the indicated RNAi lines. Arrows in D and E indicate defects in the posterior cross vein. (F–H) Control guts (F) or midguts expressing the indicated RNAi lines (G and H) with Myo1Ats at 29°C for 25 days were immunostained for Dl (red) and DRAQ5 (blue). (I) Number of Dl+ cells per field in midguts of the indicated genotypes (mean ± SD: n = 15 for each genotype). (J) Knockdown efficiency measured by RT-qPCR after 25-day expression of the indicated RNAi lines using Myo1Ats (mean ± SD: triplicates).
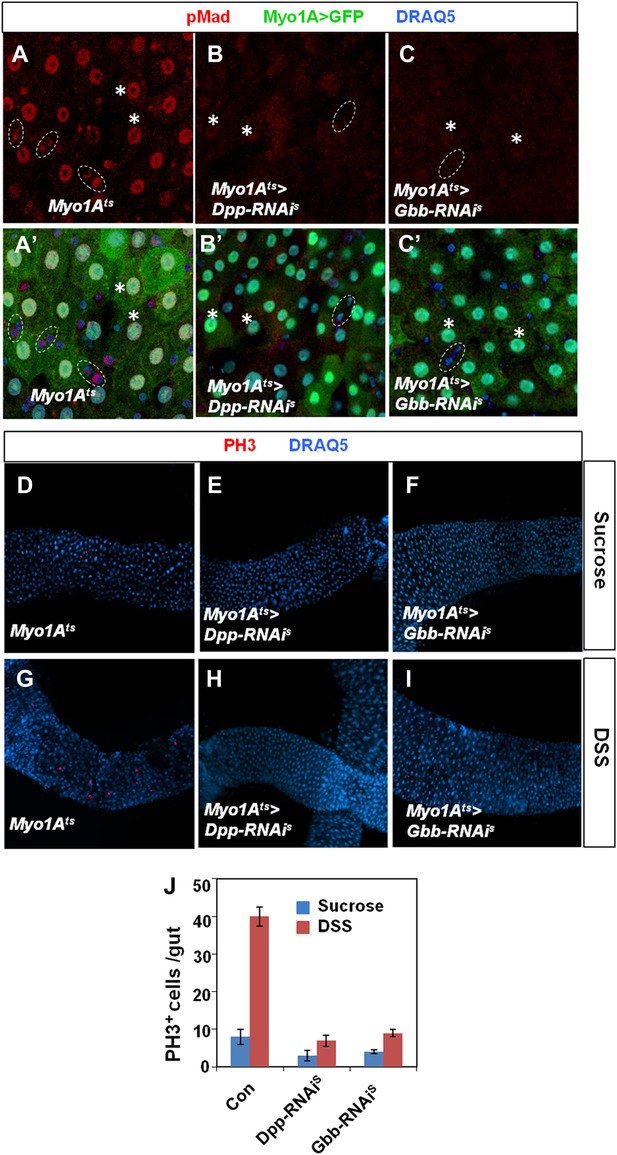
Characterization of Dpp and Gbb knockdown in ECs.
Adult midguts expressing Myo1Ats>GFP (A and A′), Myo1Ats>GFP + Dpp-RNAiS (B and B′), or Myo1Ats>GFP + Gbb-RNAiS (C and C′) at 29°C for 25 days followed by immunostaining for pMad (red), GFP (green), and DRAQ5 (blue). Control guts exhibited asymmetric pMad staining in precursor pairs (outlined by dashed circles in A and A′) and high levels of pMad in ECs (indicated by asterisks in A and A′). pMad was diminished in both precursor cells and ECs when Dpp or Gbb was knocked down in ECs (B–C′). (D–I) Adult midguts expressing Myo1Ats>GFP (D and G), Myo1Ats>GFP + Dpp-RNAiS (E and H), or Myo1Ats>GFP + Gbb-RNAiS (F and I) at 29°C for 25 days followed by Sucrose (D–F) or DSS (G–I) treatment and immunostaining for PH3 (red) and DRAQ5 (blue). Knockdown of Dpp or Gbb in ECs blocked DSS-induced ISC proliferation. (J) Quantification of PH3+ cells in midguts from adults of the indicated genotypes (mean ± SD: n = 20 for each genotype).
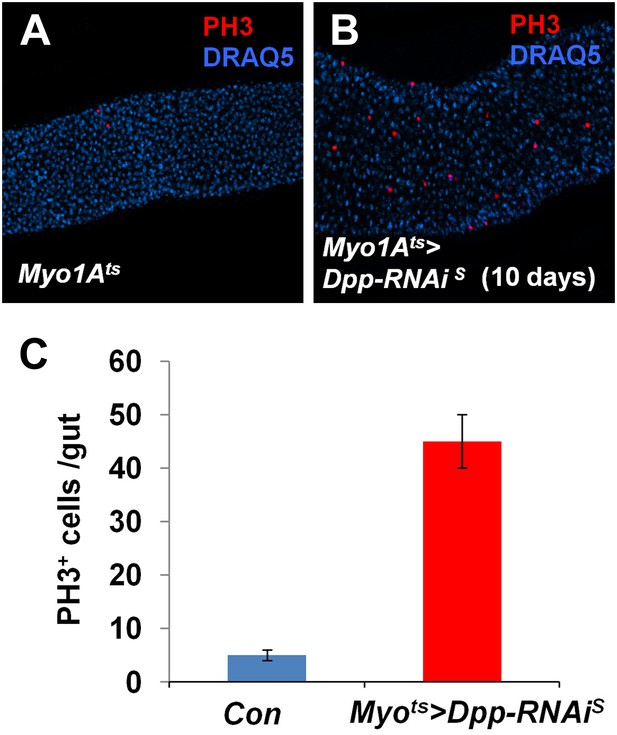
Partial loss of BMP in ECs stimulates ISC proliferation.
(A and B) Control guts (A) and guts expressing strong Dpp-RNAi line (B) for 10 days were immunostained for PH3 (red) and DRAQ5 (blue). (C) Quantification of PH3+ cells in midguts from adults of the indicated genotypes (mean ± SD: n = 20 for each genotype).
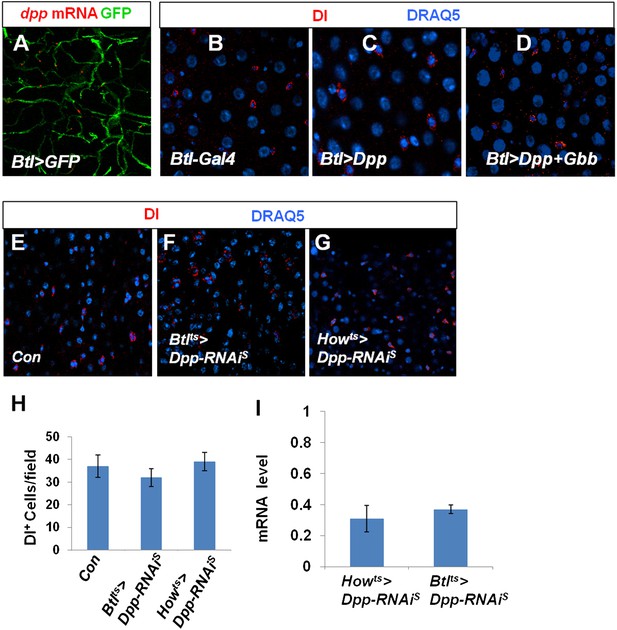
Characterization of Dpp and Gbb in trachea and VM.
(A) RNA in situ hybridization of midguts expressing Btl>GFP with a dpp probe. dpp mRNA was detected in Btl>GFP+ tracheal cells. (B–D) Adult midguts expressing Btlts-Gal4 (B), Btlts>Dpp (C) or Btlts>Dpp + Gbb (D) at 29°C for 12 days were immunostained for Dl (red) and DRAQ5 (blue). Misexpression of either Dpp alone or both Dpp and Gbb in tracheal cells failed to induce ectopic ISCs. (E–G) Control midguts (Con) (E) or midguts expressing Dpp RNAi in trachea (Btlts>Dpp-RNAiS) (F) or VM (Howts>Dpp-RNAiS) (G) at 29°C for 25 days were immunostained for Dl and DRAQ5. (H) Quantification of Dl+ cells for the indicated genotypes (mean ± SD: n = 8 for each genotype). Knockdown of Dpp in either tracheal cells or VM did not significantly affect the number of Dl+ cells in the midguts. (I) Knockdown efficiency measured by RT-qPCR after 25-day expression of the indicated RNAi lines using Howts or Btlts (mean ± SD: triplicates).
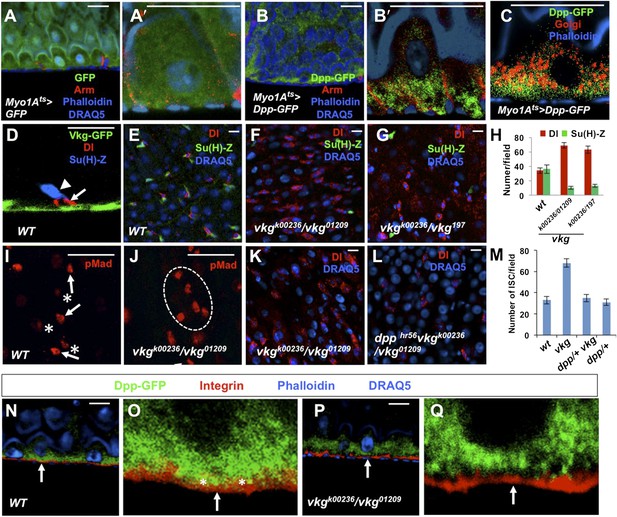
Regulation of ISC self-renewal by Vkg and BMP activity gradient.
(A–C) Low (A and B) and high (A′, B′, C) magnification sagittal views of adult midguts expressing Myo1Ats>GFP (A–A′) or Myo1Ats>Dpp-GFP (B–C) and immunostained with the indicated antibodies or dyes. Golgi is marked by an anti-Lava lamp antibody. Adult flies expressing Dpp-GFP (or GFP) with Myo1Ats were raised at 29°C for 5 days, followed by immunostaining. (D) Sagittal view of a Vkg-GFP expressing gut immunostained with the indicated antibodies or dyes. Arrows and arrowhead indicate ISC and EB, respectively. (E–G, I, J) Wild type (E and I) or vkg mutant guts (F, G, J) were immunostained for Dl (red in E–G), pMad (red in I and J), Su(H)-lacZ and DRAQ5. In control guts (I), precursor cells exhibit high (arrows) and low (asterisks) levels of pMad staining in pairs. vkg mutant guts from 10–12-day-old females contained clusters of precursor cells with high levels of pMad signal (outlined in J). (H) Quantification of Dl+ or Su(H)-Z+ cells in wild type and vkg mutant guts (mean ± SD, n = 20 for each genotype). (K–L) The ectopic Dl+ phenotype in vkg mutant was rescued by dpp heterozygosity (dpphr56/+ at 29°C). (M) Quantification of Dl+ cells in midguts of the indicated genotype (mean ± SD, n = 20 for each genotype). vkg: vkgK00236/vkg01209 ; dpp−/+ vkg: dpphr56 vkgK00236/vkg01209. (N–Q) Low and high magnification views of wild type (N–O) or vkg mutant (P–Q) midguts expressing Dpp-GFP and immunostained for Integrin/Mys, GFP and DRAQ5. Arrows point to the BM in all panels. In wild type guts, Dpp-GFP is enriched at BM as indicated by colocalization of Dpp-GFP and Integrin/Mys signals (yellow in O; asterisks), but the colocalization is greatly reduced in vkg mutant guts (Q).
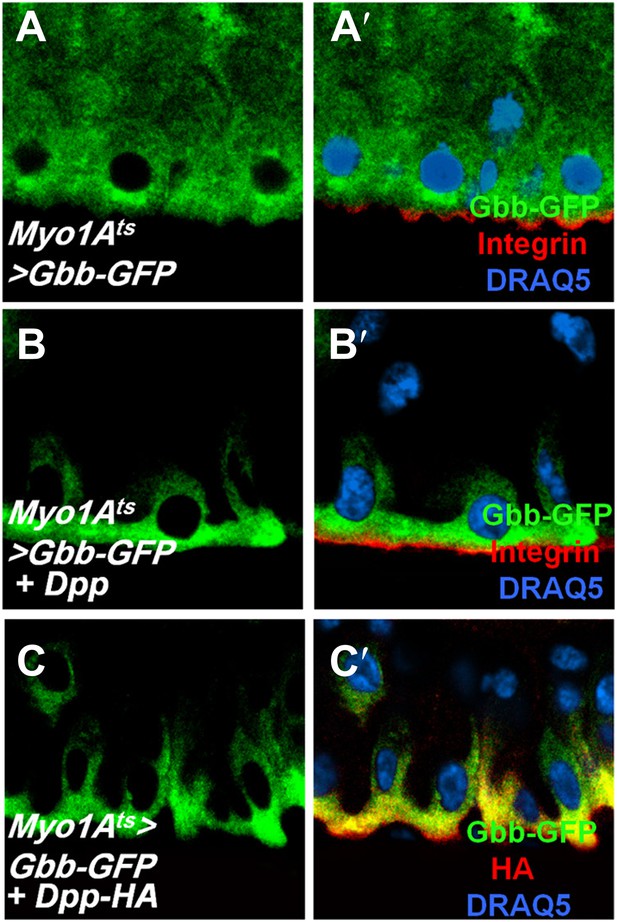
Dpp regulates Gbb subcellular localization.
(A–C′’) Sagittal views of adult midguts expressing Myo1Ats>Gbb-GFP (A and A′), Myo1Ats>Gbb-GFP + Dpp (B and B′), or Myo1Ats>Gbb-GFP + Dpp-HA (C and C′) were immunostained with antibodies against GFP (green), Integrin/Mys (red in A′ and B′) or HA (red in C′), and DRAQ5. When expressed alone, Gbb-GFP was uniformly distributed along the apical/basal axis. Coexpression of either the non-tagged or HA-tagged Dpp redistributed Gbb-GFP, resulting its enrichment on the basal side of EC.
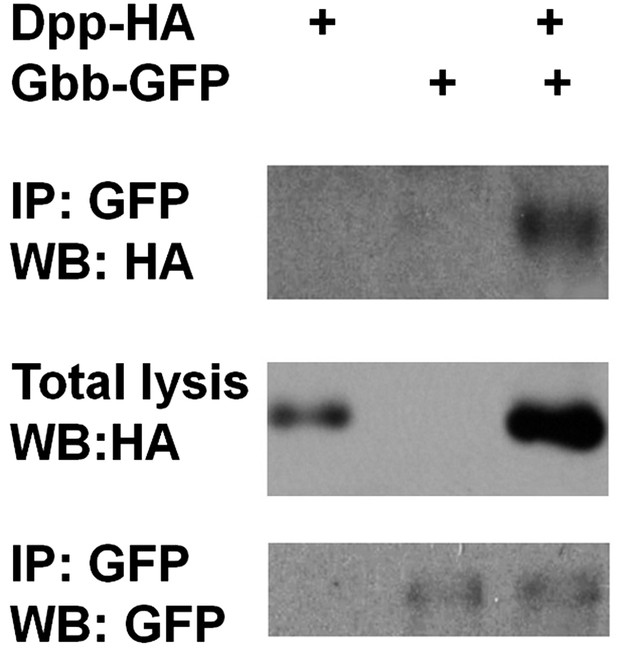
Dpp physically interacts with Gbb.
Adult midguts expressing Dpp-HA and Gbb-GFP individually or in combination with Myo1Ats at 29°C for 5 days were subjected to immunoprecipitation and western blot analysis with the indicated antibodies.
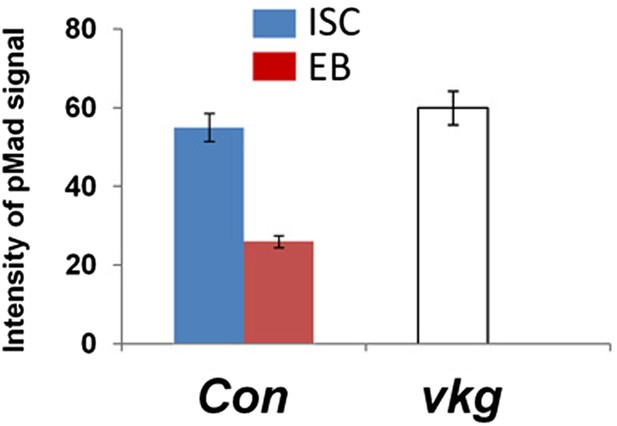
Integrated pMad levels in precursor cells of control or vkg mutant midguts.
Quantification of pMad signals in pairs of wild type ISC/EB (Con; esgts>GFP) or in clusters of vkg mutant (vkg00236/01,029) precursor cells in the posterior midguts (mean ± SD: n = 20 for control ISC/EB pairs, n > 40 for precursor cells in vkg mutant guts).
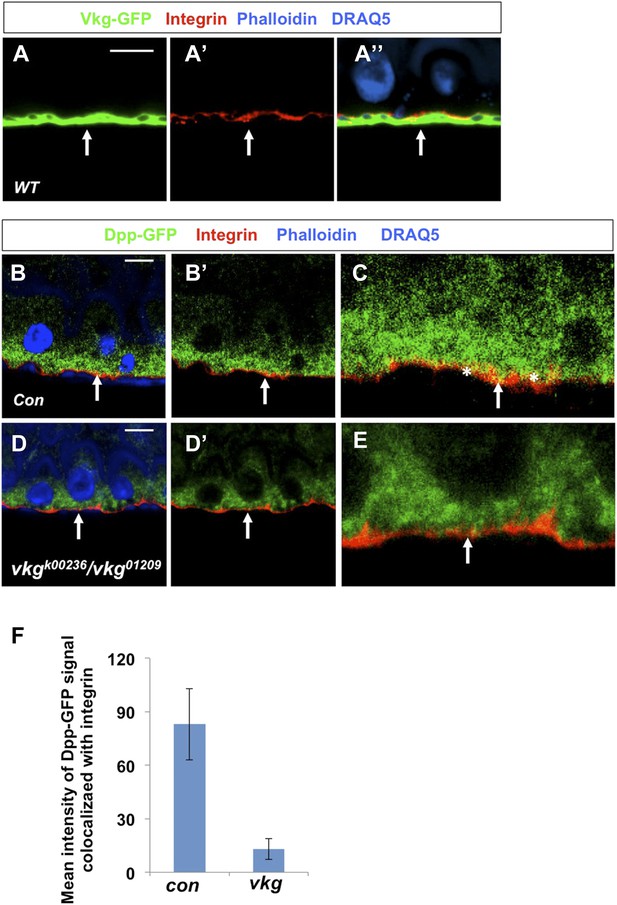
Dpp localization at the BM is diminished in vkg mutant guts.
(A–A″) Adult midguts were immunostained for the Drosophila integrin βPS subunit Integrin/Mys (red), Vkg-GFP (green), Phalloidin and DRAQ5 (blue). Integrin/Mys is enriched at the basal membrane (BM) marked by Vkg-GFP. (B–E), Low (B–B′, D–D′) and high (C and E) magnification views of wild type (B and C) or vkg mutant (D–E) midguts expressing Dpp-GFP and immunostained for Integrin/Mys (red), GFP (green), Phalloidin and DRAQ5 (blue). Dpp-GFP is enriched at the BM in wild type midguts as suggested by the overlap of Dpp-GFP and Integrin/Mys signals (yellow; indicated by asterisks in C). Dpp-GFP is no longer enriched at BM in vkg mutant guts as shown by diminished colocalization between Dpp-GFP and Integrin/Mys (E). Arrows point to the BM in all panels. (F) Quantification of the mean intensity of extracellular Dpp-GFP signals that colocalize with integrin/Mys signals in control or vkg mutant guts (mean ± SD: n = 15 for each genotype).
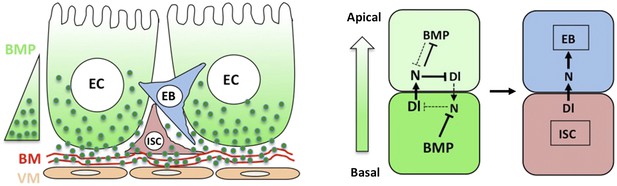
A working model for how BMP regulates ISC self-renewal.
Basal/basolateral secretion coupled with basement membrane (BM) trapping sets up an apical-basal BMP activity gradient consisting of Dpp-Gbb heterodimers. Basally localized ISC daughter cells activate higher levels of BMP signaling that promotes ISC self-renewal by antagonizing N. See text for details.





