The structure and organization of lanceolate mechanosensory complexes at mouse hair follicles
Figures
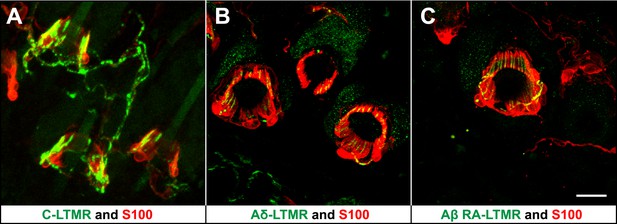
LTMRs and TSCs form palisade-like lanceolate complexes at hair follicles.
(A) On back hairy skin sections from ThCreER;Rosa26tdTomato mice, C-LTMRs are visualized using tdTomato fluorescence (green), while TSCs are labeled using S100 immunostaining (red). C-LTMRs form longitudinal lanceolate endings associated with TSCs at zigzag and awl/auchene hair follicles. (B) Back hairy skin sections from TrkBtauEGFP animals were stained with anti-GFP to label Aδ-LTMR axonal terminals and anti-S100 (red) to label TSCs. Aδ-LTMRs form longitudinal lanceolate endings associated with TSCs at zigzag and awl/auchene hair follicles. (C) Back hairy skin sections from Npy2r-GFP animals were stained with anti-GFP to label Aβ RA-LTMR axonal terminals and anti-S100 (red) to label TSCs. At a representative awl/auchene hair follicle, Aβ RA-LTMRs form longitudinal lanceolate endings associated with TSCs. Similar patterns can be seen at guard hair follicles (Li et al., 2011). Animals around 3 weeks of age were used for these experiments. Scale bar, 20 μm.
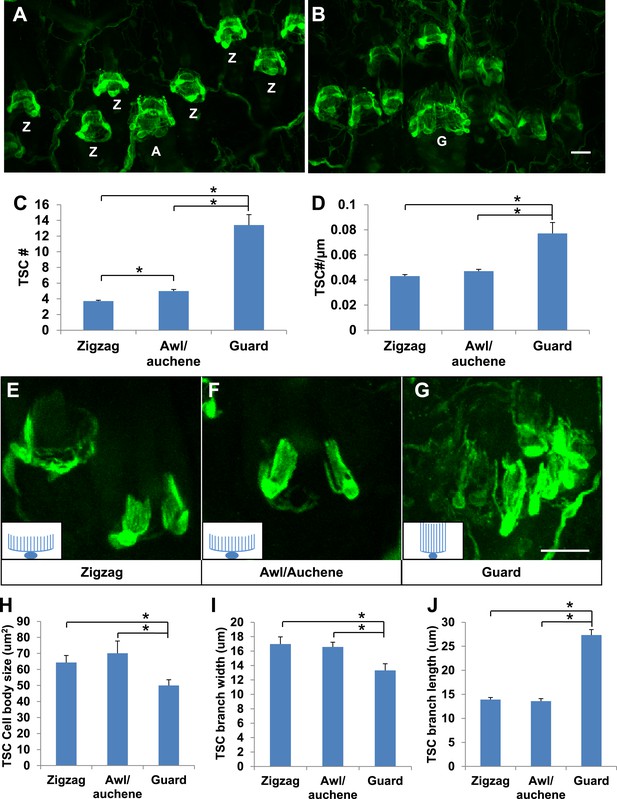
Guard, awl/auchene, and zigzag hair follicles have different numbers and morphologies of TSCs.
(A and B) Whole-mount immunostaining of back hairy skin using anti-S100 shows that awl/auchene (A) and zigzag (Z) hair follicles (in panel A) and guard (G) hair follicles (in panel B) have different numbers of TSCs. Scale bar, 20 μm. (C) Comparisons of numbers of TSCs at individual hair follicles. Awl/auchene hairs (5.0 ± 0.2 TSCs, n = 24 hair follicles) have slightly more TSCs than zigzag hairs (3.7 ± 0.1 TSCs, n = 64 hair follicles) (p<0.001). Guard hairs (13.4 ± 1.3 TSCs, n = 5 hair follicles) have many more TSCs than awl/auchene and zigzag hairs (p<0.001 for both comparisons). (D) Densities of TSCs at individual hair follicles were calculated by dividing the number of TSCs by the circumference of the hair follicles. Although TSC densities are comparable between zigzag (4.3 ± 0.1 TSCs/100 μm) and awl/auchene hairs (4.7 ± 0.2 TSCs/100 μm) (p=0.084), guard hair follicles (7.7 ± 0.9 TSCs/100 μm) have almost twofold higher densities of TSCs compared to zigzag and awl/auchene hair follicles (p<0.001 for both comparisons with zigzag and awl/auchene hair follicles). (E–G). In Plp1CreER;Rosa26YFP animals treated with 0.01 mg of tamoxifen, TSCs were sparsely labeled to visualize the morphologies of individual TSC associated with different hair follicle types. Insets in E–G are schematic images of TSCs to summarize the differences observed between TSCs associated with guard hairs vs awl/auchene and zigzag hairs. Scale bar, 20 μm. (H–J) Sizes of TSC cell bodies (H), widths of total processes of individual TSCs (I) and lengths of TSC processes (J) were measured at zigzag, awl/auchene, and guard hair follicles. Although all three parameters are comparable between zigzag and awl/auchene hair follicles, indicating similar morphologies, individual TSCs at guard hair follicles have significantly smaller cell sizes, and narrower and longer processes compared to the other two hair follicle types (Zigzag hair follicles: cell body size, 64.3 ± 4.5 μm2; width of processes, 17.0 ± 1.0 μm; length of processes, 13.9 ± 0.4 μm; n = 25 hair follicles. Awl/auchene: cell body size, 70.1 ± 7.6 μm2; width of processes, 16.6 ± 0.7 μm; length of processes, 13.6 ± 0.6 μm; n = 10 hair follicles. Guard hair follicles: cell body size, 50.0 ± 3.5 μm2, p<0.05 compared with zigzag or awl/auchene hairs; width of processes, 13.3 ± 0.9 μm, p<0.05 compared with zigzag or awl/auchene hairs; length of processes, 27.3 ± 1.1 μm, p<0.001 compared with zigzag or awl/auchene hairs; n = 16 hair follicles). Animals around 3 weeks of age were used in these whole-mount immunostaining experiments.
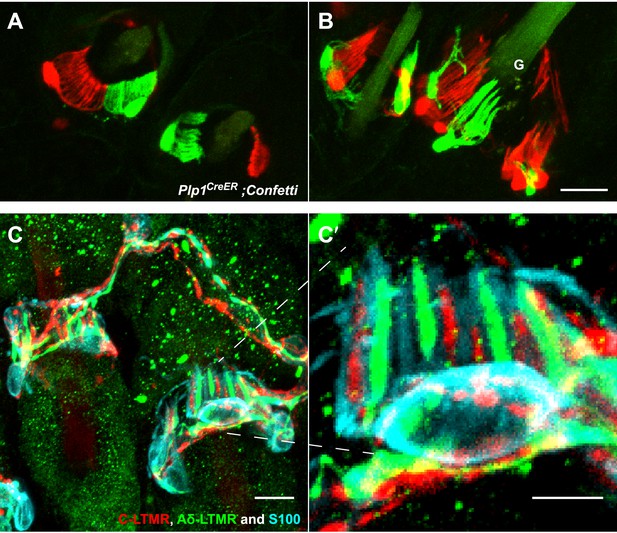
TSCs are tiled, and a single TSC can host axonal endings from multiple, physiologically distinct LTMR subtypes.
(A and B) On back hairy skin sections taken from Plp1CreER;Confetti animals, TSCs are randomly labeled with either green or red fluorescence. Neighboring TSCs at both guard (labeled with ‘G’ in B) and non-guard (A) hair follicles are tiled, exhibiting no overlapping processes. This experiment was done using 109 non-guard hair follicles and 23 guard hair follicles in two animals, all of which exhibited mosaic fluorescence labeling. 100% of these hair follicles exhibited tiled arrangements of TSCs at individual hair follicles. (C and C′) On back hairy skin sections from ThCreER;Rosa26tdTomato;TrkBtauEGFP mice, C-LTMRs were labeled with tdTomato fluorescence (red), Aδ-LTMRs were labeled with anti-GFP (green) and TSCs were stained with anti-S100 (cyan). Shown here is an example in which C-LTMR and Aδ-LTMR endings associate with different processes of the same TSC at a zigzag or awl/auchene hair follicle. Thus, a single TSC hosts more than one type of LTMR axonal terminal. Four mice were used for the triple labeling experiment and identical results were observed in each. C′ shows higher magnification of the TSC in the middle of the lanceolate complex shown in C. Scale bars, 20 μm for A and B, 10 μm in C, 5 μm in C′. Animals around 3 weeks of age were used for these experiments.
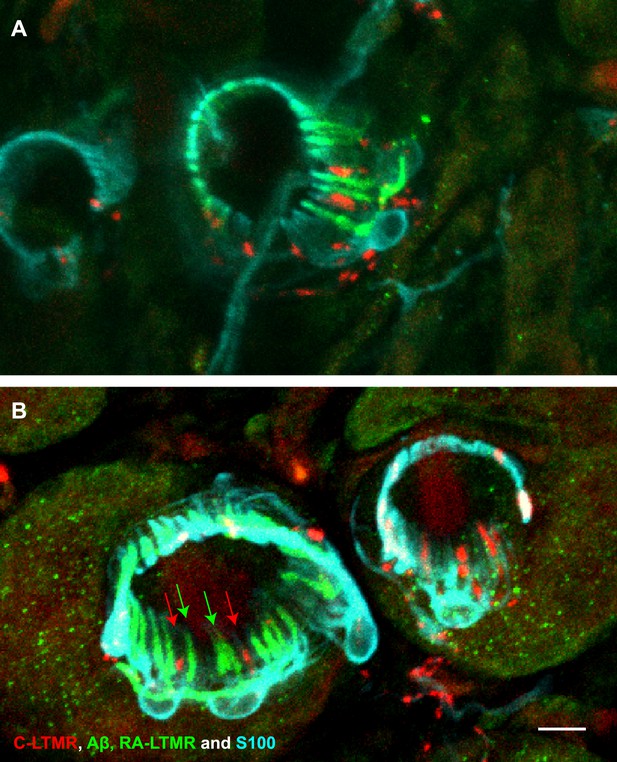
A single TSC hosts axonal endings from Aβ, RA-LTMRs, and C-LTMRs.
(A and B) On back hairy skin sections from ThCreER;Rosa26tdTomato;Npy2r-GFP mice, C-LTMRs were labeled with tdTomato fluorescence (red), Aβ, RA-LTMRs were labeled with anti-GFP (green) and TSCs were stained with anti-S100 (cyan). Shown here are two examples in which C-LTMR (highlighted by red arrows in (B) and Aβ, RA-LTMRs (highlighted by green arrows in (B) endings associate with different processes of the same TSC at awl/auchene hair follicles. Boundaries between adjacent TSCs can be roughly judged by assuming symmetric expansion of processes from their cell bodies. Scale bar, 10 μm. Three mice around 3 weeks of age were used for these experiments.
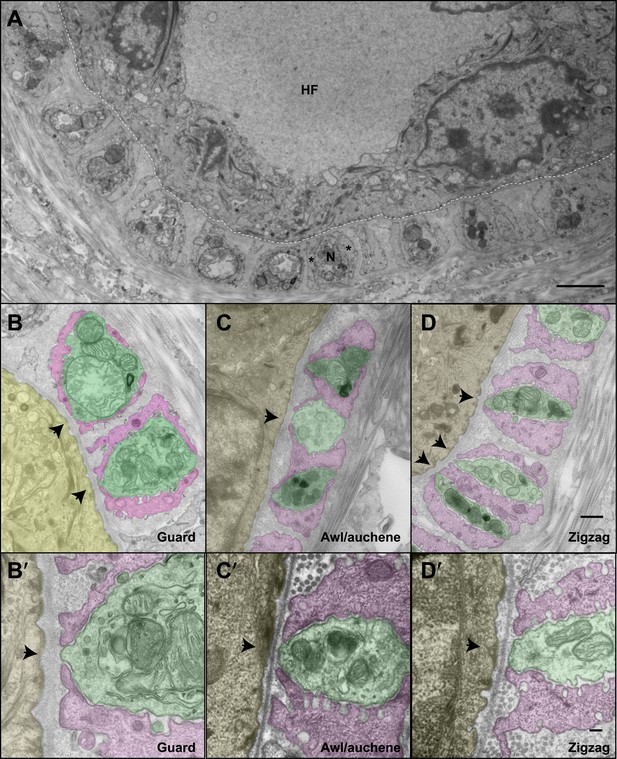
The ultrastructural relationships between LTMRs, TSCs, and hair follicle epithelial cells at the three hair follicle subtypes.
(A) A transmission electron microscopic image of a cross section through a lanceolate complex at a guard hair follicle. Repeating units of axon terminals and TSC processes are regularly arranged in a single layer surrounding the hair follicle (HF). In each unit, a mitochondria-rich axonal terminal (N) is encased by TSC processes (*). (B) A cross section of the same guard hair follicle shown in A. Axon terminals are pseudo-colored in green; TSC processes are colored in pink; the hair follicle epithelial cell is colored in yellow. Each unit is composed of one axonal terminal encased by two or three TSC processes on three sides. Axon terminals contain a large number of mitochondria. Note that small protrusions of axons (arrows) and TSC processes are precisely aligned against the basal lamina of the hair follicle. (C) A cross section of a lanceolate complex of an awl/auchene hair follicle. Each axon terminal is encased by two TSC processes on two sides. More than one axonal ending and its associated TSC processes is often packed into a single ‘unit’. Shown here is one unit composed of three axon terminals intervening among four TSC processes. Compared to guard hair follicles, awl/auchene hair follicles exhibit more area of exposed axon terminal membrane facing the outer root sheath cell of the follicle (arrow). In addition, TSC processes are thicker than those at guard hair follicles, while axon diameters are smaller. Also, the axon terminals appear heterogeneous: some have few mitochondria (the middle axon) and some many mitochondria (the other two axons). (D) A cross section of a lanceolate complex associated with a zigzag hair follicle. Similar to awl/auchene hair follicles, each axon terminal is encased by two TSC processes on two sides. One of the units in the lower left corner is composed of two axon terminals encased by three TSC processes. As with the awl/auchene complexes, and in comparison to guard hairs, areas of exposed axon terminal membrane adjacent to the hair follicle epithelial cell are large (arrows). In addition, similar to awl/auchenes, the zigzag TSC processes are thicker and axonal sections are smaller compared to guard hair follicles. Axon terminals also display varying amounts of mitochondria. (B′, C′ and D′) High magnification EM images of cross sections of guard (B′), awl/auchene (C′), and zigzag (D′) hair follicles show the gaps between TSC processes and the axon protrusions in between (arrows). Animals around 4 weeks of age were used for these experiments. Scale bars, 2 μm in A; 500 nm for B, C and D; 100 nm for B′, C′ and D′.
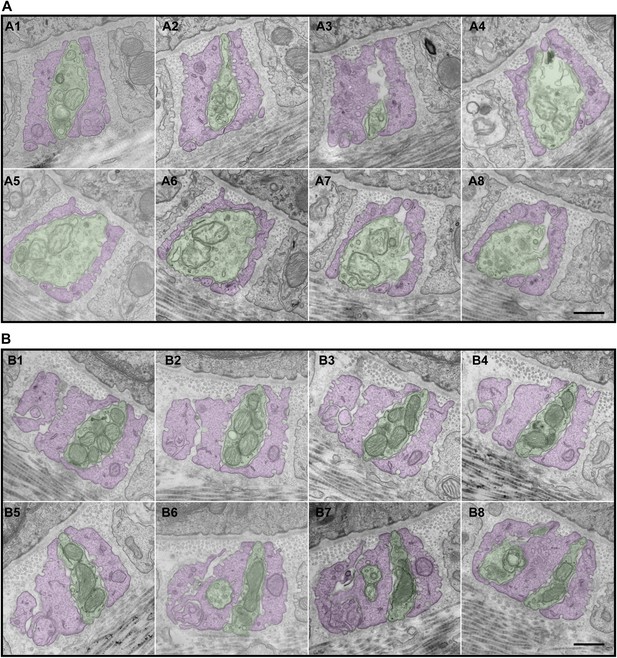
Serial EM cross-sections of C-LTMR and Aδ-LTMR axonal endings at a zigzag hair follicle.
Serial EM cross-sections spanning more than 3 µm along two different LTMR axonal terminals of lanceolate complex at a zigzag hair follicle were collected. Representative images that are approximately 0.4 μm to 0.5 μm apart were shown. (A) A1 to A8 are serial EM cross-sections of an axonal terminal that has a relatively small number of mitochondria with low electron density. Further analyses (Figure 6, Figure 6—figure supplement 2) show that this axonal ending type is likely to be C-LTMR. (B) B1 to B8 are serial EM cross-sections of an axonal terminal that is packed with mitochondria with relatively high electron density. Further analyses (Figure 6, Figure 6—figure supplement 2) show that this axonal ending type is likely to be Aδ-LTMR. Animals around 4 weeks of age were used for these experiments. Scale bar, 500 nm.
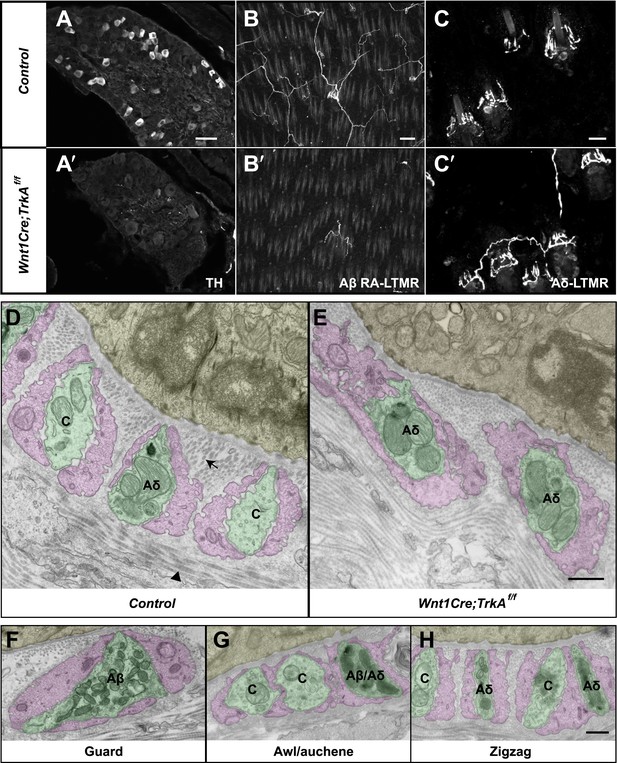
Identification of C-LTMR, Aδ-LTMR, and Aβ RA-LTMR axonal endings using EM.
(A and A′) DRG sections from Wnt1Cre;TrkAf/f (A′) and control (A) animals were stained with anti-TH. TH+ C-LTMRs are nearly completely lost in TrkA conditional knockout animals compared to control. (B and B′) Whole-mount GFP immunostaining of back skin samples from Wnt1Cre;TrkAf/f;Npy2r-GFP (B′) and control (B) animals shows that cutaneous innervation of Npy2r-GFP+ Aβ RA-LTMRs at hair follicles is almost completely lost in TrkA conditional knockout animals compared to control. (C and C′) GFP immunostaining of back skin sections from Wnt1Cre;TrkAf/f;TrkBtauEGFP (C′) and TrkBtauEGFP control (C) animals shows that innervation of hair follicles by TrkBtauEGFP+ Aδ-LTMRs remains intact in the TrkA conditional knockout animals compared to control. (D and E) Cross sections of lanceolate complexes at zigzag hair follicles from Wnt1Cre;TrkAf/f (E) and control (D) mice. Axon terminals are pseudo-colored in green; TSC processes are colored in pink; hair follicle epithelial cells are colored in yellow. As shown in Figure 4D, axon terminals at the control zigzag hair follicle have varying numbers of mitochondria. In contrast, all axons at the zigzag hair follicle from Wnt1Cre;TrkAf/f animals exhibit abundant clusters of mitochondria. Thus, axons containing few mitochondria are C-LTMRs, which are lost in TrkA conditional knockout animals, whereas axons containing abundant mitochondria are Aδ-LTMRs, which remain intact in TrkA conditional knockout animals. (F) A cross section of a lanceolate complex at a wild-type guard hair follicle. All axons associated with guard hair lanceolate complexes are densely packed with mitochondria and are derived from Aβ RA-LTMRs. (G) A cross section of a lanceolate complex at a wild-type awl/auchene hair follicle. Axons with few mitochondria are C-LTMRs; axons with abundant mitochondria are either Aβ RA-LTMRs or Aδ-LTMRs. (H) A cross section of a lanceolate complex at a wild-type zigzag hair follicle. Axons with few mitochondria are C-LTMRs, whereas axons with abundant mitochondria are Aδ-LTMRs. Animals around 4 weeks of age were used for these experiments. Scale bars, 50 μm for A and A′; 100 μm for B and B′; 20 μm for C and C′; 500 nm for D–H.
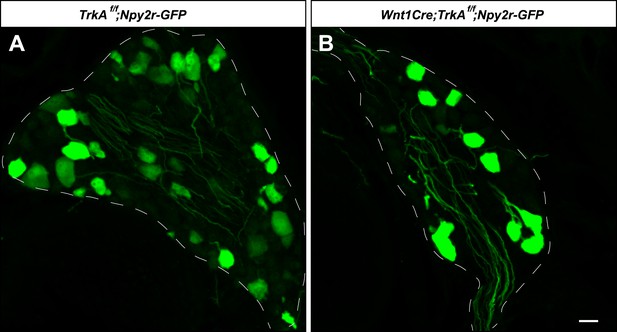
Aβ RA-LTMR neurons remain intact in Wnt1Cre;TrkAf/f animals.
(A and B) DRG sections from Wnt1Cre;TrkAf/f;Npy2r-GFP (B) and control, TrkAf/f;Npy2r-GFP (A) animals were stained with anti-GFP. Aβ RA-LTMR neurons labeled by Npy2r-GFP were still present in DRGs of these TrkA conditional knockout animals (B). Scale bar, 20 μm.
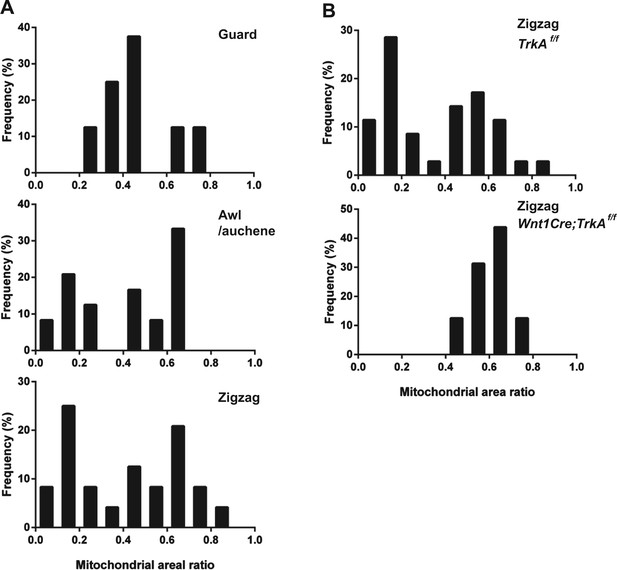
Quantification of mitochondrial abundance.
Ratios of mitochondrial area and axon area in EM cross sections of individual LTMR endings were quantified. (A) Histograms showing frequency distributions of mitochondrial area ratios of LTMR endings at guard (8 LTMR axons), awl/auchene (24 LTMR axons) and zigzag hair follicles (24 LTMR axons) from wild-type animals. Mitochondrial area ratios appear homogenously high in axons associated with guard hair follicles. In contrast, a heterogenous, bimodal distribution of mitochondria area ratios is observed in axons associated with awl/auchene and zigzag hairs. (B) Histograms showing frequency distributions of mitochondrial area ratios of LTMR endings at TrkA f/f control (35 LTMR axons) vs Wnt1Cre;TrkAf/f mutant zigzag hair follicles (16 LTMR axons). A bimodal distribution of mitochondrial area ratios is observed in control LTMR endings. In contrast, the mitochondrial abundance in LTMR endings associated with TrkA mutant hair follicles appears homogenous, exhibiting a unimodal distribution of high mitochondrial area ratios. This analysis indicates that the LTMR axonal endings at TrkA mutant zigzag hair follicles, which exhibit a high mitochondrial density, belong to Aδ-LTMRs, while those that exhibit a low mitochondria density and are absent in TrkA mutants belong to C-LTMRs. Animals around 4 weeks of age were used for these experiments.
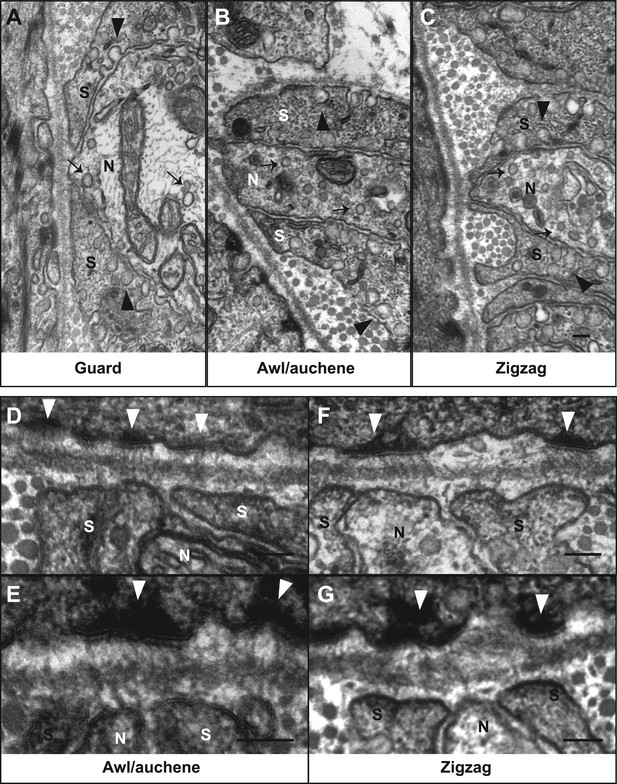
Ultrastructural features of lanceolate complexes revealed by EM using tannic acid-treated specimens.
(A–C) Cross sections of lanceolate complexes at guard, awl/auchene, and zigzag hair follicles. Small vesicles can be observed within axon terminals (arrows in A–C). TSC processes contain fine filaments that are nearly parallel to the long axis of the follicle and therefore appear as dark spots within the cytoplasm. Numerous pinocytotic vesicles are associated with both the inner and outer surfaces of TSC processes (arrowheads in A–C). (D–G) Hemidesmosomes are seen along plasma membranes of hair follicle outer root sheath cells that face LTMR axons and TSC processes (white arrowheads). Fine filament-like structures emanate from the hemidesmosomes, traverse the basal lamina, and form contacts with LTMR axon terminals and TSC processes. Axon terminals are labeled with ‘N’; TSC processes are labeled with ‘S’. Animals around 4 weeks of age were used in these experiments. Scale bars, 100 nm.
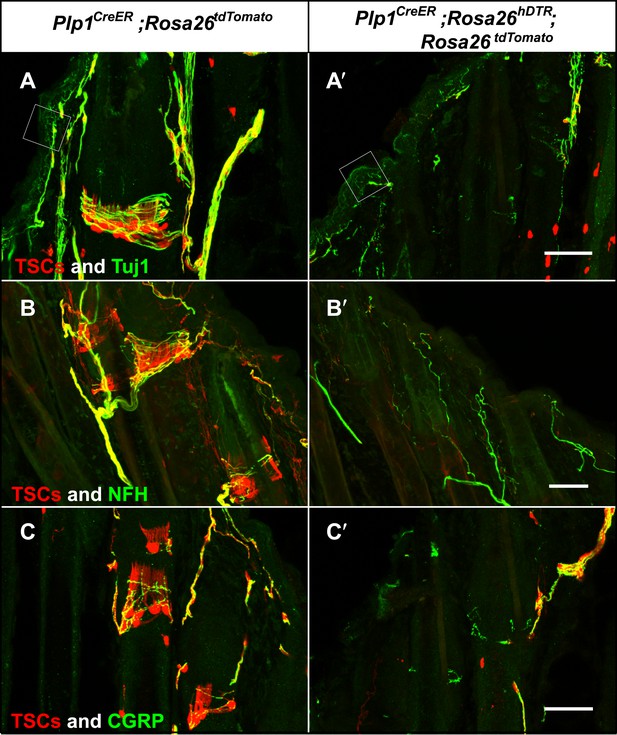
Genetic ablation of TSCs leads to loss of LTMR innervation at hair follicles.
In skin sections from Plp1CreER;Rosa26tdTomato (A–C) and Plp1CreER;Rosa26hDTR;Rosa26tdtomato (A′–C′) mice, TSCs were visualized by tdTomato fluorescence. In Plp1CreER;Rosa26hDTR;Rosa26tdtomato animals, treatments with tamoxifen and DTX lead to complete loss of TSCs at hair follicles (A′–C′). In the absence of TSCs, Tuj1 (A and A′, green) and NFH staining (B and B′, green) shows a complete loss of longitudinal axonal terminals and a partial loss of circumferential axons at the presumptive region for lanceolate complexes; CGRP+ peptidergic nociceptor fibers were also partially lost (C and C′, green). Small white squares at A and A′ highlight free nerve endings in the epidermis and dermis. Animals around 4 weeks of age were used in these experiments. Scale bars, 50 μm.
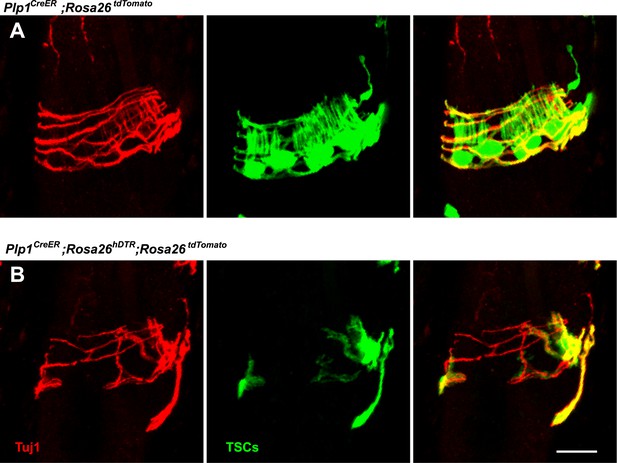
Loss of LTMR endings is coincident with degeneration of TSCs.
In skin sections from Plp1CreER;Rosa26tdTomato (A) and Plp1CreER;Rosa26hDTR;Rosa26tdtomato (B) mice, TSCs were visualized by tdTomato fluorescence. Tuj1 staining shows that at hair follicles with partial loss of TSCs, there is a corresponding loss of longitudinal lanceolate ending while circumferential endings remain.
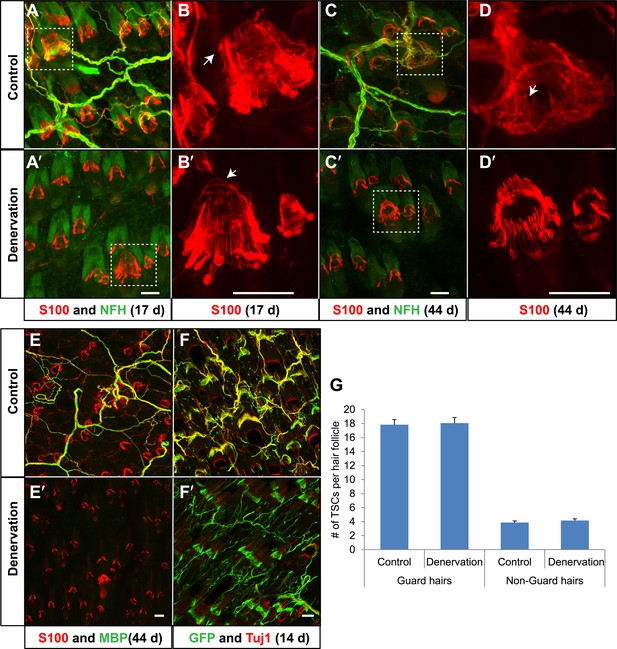
TSCs remain intact and associated with hair follicles following dorsal cutaneous nerve axotomy and distal axon degeneration.
(A and A′) Whole-mount immunostaining of S100 (red) and NFH (green) shows that 17 days after dorsal cutaneous nerve axotomy, while NFH+ cutaneous nerve have completely degenerated in the denervated skin (A′, right back skin) compared to the control skin (A, left back skin), TSCs remain intact and associated with hair follicles (A′). (B and B′) Enlarged views of boxed regions in A and A′. At control hair follicles (B), TSCs are associated with longitudinal lanceolate endings and circumferential endings (arrow). At denervated hair follicles (B′), TSCs that were associated with longitudinal lanceolate endings prior to denervation appear normal, while TSCs associated with circumferential axonal terminals (arrows) are partially lost (arrow). (C and C′) Whole-mount immunostaining of S100 (red) and NFH (green) shows that, 44 days after axotomy, TSCs in the denervated skin (C′) remain intact. (D and D′) Enlarged views of boxed regions in C and C′ show that compared to TSCs in the control skin (D), TSCs that were associated with longitudinal lanceolate endings at denervated hair follicles (D′) appear normal, while TSCs associated with circumferential axonal terminals are completely lost (arrow in D). (E and E′) Whole-mount immunostaining of S100 (red) and MBP (green) shows that, at 44 days after axotomy, while myelinating Schwann cells have completely degenerated, TSCs in the denervated skin remain intact (E′). (F and F′) In Plp1CreER;Rosa26GCamp3 animals, TSCs were induced to express GCaMP3 by tamoxifen injection before dorsal cutaneous nerve axotomy. Immunostaining for GFP (green) and Tuj1 (red) shows that, at 14 days following axotomy, while Tuj1+ cuanteous axons have completely degenerated, genetically labeled TSCs remain intact (F′). (G) Quantification of TSC numbers 5 weeks after denervation surgery shows no changes in TSC numbers at both guard hair and non-guard hair follicles in denervated skin compared to those at the control skin. n = 3. Animals around 8 weeks of age were used for all whole-mount immunostaining experiments in A–E′ and G. Plp1CreER;Rosa26GCamp3 mice around 4 weeks old were used in experiments shown in F and F′. Scale bars, 50 μm.
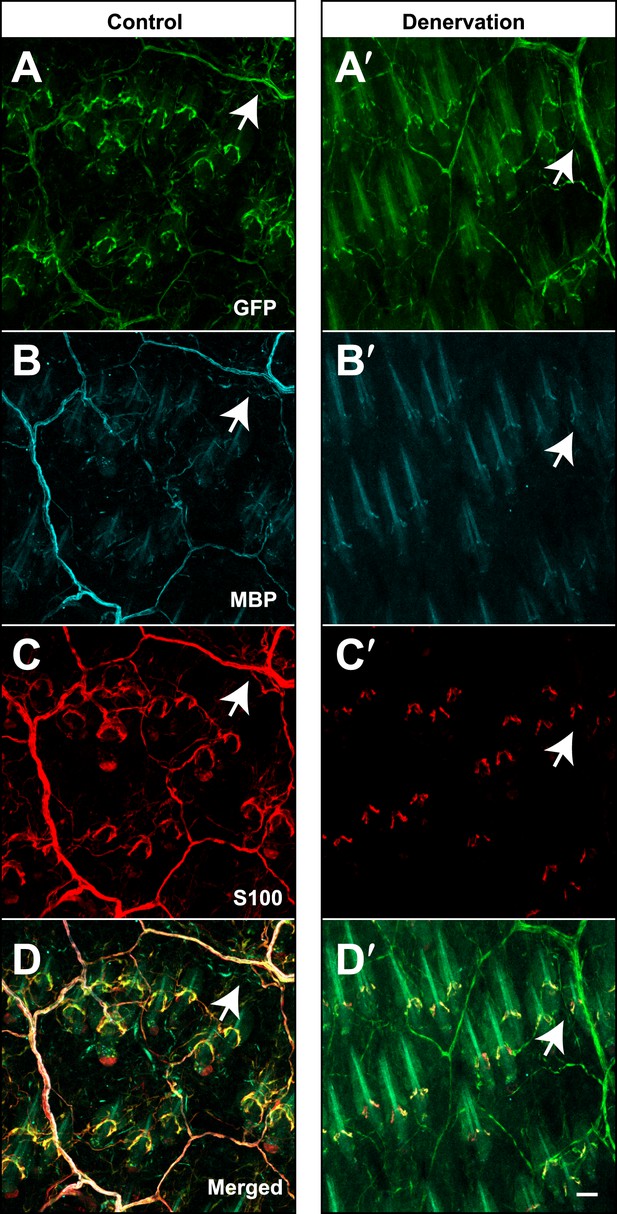
Genetically labeled immature Schwann cells after skin denervation.
Plp1CreER;Rosa26LSL-YFP mice were treated with tamoxifen before receiving skin denervation surgeries and examined 4 weeks after denervation. (A–D) On the sham-operated control side of the back skin, wholemount immunostaining with GFP (A), MBP (B) and S100 (C) show that genetically-labeled, YFP+ myelinating Schwann cells are positive for MBP and S100 (Arrows in A–D). (A′–D′) Same staining was performed on the denervated back skin. Genetically labeled, YFP+ TSCs remained at hair follicles after denervation. GFP+ sheaths that are negative for S100 and MBP can be observed (arrows in A′–D′) and they are likely to be genetically labeled immature Schwann cells. Animals around 8 weeks old were used in these experiments. Scale bar, 50 μm.
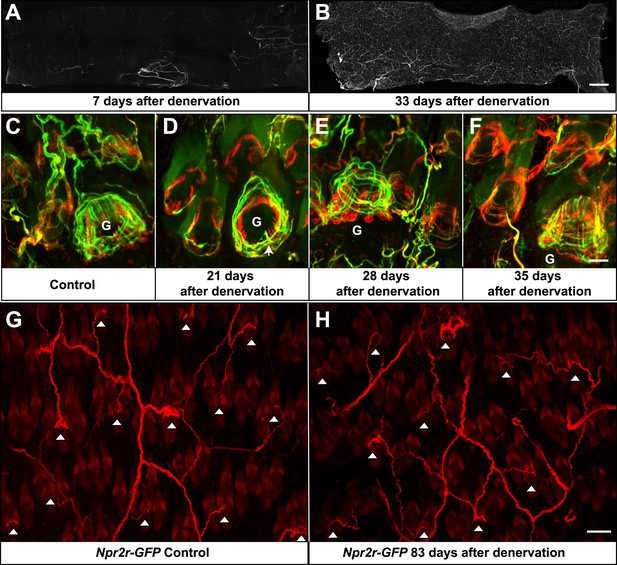
Re-innervation of TSCs following dorsal cutaneous nerve axotomy.
(A and B) Whole-mount immunostaining with NFH shows that axons are missing from denervated skin 7 days after axotomy (A), whereas nerve fibers have begun extending into the denervated area 33 days after dorsal cutaneous nerve axotomy (B). (C) In the control skin of Plp1CreER;Rosa26GCamp3 animals, whole-mount immunostaining with Tuj1 (green) and GFP (red) shows the typical morphology of lanceolate complexes at a guard hair follicle located in the lower right area of the image, as well as surrounding non-guard hair follicles. (D) 3 weeks after dorsal cutaneous nerve axotomy, re-innervation of guard hair follicles in the form of circumferential endings can be observed. A few longitudinal lanceolate endings can be occasionally observed (arrow). A small degree of re-innervation can also be seen in the surrounding non-guard hair follicles. (E) 4 weeks after dorsal cutaneous nerve axotomy, longitudinal lanceolate endings begin to form at re-innervated guard hair follicles. Circumferential endings can also be observed in the surrounding non-guard hair follicles. (F) 5 weeks after axotomy, lanceolate complexes at re-innervated guard hair follicles are comparable to those in the control, uninjured skin. More innervation of the surrounding non-guard hair follicles can also be seen. Guard hairs are labeled with ‘G’ in panels C to F. (G and H) Npy2r-GFP animals were subjected to dorsal cutaneous nerve axotomy, as above. G shows whole-mount GFP immunostaining of the control, non-denervated skin (left back hairy skin), while H shows GFP immunostaining of denervated skin (right back hairy skin) 83 days after axotomy. In control, uninjured skin, GFP+ Aβ RA-LTMRs form longitudinal lanceolate endings at guard and awl/auchene hair follicles (G, white arrowheads). 83 days after axotomy, GFP+ Aβ RA-LTMRs re-innervate guard and awl/auchene hair follicles in the denervated skin (H, white arrowheads), indicating that the specificity of the hair follicle subtype innervation pattern is conserved during the re-innervation processes. Many GFP+ axonal terminals in the re-innervated skin form longitudinal lanceolate endings that are comparable to those in the control skin. Scale bars, 2 mm for A and B; 20 μm from C to F; 50 μm for G and H.
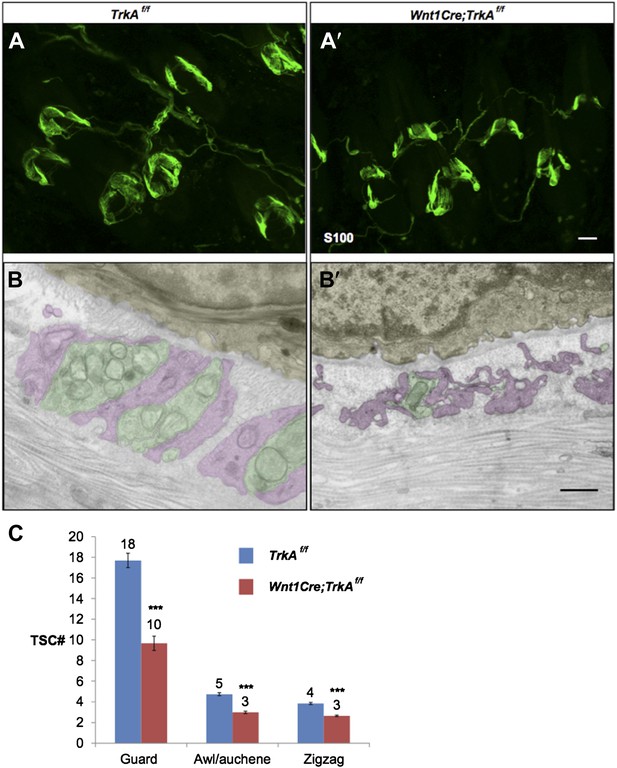
Characterization of TSCs at hair follicles of Wnt1Cre;TrkAf/f conditional mutant mouse.
A and A’. Whole mount immunostaining with anti-S100 shows that numbers of TSCs at hair follicles from of Wnt1Cre;TrkAf/f conditional mutants (A’) appear slightly less than those in control animals (A). Scale bar, 20 μm.
B and B’. Cross sections of lanceolate complexes at zigzag hair follicles from Wnt1Cre;TrkAf/f (B’) and control (B) mice. Axon terminals are pseudo-colored in green; TSC processes are colored in pink; hair follicle epithelial cells are colored in yellow. TSCs from TrkA conditional knockout hair follicles appear hypotrophic compared to control TSCs. Scale bar, 500 nm.
C. Quantification of numbers of TSCs at individual guard, awl/auchene and zigzag hairs from control mice and TrkA conditional knockout mice. In control animals, there are 17.3 ± 0.7 TSCs at individual guard hair follicles (n=13 guard hairs), 4.7 ± 0.1 TSCs at awl/auchene hair follicles (n=34 awl/auchene hairs) and 3.8 ± 0.1 TSCs at zigzag hair follicles (n=54 zigzag hairs). In TrkA conditional knockout animas, there are 9.7 ± 0.7 TSCs at individual guard hair follicles (n=15 guard hairs), 3.0 ± 0.1 TSCs at awl/auchene hair follicles (n=44 awl/auchene hairs) and 2.6 ± 0.1 TSCs at zigzag hair follicles (n=83 zigzag hairs) (p<0.001 for comparisons between all three hair follicle subtypes).
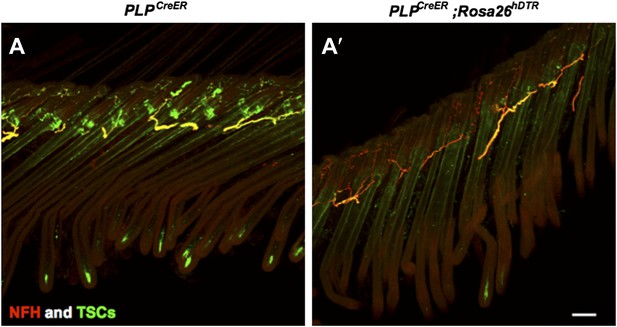
Characterization of cutaneous nerve upon TSC ablation.
Immunostaining with anti-S100 and anti-NFH was performed on skin sections from PLPCreER (A) and PLPCreER;Rosa 26hDTR mice (A’) to label TSCs and myelinated cutaneous nerves. In the absence of TSCs (A’), many fibers remain in the dermis, while no terminals forming lanceolate endings structures remain at the hair follicles. Scale bar, 100 μm.





