SLY1 and Syntaxin 18 specify a distinct pathway for procollagen VII export from the endoplasmic reticulum
Figures
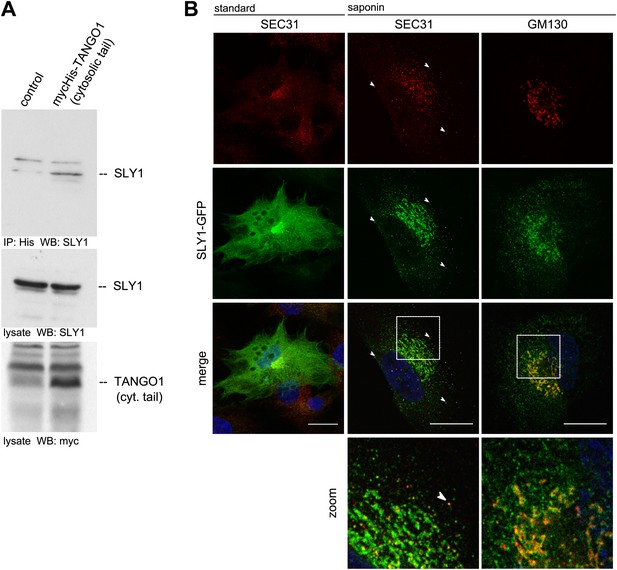
SLY1 immunoprecipitates with TANGO1 and localizes to ER exit sites.
(A) Lysates from Myc-His-TANGO1 (cytoplasmic tail) transfected or control HeLa cell lysates were incubated with Nickel beads, bound proteins (75%) and total lysates (10%) were separated by SDS-PAGE, endogenous SLY1 and Myc-His-TANGO1 (cytoplasmic tail) was detected by Western Blot using SLY1 and Myc antibodies. (B) RDEB/FB/C7 cells stably expressing SLY1-GFP (green) were fixed with 4% PFA and permeabilized with Triton-X100 or pre-extracted with saponin, washed and then fixed with 4% PFA prior to permeabilization with Triton-X100. Cells were labeled with an anti-SEC31 or an anti-GM130 antibody (red) and DAPI (blue, Scale bar: 20 µm); arrowheads indicate colocalization of SLY1-GFP and SEC31.
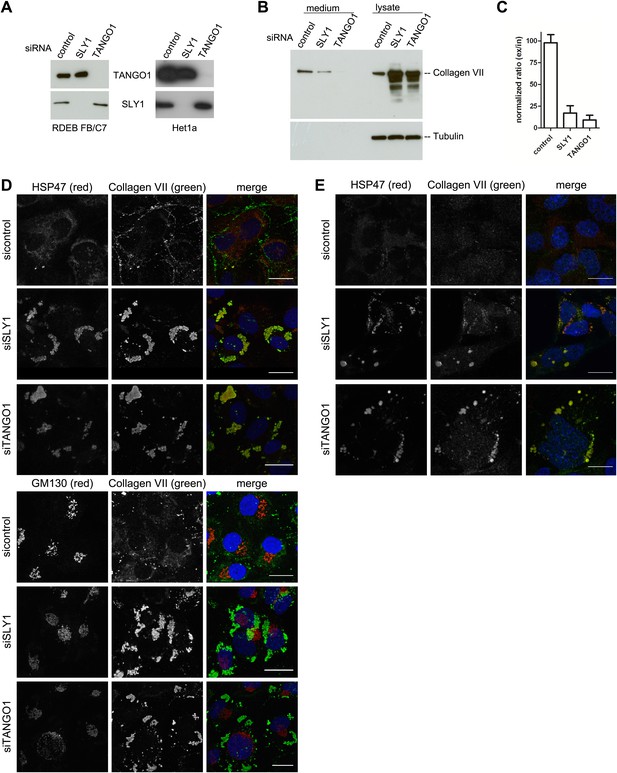
SLY1 knockdown by siRNA inhibits Procollagen VII secretion.
RDEB/FB/C7 cells were transfected with siRNAs directed against SLY1, TANGO1, or a scrambled siRNA. Het1a cells were transfected with the same set of siRNAs. (A) Knockdown efficiency was tested after 72 hr by western blotting cell lysates with anti-TANGO1 or anti-SLY1 antibodies. (B) PC VII secretion was measured by western blotting RDEB/FB/C7 cell lysates and supernatants collected for 20 hr in the presence of ascorbic acid with an anti-Collagen VII antibody. Equal protein loading and cell lysis were controlled by blotting with an anti-Tubulin antibody. (C) In three independent experiments, intensities of the PC VII signal in the lysate and the supernatant was recorded by densitometry. The ratio of external vs internal Collagen VII was normalized to quantify secretion in control cells as 100%; Error bars: standard error of the mean (SEM). siRNA-treated RDEB/FB/C7 (D) or Het1a (E) cells were seeded on coverslips and 20 hr after addition of ascorbic acid, cells were fixed and visualized with the indicated antibodies and DAPI (blue) by fluorescence microscopy (scale bars: 20 µm).
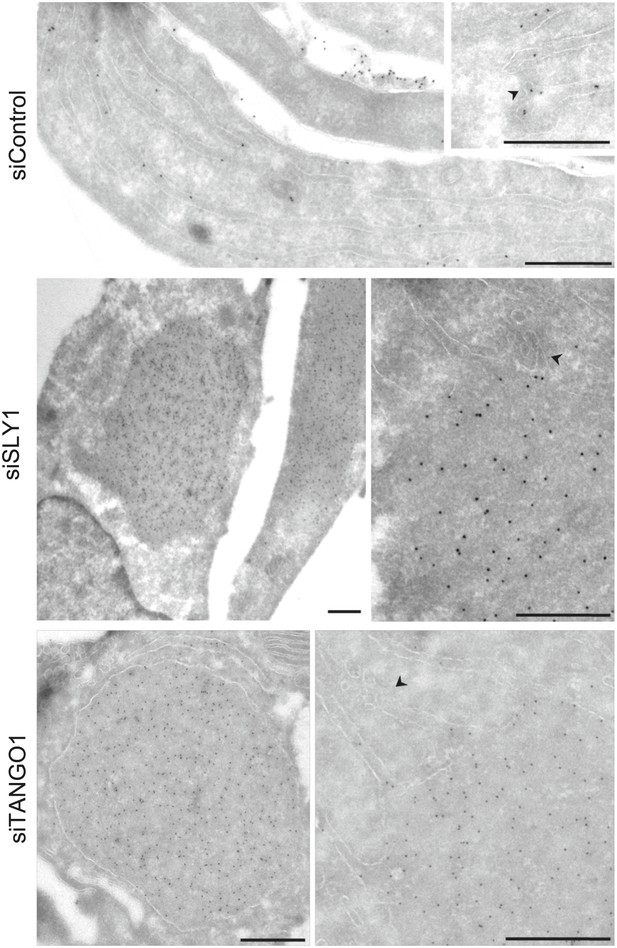
Immunoelectron microscopy localizes intracellular Procollagen VII in SLY1 knockdown cells to the ER.
Control cells and cells depleted for SLY1 or TANGO1 were fixed for immunoelectron microscopy. Ultrathin cryosections were labeled with a polyclonal rabbit antibody against Collagen VII followed by 10 nm protein A-gold complex. Arrowheads mark ER exit sites. Bars, 250 nm.
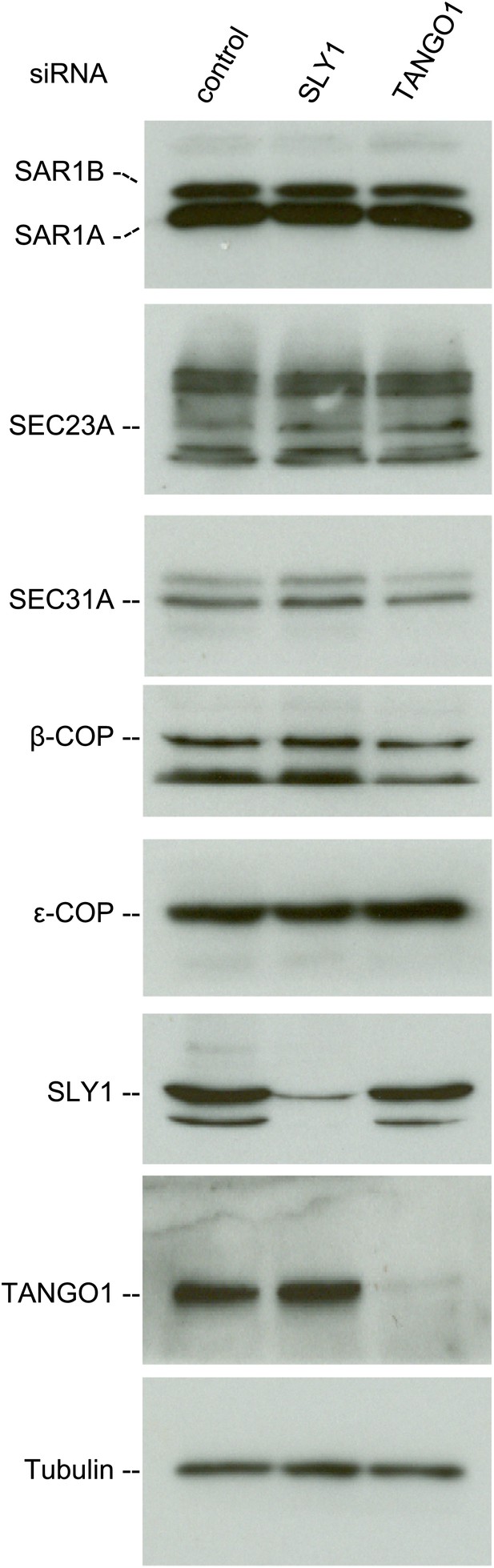
SLY1 knockdown does not affect the levels of COPI and COPII components.
Cell lysates from RDEB/FB/C7 cells transfected with siRNAs oligos against SLY1, TANGO1, or a scrambled siRNA were western blotted with antibodies to the indicated COPI and COPII components.
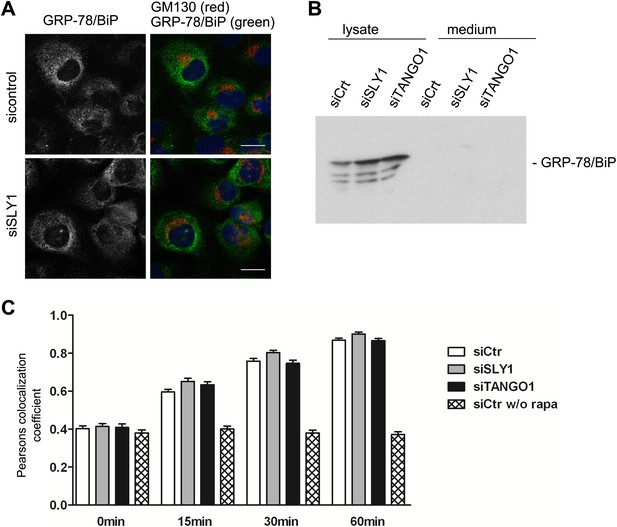
SLY1 knockdown does not affect retrograde trafficking.
(A) RDEB/FB/C7 cells depleted of SLY1 or control cells were visualized using an anti-GRP78/BIP, an anti-GM130 antibody and DAPI by fluorescence microscopy (scale bars: 20 µm). (B) Lysates and 20 hr medium of SLY1, TANGO1 or control knockdown RDEB/FB/C7 cells were analyzed by SDS-PAGE and western blotted with an anti-GRP78/BIP antibody. (C) In HeLa cells transfected with the indicated siRNAs, the colocalization of FKBP-ERGIC53-GFP and the ER localized Ii-FRAP-HA was determined at each indicated time points after addition of Rapamycin. Trapping, and thus colocalization, was measured by calculating the Pearsons colocalization coefficient between the GFP signal and the Alexa594-stained Ii-FRAP-HA. The average values of at least 30 cells analyzed per experiment for each condition are shown; Error bars: SEM.
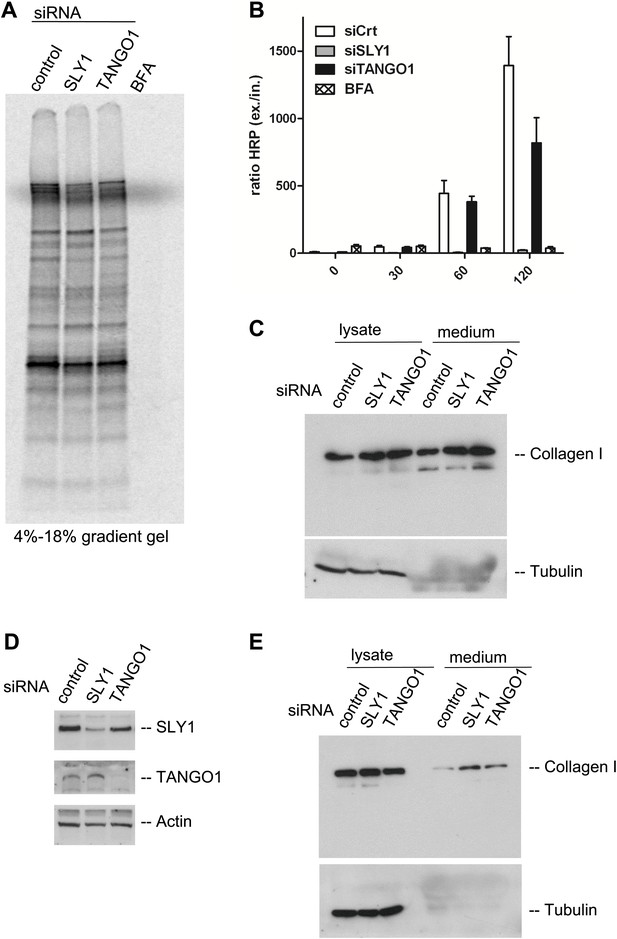
SLY1 knockdown does not block the export of endogenous secretory cargo.
(A) RDEB/FB/C7 cells depleted for SLY1 or TANGO1 or control cells were pulsed with 35S-methionine for 20 min and chased for 2 hr in complete medium that included 5 mg/ml BFA where indicated. The medium from the cells was collected and analyzed by SDS-PAGE and autoradiography. (B) HeLa ss-HRP cells were transfected with control siRNA, SLY1 or TANGO1 specific siRNA oligos or treated with BFA as a positive control for total block in secretion. At the indicated time points, the medium and cell lysates were harvested to measure HRP activity. The graph shows the ratio of secreted to intracellular ss-HRP activity. Average values of three independent experiments are shown; Error: SEM. (C) RDEB/FB/C7 cells were transfected with control siRNA, SLY1 or TANGO1 specific siRNA oligos. Collagen I secretion was measured by western blotting of RDEB/FB/C7 cell lysates and supernatants collected for 20 hr in the presence of ascorbic acid with an anti-Collagen I antibody. The samples were western blotted with an anti-Tubulin antibody to monitor loading control and cell lysis. (D) Saos2 cells were transfected with control, SLY1 or TANGO1 siRNAs. Knockdown efficiency was tested after 72 hr by western blotting cell lysates with anti-TANGO1 or anti-SLY1 antibodies. Actin was used as a loading control. (E) Collagen I secretion was measured by western blotting Saos2 cell lysates and supernatants collected for 20 hr in the presence of ascorbic acid with an anti-Collagen I antibody. The samples were also western blotted with an anti-Tubulin antibody to monitor loading control and cell lysis.
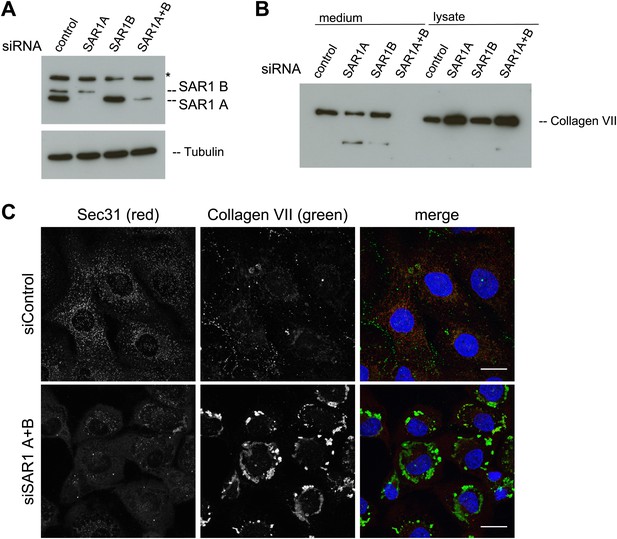
SAR1 A and B knockdown blocks Collagen VII secretion.
RDEB/FB/C7 cells were transfected with siRNAs directed against SAR1A, SAR1B, both or a scrambled control siRNA. (A) Knockdown efficiency was determined after 72 hr by western blotting cell lysates with an anti-SAR1 antibody. Tubulin was used as a loading control. *-unspecific band. (B) Collagen VII secretion was measured by western blotting RDEB/FB/C7 cell lysates and supernatants collected for 20 hr in the presence of ascorbic acid using an anti-Collagen VII antibody. (C) RDEB/FB/C7 cells treated with ascorbic acid were seeded on coverslips and 72 hr after transfection with control or SAR1 A and B siRNA, the cells were fixed and visualized by fluorescence microscopy with the indicated antibodies and DAPI (blue, scale bars: 20 µm).
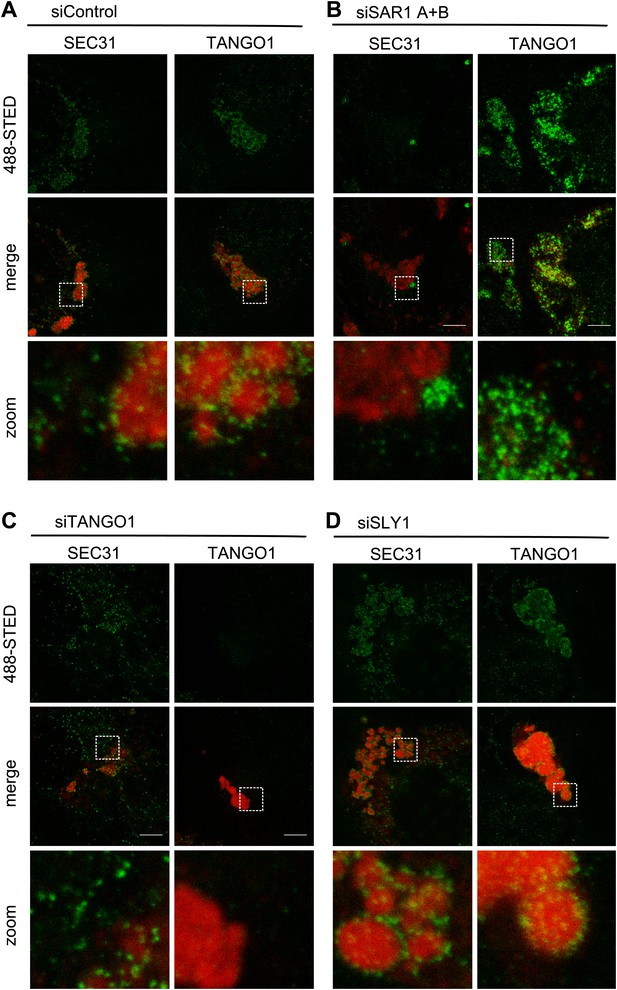
SLY1 is required for post coat assembly events in PC VII export.
RDEB/FB/C7 cells were transfected with control siRNA (A) or siRNAs directed against SAR1 A+B (B), TANGO1 (C) or SLY1 (D). Cells were incubated in medium containing ascorbic acid for 72 hr. In control cells (A) protein export from the ER was arrested for 3 hr at 15°C and cells were fixed after a 30 min release to 37°C. All other samples were fixed without a 15°C temperature block (B–D). The ER exit sites were visualized using either anti-SEC31 or anti-TANGO1 antibodies (green) in STED mode. PC VII patches were visualized using either anti-Collagen VII (in combination with anti-SEC31) or the colocalizing HSP47 (in combination with anti-TANGO1) antibodies (red) in normal confocal mode. Edge length of the zoom boxes is approximately 5 µm.
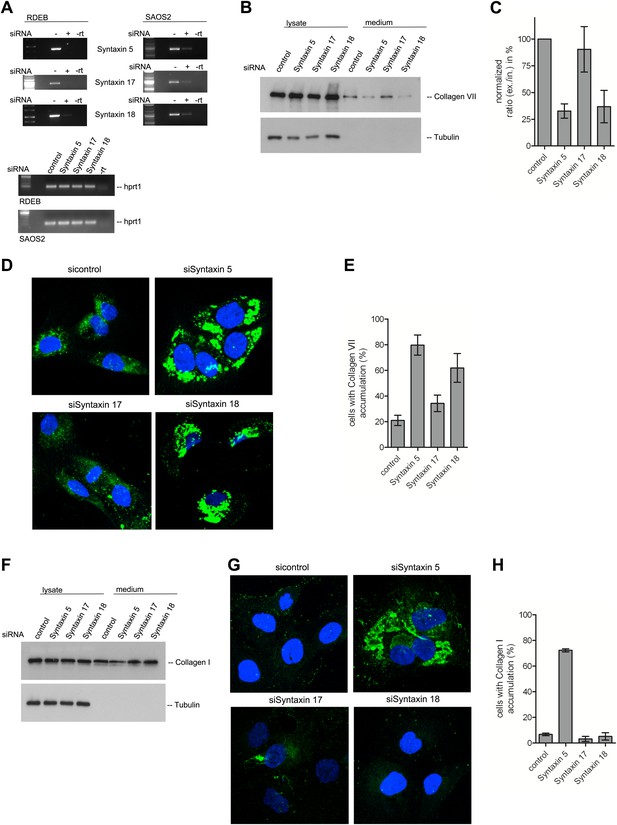
The t-SNARE Syntaxin 18 is necessary for Procollagen VII but not Procollagen I export.
To evaluate PC VII (B–E) and PC I export (F–H), RDEB/FB/C7 and Saos2 cells were transfected with siRNAs directed against STX5, STX17, STX18 or a scrambled siRNA. (A) Knockdown efficiency was assessed 48 hr after transfection by RT-PCR. PC VII secretion (B) and PC I secretion (F) was measured by western blotting cell lysates and supernatants collected for 20 hr in the presence of ascorbic acid from RDEB/FB/C7 and Saos2 cells, respectively. Equal protein loading and cell lysis was controlled by blotting with an anti-Tubulin antibody. (C) Intensities of Collagen VII in the lysate and the supernatant was recorded by densitometry in four independent experiments. The ratio of external vs internal Collagen VII was normalized to quantify secretion in control cells as 100%; Error bars: standard error of the mean (SEM). (D and G) siRNA treated RDEB/FB/C7 cells were seeded on coverslips and 20 hr after addition of ascorbic acid, cells were fixed and visualized with Collagen VII (D) or Collagen I (G) antibodies (green), and DAPI (blue) by fluorescence microscopy. The percentage of cells that accumulate PC VII (E) or PC I (H) intracellularly was determined by counting at least 30 cells in five random fields. The number of cells accumulating PC VII in the case of STX5 and STX18 siRNA was significantly different from control cells (p<0.05). Accumulation of PC VII in STX17 siRNA cells was not significantly different from the control situation (p>0.1). Error bars: standard error of the mean (SEM).
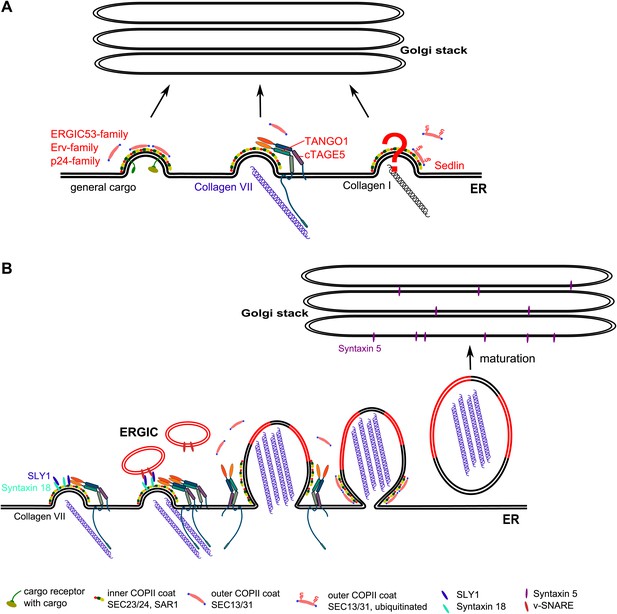
A working model for procollagen export from the ER.
(A) Multiple exit routes from the ER. Cargo receptors of the ERGIC53, ERV, and p24 families bind secretory cargoes in the lumen and the inner COPII coat on the cytoplasmic side of the ER. These receptors bound to cargoes are collected into COPII vesicles for export from the ER. TANGO1 connects PC VII in the lumen of the ER with COPII coats on the cytoplasmic side of the ER. The mechanism by which PC I is connected with the COPII components is not known. Sedlin and ubiquitination of SEC31 are required for PC I secretion but their role in PC VII export is not known. (B) Building a Procollagen VII containing mega carrier by fusion of recycling ERGIC membranes. Post concentration of PC VII by TANGO1 at the ER exit sites, many of the ER exit sites are concentrated to generate a patch enriched in PC VII. This patch containing STX18 then promotes SLY1 dependent fusion of membranes from the ERGIC. Accretion of membranes by this process grows PC VII-enriched ER patch. The COPII coats and the TANGO1 remain at the neck of the growing patch. These components at the neck promote fission and the resulting mega container enriched in PC VII and ERGIC membrane components is in fact the first post ER compartment that matures to move PC VII forward.
Tables
SNAREs involved in the early secretory pathway
| Name | t- or v-SNARE | Sub-cellular localization | Secretion pathway | Cargoes associated |
|---|---|---|---|---|
| STX 18 | t | ER (Nakajima et al., 2004; Itakura et al., 2012) | Golgi to ER, ER to Golgi | Collagen VII (this study), ssGFP (Gordon et al., 2010) |
| STX 17 | t | ER (Itakura et al., 2012) | Autophagy | GFP-LC3 (Itakura et al., 2012) |
| USE1/P31 | t | ER (Nakajima et al., 2004; Okumura et al., 2006) | Golgi to ER | ERGIC-53, KDEL-R (Aoki et al., 2009) |
| SEC20 | t | ER (Nakajima et al., 2004) | Golgi to ER, ER to Golgi | ssGFP (Gordon et al., 2010) |
| STX 5 | t | Golgi (Rowe et al., 1998; Aoki et al., 2009) | ER to Golgi, Golgi to ER | Collagen VII (this study), Collagen I (this study), ssGFP (Gordon et al., 2010) |
| BET1 | t | ER-ERGIC-Golgi (Hay et al., 1996; Uemura et al., 2009) | ER to Golgi | Chylomicrons (Siddiqi et al., 2006) |
| BET1L | t | Golgi (Tai et al., 2004) | unknown | |
| SEC22B | v | ER-ERGIC-Golgi (Hay et al., 1996; Tai et al., 2004) | ER to Golgi, Golgi to ER | ssGFP (Gordon et al., 2010) |
| YKT6 | v | ER-ERGIC-Golgi (Zhang and Hong, 2001; Volchuk et al., 2004) | ER to Golgi, Golgi to ER | ssGFP (Gordon et al., 2010) |
| VTI1a | v | ER-ERGIC-Golgi (Flowerdew and Burgoyne, 2009) | ER to Golgi | Chylomicrons (Siddiqi et al., 2006) |
-
t, targeting; v, vesicular; ER, endoplasmic reticulum; ERGIC, ER to Golgi intermediate compartment; ssGFP, signal sequence GFP.





