Loss of Cdc42 leads to defects in synaptic plasticity and remote memory recall
Figures
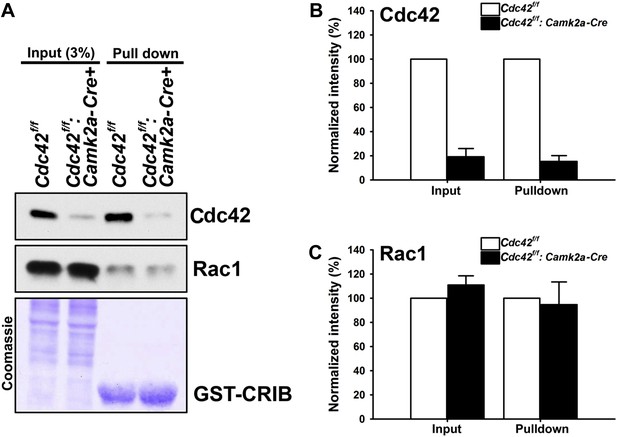
Loss of hippocampal Cdc42 in Cdc42f/f: Camk2a-Cre mice.
(A) Top and middle panels, representative western blots of Cdc42 and Rac1 levels for input (left two lanes) and GST-CRIB pulldowns (right two lanes). Bottom panel is a representative coomassie stain showing equivalent amounts of total protein (left two lanes) or GST-CRIB fusion protein (right two lanes). (B and C) Graphs depicting the quantification of (B) Cdc42 or (C) Rac1 GTPases from western blot analysis of GST-CRIB pulldowns from hippocampal lysates. n = 4 for each group. *p<0.0001.
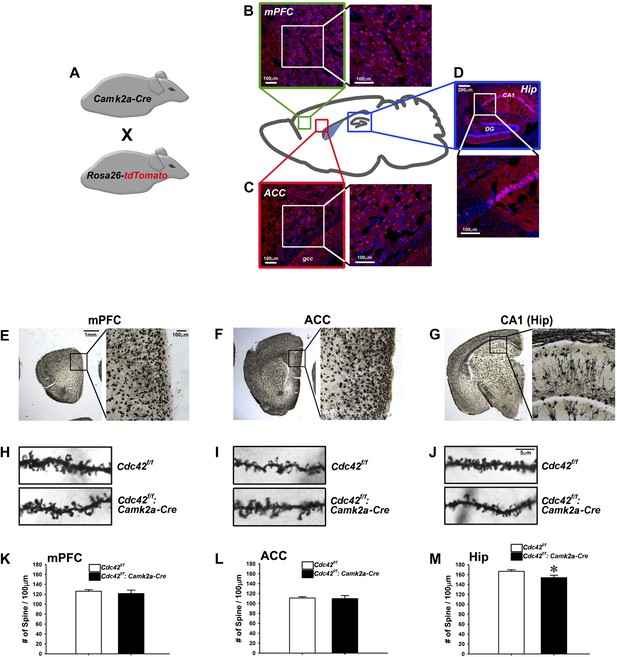
Analysis of dendritic spines in Cdc42f/f: Camk2a-Cre mice.
(A) Schematic of breeding for the analysis of Camk2a-cre expression analysis in B–D. (B–D) Representative images of cre-dependent tdTomato expression in (B) medial pre-frontal cortex (mPFC), (C) anterior cingulate cortex (ACC), and (D) Hippocampus (Hip). (E–G) Representative images of golgi stained tissue sections from (E) the mPFC, (F) the ACC, and (G) CA1 hippocampal region. (H–J) Representative images of individual dendritic segments from the (H) the mPFC, (I) ACC, or (J) CA1 hippocampal region (top panels) control Cdc42f/f or (bottom panels) cKO Cdc42f/f: Camk2a-Cre mice. (K–M) Graphs depicting the quantitative analysis of spines per 100 micron of dendritic seqements for each genotype from each region in H–J. n = 5 for each group. *p<0.05.
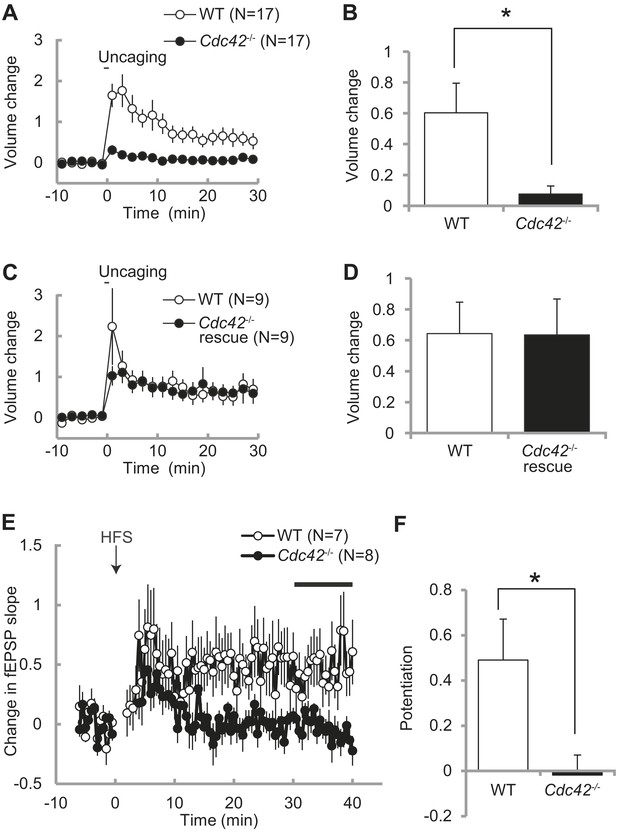
Impaired structural and functional synaptic plasticity in Cdc42f/f: Camk2a-Cre mice.
(A) Single spine volume changes in response to glutamate uncaging for (open circle) WT control Cdc42f/f or (closed circle) cKO Cdc42−/− mice. (B) Mean responses for minutes 20–30 between each genotype from (A) showing a significant impairment in cKO spines. N = 15 spines/15 slices for each group, *p<0.05. (C) Same as in (A) except the cKO neurons are co-transfected with a Cdc42 expression construct (rescue). (D) Mean responses for minutes 20–30 between WT and cKO Cdc42−/− rescue spines are shown. N = 9 spines/9 slices for each group. (E) Graph depicting changes in fEPSP slope in response to high-frequency stimulation (HFS; 100 pulses at 100 Hz; three times with 20 s intervals) of the SC–CA1 pathway (time zero). (F) Mean fEPSP potentiation for minutes 30–40 was significantly reduced in Cdc42−/− hippocampal slices when compared to WT littermates. N = 7 and 8 slices for WT and Cdc42−/−, respectively. *p<0.05.
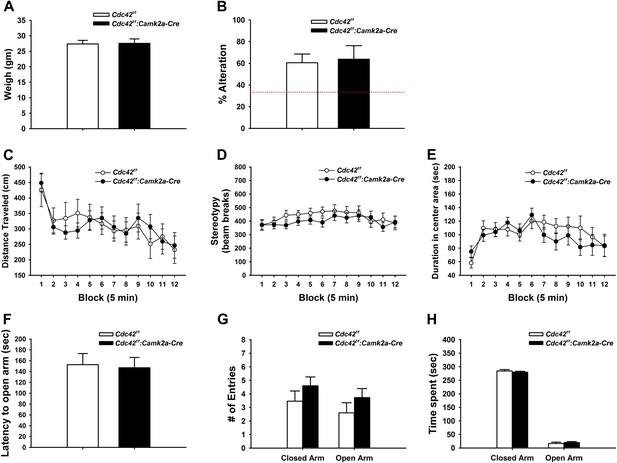
Behaviors unaffected by loss of Cdc42.
(A) Average body weight of Cdc42f/f (control, open bar) or Cdc42f/f: Camk2a-Cre mice (cKO, black bar). (B) Percent alternation in the Y-maze for both genotypes. Dashed line indicates the expected percent correct alternation that would be observed by chance. (C–E) Analysis of Open Field exploration behavior for (C) distance traveled, (D) stereotypy, or (E) duration spent in the center of the field. (F–H) Analysis of zero maze exploration behavior for (F) latency to enter the open arm, (G) number of entries to both the closed and open arms of the maze, and (H) total time spent in each arm. n = 14 for each group.
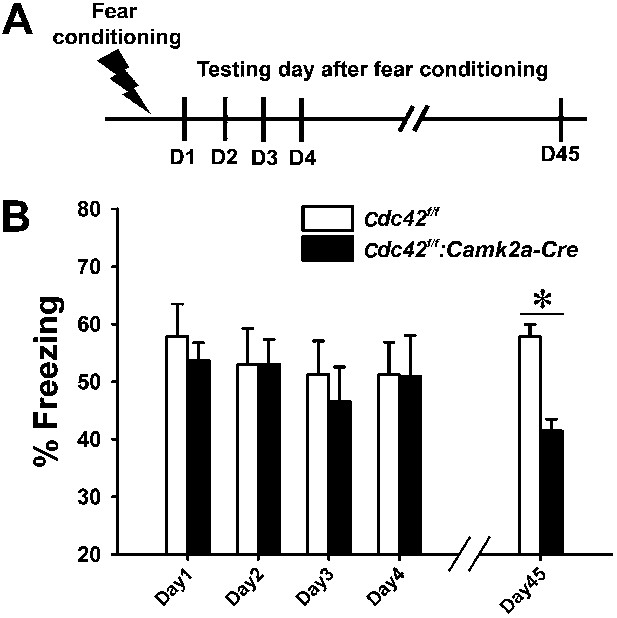
Cdc42 cKO mice exhibit reduced memory recall in the fear conditioning learning and memory paradigm.
(A) Schematic of the fear conditioning protocol in which the mice receive a mild aversive foot-shock on day 1 (D1) in a conditioning chamber. Freezing upon placement in the chamber (without shock) was assessed during acquisition (day 1) or for long-term (days 2–4) or remote memory (day 45). (B) Graph depicting the average percent time spent freezing at each time point for each genotype. n = 14 for each group. *p<0.001.
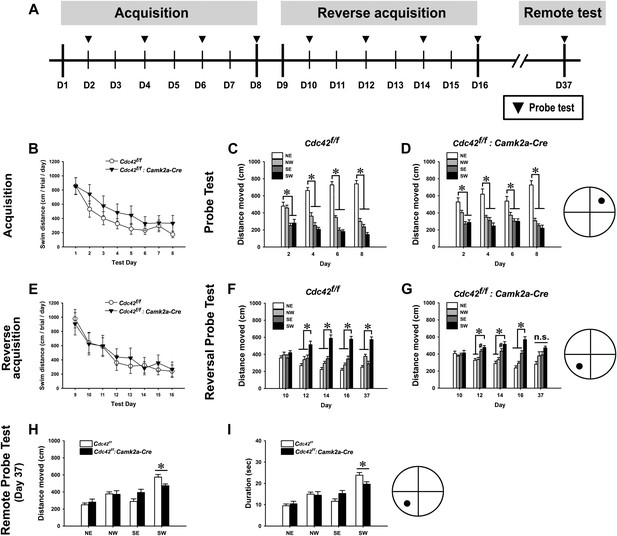
Cdc42 is essential for normal memory recall in the water maze test.
(A) Schematic of the water maze testing schedule showing the acquisition phase during days (D) 1–8, platform reversal phase during D9-16, and remote probe trial test on D37. No significant differences were observed between control Cdc42f/f and Cdc42f/f: Camk2a-Cre cKO mice during water maze acquisition phase as measured by (B) total swim distance to the platform, or in distance moved in the target vs non-target quadrants for (C) control or Cdc42 cKO mice. (E) Swim distances to the platform during acquisition was also unaffected in the Cdc42 cKO mice during reversal learning. (F) Control mice spent significantly more time in the target vs non-target quadrants during the remote memory probe trial on day 37, however (G) Cdc42 cKO mice did not distinguish between these quadrants. There were significant differences between the control and Cdc42 mice in both the (H) distance moved and (I) duration of time spent within the target (SW) quadrant during the remote memory probe trial. n = 8 for WT; n = 10 for cKO. *ps < 0.05, # = no significant difference from target quadrant.





