Integrated control of transporter endocytosis and recycling by the arrestin-related protein Rod1 and the ubiquitin ligase Rsp5
Figures
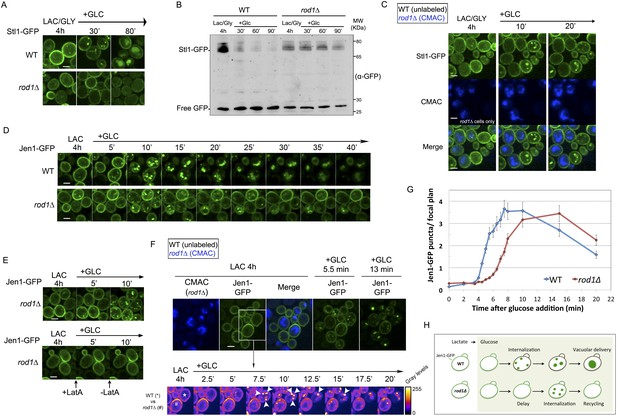
Dual function of Rod1 in transporter internalization and post-endocytic sorting.
(A) Rod1 is required for the glucose-induced endocytosis of Stl1, the glycerol/proton symporter, from the plasma membrane to the vacuole. WT (ySL1146) and rod1Δ (ySL1153) cells were grown in lactate/glycerol medium to induce Stl1-GFP expression and targeting to the plasma membrane. Cells were treated with glucose for the indicated times and imaged for Stl1-GFP localization. Scale bar = 2.5 µm. (B) Stl1-GFP degradation in response to glucose requires Rod1. WT (ySL1146) and rod1Δ (ySL1153) cells expressing Stl1-GFP were grown as in A. Crude extracts were prepared at the indicated times and were immunoblotted with anti-GFP antibodies. (C) Rod1 is required for Stl1 internalization in response to glucose. WT (ySL1146) and rod1Δ (ySL1153) cells were grown in lactate/glycerol medium to induce Stl1-GFP expression and targeting to the plasma membrane. rod1Δ cells were then labeled with CMAC and were co-injected with WT cells into the microfluidics device in lactate/glycerol medium, before glucose was added. Images taken at 10 and 20 min after glucose addition are shown. Scale bar = 2.5 µm. See also Video 1. (D) Jen1-GFP is internalized upon glucose treatment even in the absence of Rod1. Lactate-grown WT (ySL1150) and rod1Δ (ySL743) cells expressing Jen1-GFP were injected into a microfluidics device in lactate medium. Cells were imaged over time after glucose addition. Scale bar = 2.5 µm. (E) The appearance of Jen1-GFP-positive puncta in the rod1Δ mutant is inhibited by latrunculin A (LatA). Left panel, rod1Δ (ySL743) cells expressing Jen1-GFP were grown on lactate medium and injected into the microfluidics device in lactate medium, before glucose was added. Right panel, glucose and LatA were simultaneously added. After 5 min, LatA was removed and cells were fueled only with glucose medium. Scale bar = 2.5 µm. (F) rod1Δ cells display a kinetic delay in Jen1 internalization. Top, WT (ySL1150) and rod1Δ (ySL743) cells expressing Jen1-GFP were grown on lactate medium. The rod1Δ cells were then labeled with CMAC and were co-injected with WT cells into the microfluidics device in lactate medium, before glucose was added. Images taken at 5 and 13 min after glucose addition are shown. Bottom, images representative of WT and rod1Δ cells are shown at various times and are shown in false colors to visualize Jen1 fluorescence intensity. Arrowheads indicate strongly fluorescent vesicles, presumably late endosomes, which do not appear in the rod1Δ mutant. Scale bar = 2.5 µm. See also Video 3. (G) Quantification of the experiment shown in F. The mean number (±SEM) of vesicles in a focal plane for each strain (30 cells/strain, n = 3) was plotted as a function of time. (H) Graphical representation of the phenotype observed in rod1Δ cells. A fraction of Jen1 is internalized but recycles to the cell membrane.
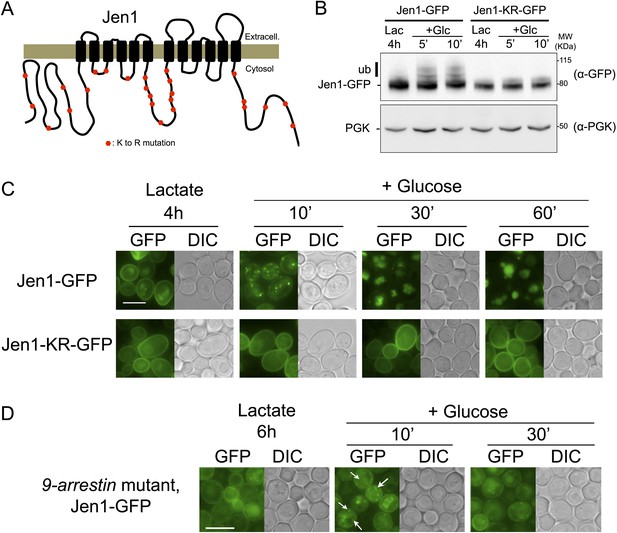
Jen1 ubiquitylation is required for its glucose-induced endocytosis.
(A) Schematic of the lysine-to-arginine mutations introduced in the cytosolic loops of Jen1 to generate the Jen1-KR construct. (B) Jen1-KR-GFP is not ubiquitylated in response to glucose. WT cells carrying a plasmid-encoded Jen1-GFP (pSL161) or the same plasmid bearing the KR mutations (pSL163) were grown on lactate medium and glucose was added for the indicated times. Crude extracts were immunoblotted with the indicated antibodies. Yeast PGK (phosphoglycerate kinase) is used as a loading control. Appearance of the glucose-induced higher molecular weight species of Jen1, which were previously shown to correspond to Jen1 ubiquitylated adducts (Paiva et al., 2009; Becuwe et al., 2012b), do not appear upon mutations of Jen1 lysines. (C) Jen1-KR-GFP is not internalized in response to glucose. WT cells carrying a plasmid-encoded Jen1-GFP (pSL161) or the same plasmid bearing the KR mutations (pSL163) were grown on lactate medium, then glucose was added and cells were imaged at the indicated times. Upon glucose addition, Jen1-GFP is internalized into vesicles and then is targeted to the vacuole, but Jen1-KR-GFP remains stable at the plasma membrane. Note the partial endoplasmic reticulum (ER) labeling of Jen1-KR-GFP, showing that this construct displays a mild defect in ER exit to the secretory pathway. Scale bar = 5 µm. (D) Deletion of nine genes encoding arrestin-related proteins is not sufficient to abolish Jen1 internalization. The 9-arrestin strain (strain EN60, a kind gift from Hugh Pelham: art1Δ ecm21Δ aly2Δ rod1Δ art5Δ aly1Δ rog3Δ csr2Δ art10Δ) (Nikko and Pelham, 2009) expressing Jen1-GFP tagged at its endogenous genomic locus (ySL1318) was grown in lactate medium. Glucose was then added to the medium and Jen1-GFP localization was monitored at the indicated times. Vesicles resulting from Jen1-GFP internalization can still be observed in this mutant after glucose addition (white arrows). Scale bar = 5 µm.
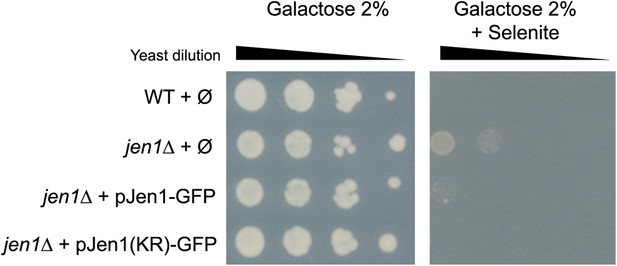
Jen1-KR-GFP is a functional protein.
Jen1 transports selenite (McDermott et al., 2010), which is used here as a readout for Jen1 activity. WT and jen1Δ strains carrying either an empty vector (Ø), a plasmid-encoded Jen1-GFP (pSL161) or the same plasmid bearing the KR mutations (pSL163) were grown on galactose medium (control plate) or galactose + 300 µM selenite. Note that growth on galactose is required to derepress the expression of the JEN1 gene and to reveal its selenite transport activity at the plasma membrane.
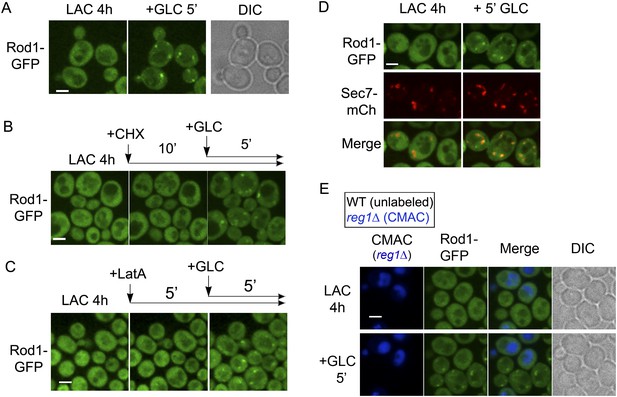
Rod1 is dynamically recruited to the trans-Golgi network when endocytosis is triggered.
(A) Rod1-GFP re-localizes from the cytosol to punctate structures in response to glucose. Lactate-grown cells (ySL542) expressing Rod1-GFP were injected into a microfluidics device in lactate medium, and were then imaged over time after glucose addition. Scale bar = 2.5 µm. See also Video 4. (B) Inhibition of translation using cycloheximide (CHX) does not alter Rod1-GFP re-localization. Cells (ySL542) expressing Rod1-GFP were grown on lactate medium and injected into a microfluidics device in lactate medium. Cells were then treated with 100 µg/ml CHX for 10 min in lactate medium and imaged over time after addition of glucose in the presence of CHX. Scale bar = 2.5 µm. (C) Disruption of the actin cytoskeleton using latrunculin A (LatA) does not alter Rod1-GFP re-localization. Cells (ySL542) expressing Rod1-GFP were grown on lactate medium and injected into a microfluidics device in lactate medium. Cells were then treated with 0.2 mM LatA for 5 min in lactate medium, then imaged over time after addition of glucose in the presence of LatA. Scale bar = 2.5 µm. (D) Rod1 co-localizes with the trans-Golgi network marker, Sec7-mCherry, in response to glucose. Cells (ySL638) expressing both Rod1-GFP and Sec7-mCh were grown on lactate medium and injected into a microfluidics device in lactate medium. Cells were then imaged over time after glucose addition. Scale bar = 2.5 µm. See also Video 5. (E) Rod1 does not localize to the TGN in response to glucose in the mutant for the regulatory subunit of protein phosphatase 1, reg1Δ. WT (ySL542) and reg1Δ (ySL600) cells expressing Rod1-GFP were grown on lactate medium. The reg1Δ cells were then labeled with CMAC and were co-injected with WT cells into the microfluidics device in lactate medium, before glucose was added. Images taken before and after 5 min of glucose treatment are shown. Scale bar = 2.5 µm.
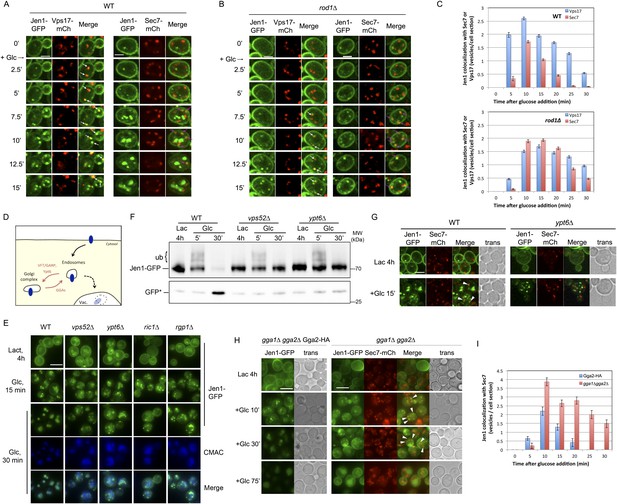
Jen1 transits through the TGN during its endocytosis and requires Rod1 for exit from the TGN to the vacuole.
(A) Jen1 co-localizes with the TGN marker, Sec7-mCh, during its trafficking to the vacuole in wild-type cells. WT cells expressing Jen1-GFP and either Vps17-mCh (ySL1168), a marker of the early endosomal compartment, or Sec7-mCh (ySL1165) were grown on lactate medium. Of note, little or no co-localization was observed between Sec7-mCh and Vps17-GFP (Figure 4—figure supplement 1). The Sec7-mCh-expressing cells were labeled with CMAC and were co-injected with the Vps17-mCh-expressing cells into the microfluidics device in lactate medium, before glucose was added. Cells have been cropped to show only one Vps17-mCh expressing cell (left) or one Sec7-mCh expressing cell (right). The uncropped picture is displayed in Figure 4—figure supplement 2, and the full video in Video 6. Co-localization events between Jen1-GFP and either Vps17-mCh or Sec7-mCh are indicated with white arrows or pink arrows, respectively. Scale bar = 2.5 µm. Of note, the co-localization of another transporter, Dip5, with Sec7-mCh after its endocytosis was also observed (see Figure 4—figure supplement 3). (B) Jen1 also co-localizes with the TGN marker, Sec7-mCh, after internalization in the rod1Δ mutant. rod1Δ cells expressing Jen1-GFP and either Vps17-mCh (ySL1177), an endosomal marker, or Sec7-mCh (ySL1176) were grown on lactate medium. As in panel A, the Sec7-mCh-expressing cells were labeled with CMAC and were co-injected with Vps17-mCh-expressing cells into the microfluidics device in lactate medium, before glucose was added. Cells have been cropped to show only one Vps17-mCh expressing cell (left) or one Sec7-mCh expressing cell (right). The uncropped picture is displayed in Figure 4—figure supplement 4, and the full video in Video 7. Co-localization events between Jen1-GFP and either Vps17-mCh or Sec7-mCh are indicated with white arrows or pink arrows, respectively. Scale bar = 2.5 µm. (C) Quantification of the co-localization of Jen1-GFP puncta with either Vps17-mCh or Sec7-mCh puncta in WT (top) or rod1Δ (bottom) cells (20 cells, n = 3). Jen1 co-localizes successively with Vps17 and Sec7. Furthermore, Jen1 co-localizes more robustly with Sec7-mCh in the rod1Δ mutant. See also Figure 4—figure supplement 5. (D) Schematic showing the place of action of the VFT/GARP complex, Ypt6 and the GGA proteins in endosome-to-Golgi trafficking. (E) Deletions of VPS52 (VFT/GARP complex), YPT6 or genes encoding the Ypt6 GEF complex (RGP1 and RIC1) abolish Jen1 trafficking to the vacuole. Lactate-grown WT (ySL1150), vps52Δ (ySL1369), ypt6Δ (ySL1175), ric1Δ (ySL1630) and rgp1Δ (ySL1631) cells expressing Jen1-GFP were imaged before or after the addition of glucose at the indicated time. At t = 30 min Glc treatment, CMAC staining was used to visualize the localization of the vacuole. Scale bar = 5 µm. (F) Deletions of VPS52 or YPT6 prevent the vacuolar degradation of Jen1-GFP. Crude extracts from lactate-grown WT (ySL1150), vps52Δ (ySL1369) and ypt6Δ (ySL1175) cells expressing Jen1-GFP were prepared at the indicated times before and after glucose addition, and were immunoblotted with anti-GFP antibodies to reveal the full-length Jen1-GFP and its degradation product (free GFP). (G) Deletion of YPT6 abrogates Jen1 co-localization with Sec7-mCh. Lactate-grown WT cells (ySL1165) or ypt6Δ cells (ySL1526) expressing Jen1-GFP and Sec7-mCh were injected in a microfluidics device and imaged before (Lactate) and 15 min after glucose addition. Co-localization between Jen1-GFP and Sec7-mCh is indicated with arrowheads. (H) Deletions of GGA1 and GGA2, encoding redundant Golgi-localized clathrin adaptor proteins, alter Jen1 trafficking to the vacuole. Strains gga1Δgga2Δ (ySL1307) or gga1Δ gga2Δ expressing Gga2-HA (ySL1308), used as a positive control, both expressing Jen1-GFP were grown on lactate medium, and imaged before and after the addition of glucose at the indicated times. The gga1Δ gga2Δ cells also express Sec7-mCherry, which allows evaluating of Jen1-GFP co-localization with the TGN (arrowheads). Scale bar = 5 µm. (I) Quantification of the co-localization of Jen1-GFP puncta with Sec7-mCh puncta in gga1Δgga2Δ (ySL1307) cells or gga1Δ gga2Δ cells expressing Gga2-HA (ySL1619) (20 cells, n = 3) over time after glucose addition. Deletion of the GGA genes leads to a transient accumulation of Jen1 at the TGN.
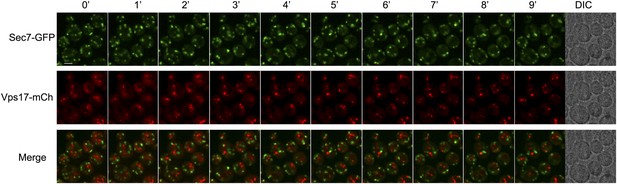
Sec7 and Vps17 localize to distinct compartments.
WT cells expressing Sec7-GFP and Vps17-mCh (ySL1531) were injected in the microfluidic device and observed every minute during 9 min. Sec7-GFP-positive vesicles never co-localize with Vps17-mCh-positive vesicles, showing that they represent two different compartments within the cell.
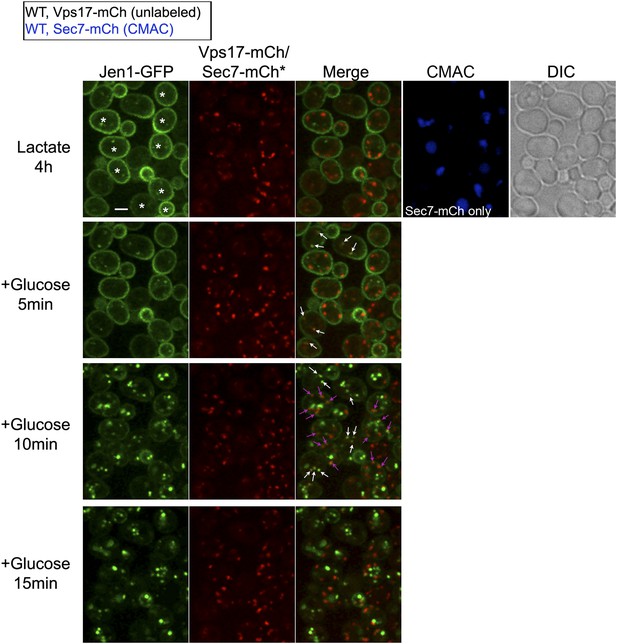
Jen1 traffics through the TGN in the course of its endocytosis in wild-type cells.
Uncropped pictures corresponding to the panel presented in Figure 3A. See corresponding legend for details. Cells expressing Sec7-mCh were marked with an asterisk. Co-localization events between Jen1-GFP and either Vps17-mCh or Sec7-mCh are indicated with white arrows or pink arrows, respectively. Scale bar = 2.5 µm.
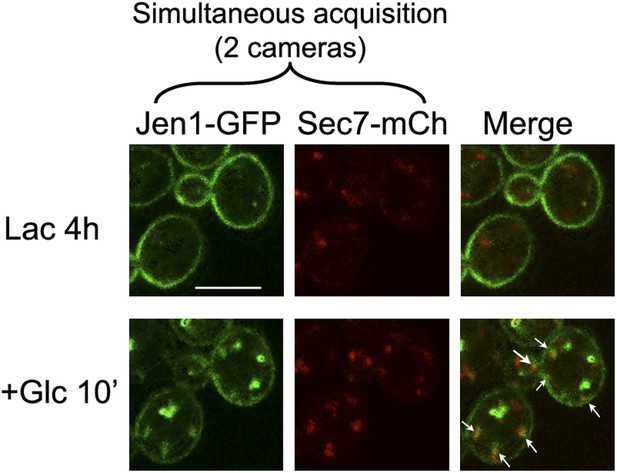
Jen1-GFP and Sec7-mCh co-localize to the same compartment when observed simultaneously.
WT cells expressing Jen1-GFP and Sec7-mCh (ySL1165) were grown on lactate medium, and were injected in the microfluidics device in lactate medium, before glucose was added. Cells were imaged at the indicated time with a Revolution xD TuCam system (Andor) allowing the simultaneous acquisitions of the red and green channels. Arrows illustrate examples of co-localizations.
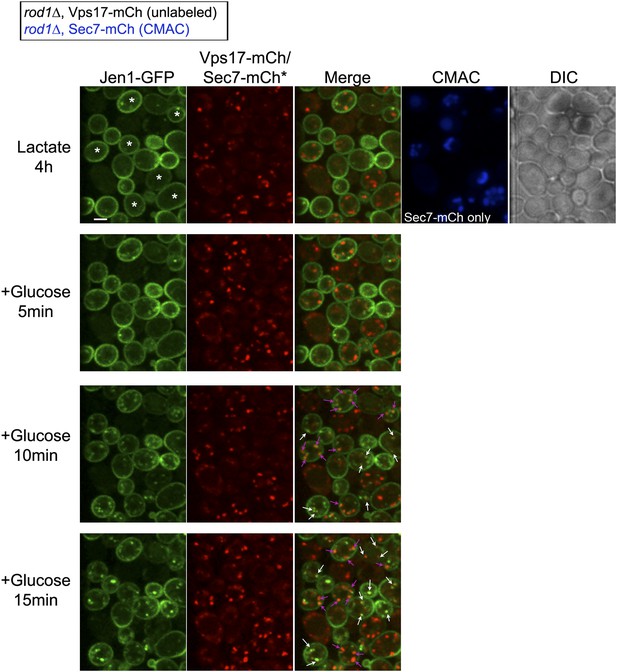
Jen1 also co-localizes to the TGN in rod1Δ mutant cells.
Uncropped pictures corresponding to the panel presented in Figure 3B. See corresponding legend for details. Cells expressing Sec7-mCh were marked with an asterisk. Co-localization events between Jen1-GFP and either Vps17-mCh or Sec7-mCh are indicated with white arrows or pink arrows, respectively. Scale bar = 2.5 µm.
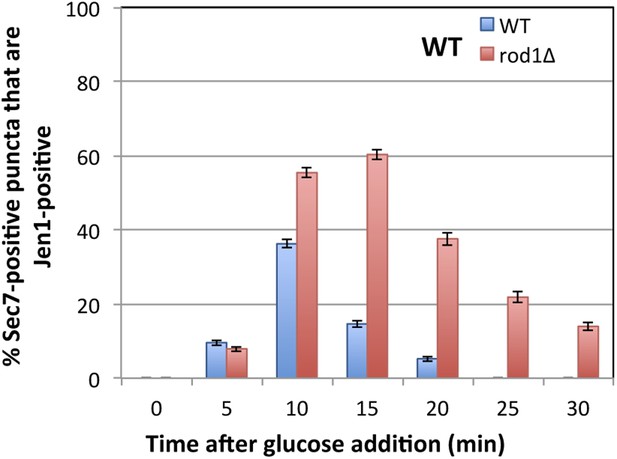
Quantification of Sec7-mCh puncta that are also Jen1-GFP positive in WT and rod1Δ cells.
This quantification was based on the data presented in Figure 4A,B. Jen1 co-localizes more robustly with Sec7-mCh in the rod1Δ mutant.
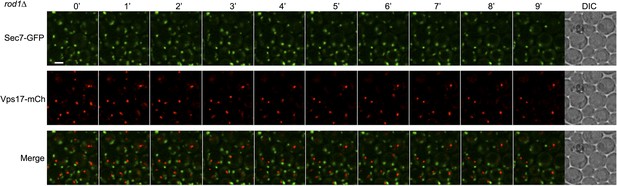
Sec7 and Vps17 localize to distinct compartments in rod1Δ cells.
rod1Δ cells expressing Sec7-GFP and Vps17-mCh (ySL1602) were injected in the microfluidic device and observed every minute during 9 min. Sec7-GFP-positive vesicles never co-localize with Vps17-mCh-positive vesicles, showing that they represent two different compartments within the cell.
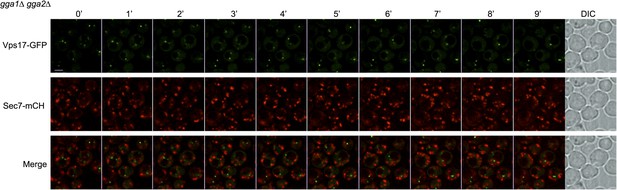
Sec7 and Vps17 localize to distinct compartments in gga1Δgga2Δ cells.
gga1Δgga2Δ cells expressing Vps17-GFP and Sec7-mCh (ySL1615) were injected in the microfluidic device and observed every minute during 9 min. Vps17-GFP-positive vesicles never co-localize with Sec7-mCh-positive vesicles, showing that they represent two different compartments within the cell.
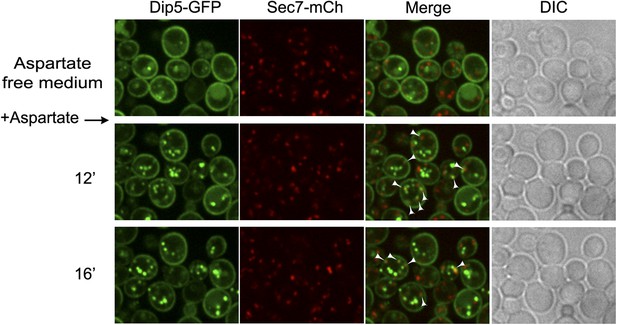
Dip5-GFP traffics through the TGN in the course of its endocytosis in WT cells.
WT cells expressing Dip5-GFP and Sec7-mCh (ySL956) were grown on aspartate-free medium, and injected into the microfluidics device in the same medium. Aspartic acid was then added to the medium (200 µg/ml) and cells were imaged at the indicated times. Arrowheads also indicate examples of co-localizations between the Sec7-mCh and Dip5-GFP. Scale bar = 2.5 µm.
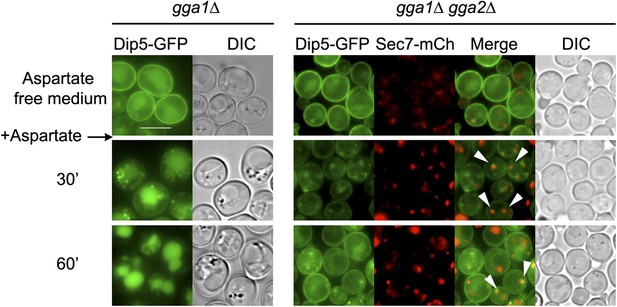
Deletions of GGA1 and GGA2, encoding redundant Golgi-localized clathrin adaptor proteins, alter Dip5 trafficking to the vacuole after endocytosis.
Strains gga1Δgga2Δ (ySL1323) and gga1Δgga2Δ expressing Gga2-HA (ySL1322), used as a positive control, both expressing Dip5-GFP genomically tagged at its endogenous locus were grown on aspartate-free medium, and imaged before and after the addition of aspartic acid (200 µg/ml) at the indicated times. The gga1Δ gga2Δ cells also express Sec7-mCherry, allowing to evaluate the localization of Dip5-GFP to the TGN during its endocytosis. Contrary to what is observed in the control, Dip5-GFP fails to reach the vacuolar lumen in gga1Δ gga2Δ cells. Arrowheads also indicate examples of co-localizations between the Sec7-mCh and Dip5-GFP Scale bar = 5 µm.
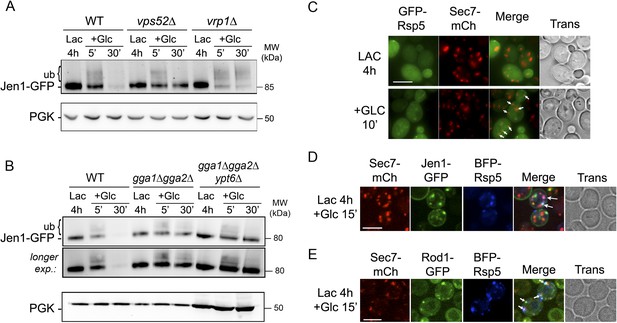
The prolonged presence of glucose is required for the full endocytosis of Jen1.
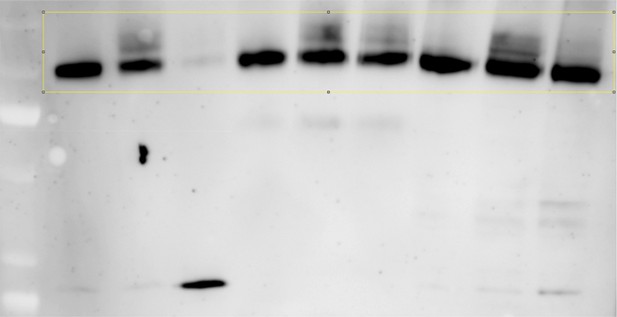
(A) Jen1 is deubiquitylated after endocytosis. WT (ySL1150), vps52Δ (ySL1369) and vrp1Δ (ySL1610) cells expressing Jen1-GFP were grown in lactate medium and treated with glucose. Crude extracts were prepared at the indicated times and were immunoblotted with the indicated antibodies. In contrast to the situation in the vps52Δ mutant, in which Jen1 ubiquitylation vanishes over time, Jen1 ubiquitylation remains stable in the endocytic mutant vrp1Δ. (B) Jen1 ubiquitylation at the TGN requires retrograde sorting from endosomes to the TGN. WT (ySL1636), gga1Δgga2Δ (ySL1638) and ypt6Δ gga1Δ gga2Δ (ySL1639) cells expressing Jen1-GFP were grown in lactate medium and treated with glucose for the indicated time. Crude extracts were prepared and immunoblotted with antibodies against GFP. An increased ubiquitylation of Jen1-GFP is observed in the gga1Δgga2Δ mutant, that is abolished upon the additional deletion of YPT6. Note that Jen1-GFP is expressed at a lower level in the triple ypt6Δ gga1Δ gga2Δ mutant, therefore samples were loaded so that a comparable signal is observed in each strain. (C) A fraction of the ubiquitin ligase Rsp5 re-localizes to the TGN upon glucose addition. Cells (ySL1011) expressing both Sec7-mCh and GFP-Rsp5 were imaged after growth on lactate medium, and 10 min after glucose addition. Scale bar = 5 µm. (D) Rsp5 co-localizes with Jen1 at the TGN. Cells (ySL1622) expressing Sec7-mCh, Jen1-GFP and BFP-Rsp5 were grown in lactate medium, and imaged 15 min after glucose addition. Scale bar = 5 µm. (E) Rsp5 co-localizes with Rod1 at the TGN. Cells (ySL1625) expressing Sec7-mCh, Rod1-GFP and BFP-Rsp5 were grown in lactate medium, and imaged 15 min after glucose addition. Scale bar = 5 µm.
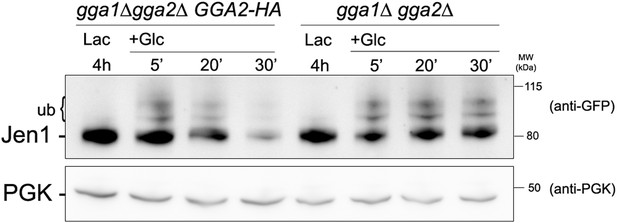
Jen1 accumulates in an ubiquitylated form in the gga1Δ gga2Δ mutant.
Strains gga1Δgga2Δ (ySL1307) or gga1Δ gga2Δ expressing Gga2-HA (ySL1308), used as a positive control, both expressing Jen1-GFP were grown on lactate medium, and crude extracts were prepared before and after the addition of glucose at the indicated times. Crude extracts were immunoblotted with the indicated antibodies.
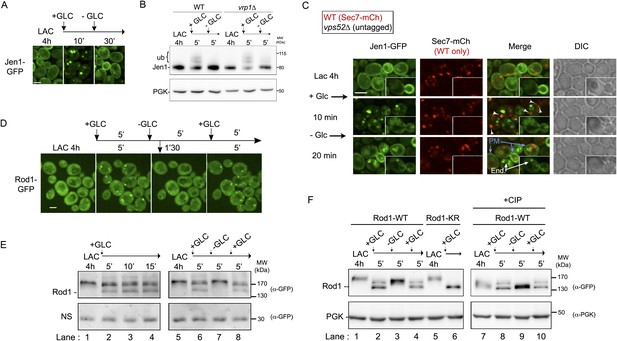
The control of Jen1 trafficking at the TGN allows the recycling of internalized transporters back to the cell membrane.
(A) Jen1 endocytosis is reversible upon glucose removal. Lactate-grown WT cells expressing Jen1-GFP (ySL1150) were injected into a microfluidics device in lactate medium. Cells were then imaged over time, before and after 10 min glucose addition, and then 20 min after glucose removal. Scale bar = 2.5 µm. (B) The persistence of the glucose signal is required to maintain Jen1 ubiquitylation. Lactate-grown WT cells expressing Jen1-GFP (ySL1150) were treated with glucose for 5 min. Cells were then briefly centrifuged and incubated back into lactate medium for 5 min. Crude extracts were prepared at each step and were immunoblotted with the indicated antibodies. The high-molecular weight species of Jen1 appearing upon glucose addition correspond to ubiquitylated Jen1 (see Becuwe et al., 2012b) (see also Figure 2B). The same extracts prepared from vrp1Δ cells show that Jen1 deubiquitylation observed in WT cells is not due to Jen1 degradation but to an active deubiquitylation process. (C) Lactate-grown WT (ySL1165) cells expressing both Jen1-GFP and Sec7-mCh, and vps52Δ (ySL1369) cells expressing only Jen1-GFP were co-injected into the microfluidics device in lactate medium, before glucose was added for 10 min and then removed. Cells were imaged at the indicated times. Arrowheads indicate examples of co-localization of Jen1-GFP and Sec7-mCh in WT cells, and arrows show the plasma membrane localization of Jen1-GFP after glucose removal in WT cells. Note the polarized distribution of Jen1-GFP after recycling in WT cells, but not in vps52Δ cells (inset). PM, plasma membrane; end: endosomes. Scale bar = 2.5 µm. See also Video 8. (D) Jen1 recycling correlates with the loss of Rod1 localization to the TGN. Lactate-grown cells (ySL542) expressing Rod1-GFP were injected into a microfluidics device in lactate medium. Cells were then subjected to 5-min pulses of glucose addition/removal and imaged simultaneously. Only the first three cycles are shown here. Scale bar = 2.5 µm. See also Video 9. (E–F) Dynamics of Rod1 post-translational modifications upon glucose/lactate cycles. Lactate-grown WT cells expressing a plasmid-borne Rod1-GFP were treated with glucose for 15′, or subjected to glucose addition/removal as indicated. Crude extracts were prepared at each step and were immunoblotted with the indicated antibodies. A non-specific (NS) cross-reacting band is used as a loading control. The high-molecular weight species of Rod1-GFP observed in the lactate samples correspond to phosphorylation (panel E, lanes 1, 5 and 7), because phosphatase treatment (CIP) abolishes the migration shift (panel F, lanes 7 and 9). The glucose-induced doublet (panel E, lanes 2, 3, 4, 6 and 8) corresponds to ubiquitylated (higher band) and non-ubiquitylated (lower band) forms of Rod1, because mutation of its ubiquitylation sites (using a plasmid encoding Rod1-KR) abolishes the migration shift (panel F, lane 6), as described previously (Becuwe et al., 2012b). PGK is used as a loading control in panel F.
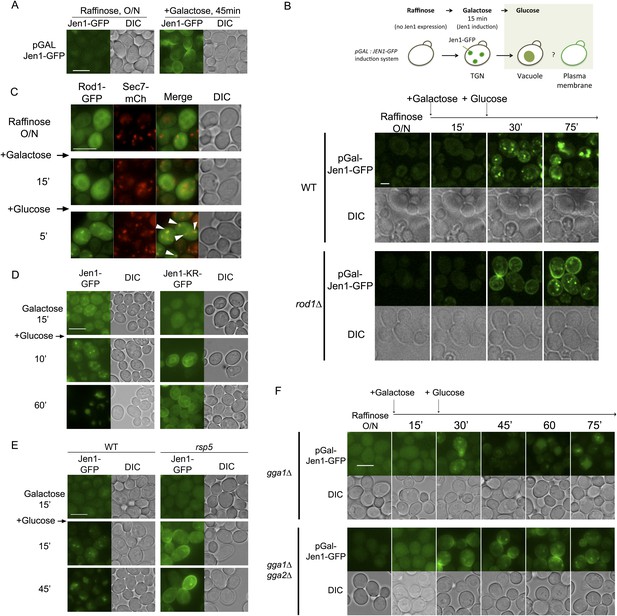
Rod1 promotes Jen1 exit from the secretory pathway to the vacuole.
(A) A galactose-inducible Jen1-GFP is targeted to the plasma membrane in galactose medium. WT cells (ySL1083) expressing a galactose-inducible Jen1-GFP were grown in raffinose medium overnight, and transferred to galactose medium for 45 min to allow Jen1-GFP expression and targeting to the plasma membrane. Scale bar = 5 µm. (B) Rod1 is required for the glucose-induced retargeting of Jen1 from the secretory pathway to the vacuole. Top, outline of the experiment. A 15-min pulse of galactose allows the synthesis of Jen1-GFP, and the effect of glucose on the sorting of neosynthesized Jen1-GFP is then monitored. Bottom, WT (ySL1083) and rod1Δ (ySL781) cells both expressing a galactose-inducible Jen1-GFP were grown overnight on raffinose medium. After 15 min of galactose induction, glucose was then added to the cells and Jen1-GFP fluorescence was followed over time. Scale bar = 2.5 µm. See also Video 10. Scale bar = 2.5 µm. Note that the sorting of neosynthesized Jen1 to the vacuole in response to glucose does not require targeting to the plasma membrane and endocytosis, see Figure 7—figure supplement 1. (C) Rod1 localizes to the TGN when transferred from galactose to glucose medium. Cells (ySL638) co-expressing Rod1-GFP and Sec7-mCh were grown overnight on raffinose medium, and observed 15 min after galactose addition and then and 5 min after glucose addition. Arrowheads indicate co-localization events between Rod1-GFP and Sec7-mCH. Scale bar = 5 µm. (D) The non-ubiquitylatable Jen1-KR-GFP construct fails to be targeted from the secretory pathway to the vacuole in response to glucose. WT cells expressing either a plasmid-encoded, galactose inducible Jen1-GFP (ySL1083) or the mutant Jen1-KR-GFP (ySL1339) construct were grown as in Figure 7B, and imaged at the indicated times. Note that the Jen1-KR-GFP presents defects in ER exit upon synthesis (ER labeling observed throughout the experiment) but still manages to reach the plasma membrane, and is not associated with the vacuole. Scale bar = 5 µm. (E) The sorting of neosynthesized Jen1 to the vacuole in response to glucose requires Rsp5. WT (ySL1083) and npi1 (a hypomorphic rsp5 mutant; ySL1556) cells both expressing a galactose-inducible Jen1-GFP were grown overnight on raffinose medium. After 15 min of galactose induction, glucose was then added to the cells and Jen1-GFP fluorescence was followed over time. Scale bar = 5 µm. (F) The Golgi-localized clathrin adaptor proteins, Gga1 and Gga2, are required for the glucose-induced retargeting of Jen1 from the secretory pathway to the vacuole. Strains gga1Δgga2Δ (ySL1311) or gga1Δgga2Δ expressing Gga2-HA (ySL1310), used as a positive control, both expressing Jen1-GFP from a plasmid-encoded, galactose-inducible construct were induced as in Figure 7B, and imaged at the indicated times. Scale bar = 5 µm.
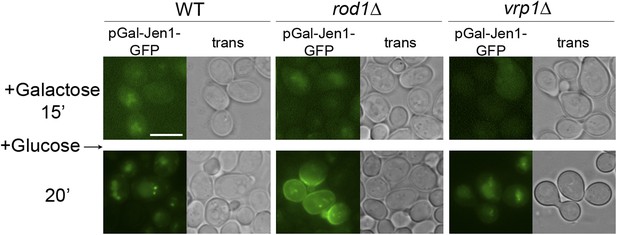
The sorting of neosynthesized Jen1 to the vacuole in response to glucose does not require targeting to the plasma membrane and endocytosis.
WT (ySL1083), rod1Δ (ySL781) and vrp1Δ (ySL1650) cells both expressing a galactose-inducible Jen1-GFP were grown overnight on raffinose medium. After 15 min of galactose induction, glucose was then added to the cells and Jen1-GFP fluorescence was followed over time.
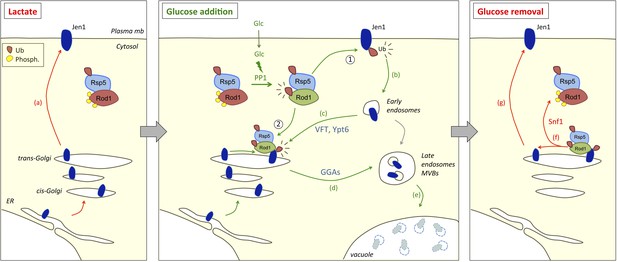
Working model for the dual function of Rod1 in the regulation of transporter endocytosis and recycling.
Left, in lactate medium, Jen1 is synthesized and targeted to the plasma membrane. Although Rod1 interacts with Rsp5 (Becuwe et al., 2012b), it is inactive (phosphorylated) and cytosolic. Middle, In the presence of glucose, Rod1 is activated by a protein phosphatase 1 (PP1)-dependent dephosphorylation and its subsequent Rsp5-mediated ubiquitylation (Becuwe et al., 2012b). Rod1 promotes transporter internalization at the plasma membrane (1), but also localizes to the TGN (2). There, Rod1 controls the fate of internalized transporters that have been retrograde-trafficked by a VFT- and Ypt6-dependent pathway, and also that of transporters coming from the secretory pathway. Transporter sorting from the TGN to the vacuole requires Rod1 and the clathrin adaptors Gga1 or Gga2 (GGAs). Right, Upon glucose removal, shortly after endocytosis is initiated, Rod1 is rephosphorylated (likely by the kinase Snf1) (Becuwe et al., 2012b), leading to Rod1 dissociation from the TGN and Jen1 recycling to the cell membrane.
Videos
Rod1 is required for the glucose-induced internalization of the glycerol/proton symporter Stl1.
WT and rod1Δ (CMAC-positive) cells expressing Stl1-GFP were grown in lactate/glycerol medium and simultaneously observed for 20 min after glucose addition. See also Figure 1C.
Jen1-GFP is internalized upon glucose treatment even in the absence of Rod1.
WT cells (left) and in rod1Δ cells (right) expressing Jen1-GFP were grown in lactate medium and observed for 45 min after glucose addition. See also Figure 1D.
rod1Δ cells display a kinetic delay in Jen1 internalization.
WT and rod1Δ (CMAC positive) cells expressing Jen1-GFP were visualized simultaneously during 20 min after glucose addition (left). Images of the same video were treated in ImageJ using the ‘Fire’ lookup table (LUT) to visualize pixel intensity (right). See also Figure 1F.
Rod1-GFP relocalizes from the cytosol to punctate structures in response to glucose.
WT cells expressing Rod1-GFP were grown in lactate medium and visualized for 60 min after glucose addition. See also Figure 3A.
Rod1 co-localizes with the trans-Golgi network marker, Sec7-mCherry, in response to glucose.
WT cells expressing both Rod1-GFP and Sec7-mCh were grown during 4 hr in lactate medium and visualized during 45 min after glucose addition. Merge of Rod1-GFP fluorescence (left panel) and Sec7-mCh fluorescence (middle panel) is observed on the right panel. See also Figure 3D.
Uncropped video corresponding to Figure 4A.
Jen1-GFP co-localizes with the endosomal marker Vps17-mCh and the TGN marker Sec7-mCh during its trafficking to the vacuole in wild-type cells. WT cells expressing either both Jen1-GFP and Sec7-mCh (indicated with a white asterisk on the first image), or both Jen1-GFP and Vps17-mCh, were grown in lactate medium and visualized simultaneously for 45 min after glucose addition. A merge of Jen1-GFP fluorescence (left panel) and Vps17-mCh/Sec7-mCh fluorescence (middle panel) is shown on the right panel.
Uncropped video corresponding to Figure 4B.
Jen1-GFP co-localizes with the endosomal marker Vps17-mCh and the TGN marker Sec7-mCh during its trafficking to the vacuole in rod1Δ cells. rod1Δ cells expressing either both Jen1-GFP and Sec7-mCh (indicated with a white asterisk on the first image), or both Jen1-GFP and Vps17-mCh, were grown in lactate medium and visualized simultaneously for 45 min after glucose addition. A merge of Jen1-GFP fluorescence (left panel) and Vps17-mCh/Sec7-mCh fluorescence (middle panel) is shown on the right panel.
Jen1 recycles back to the plasma membrane via the TGN.
WT cells expressing both Jen1-GFP and Sec7-mCh, and vps52Δ cells expressing only Jen1-GFP were grown 4 hr in lactate medium and observed simultaneously for 10 min of glucose addition and 20 min after glucose removal. Co-localization between Jen1-GFP and Sec7-mCh in WT cells is indicated by white arrows in the merge panel (second on the right). See also Figure 6C.
Jen1 recycling correlates with the loss of Rod1-localization to the TGN.
WT cells expressing Rod1-GFP were grown for 4 hr in lactate medium and observed during 3 cycles of glucose addition/removal (5-min pulses). See also Figure 6D.
Rod1 is required for the glucose-induced retargeting of Jen1 from the secretory pathway to the vacuole.
WT cells (left panel) and rod1Δ cells (right panel) expressing Jen1-GFP under a galactose-inducible promoter were grown in raffinose medium overnight and simultaneously observed for 15 min during galactose induction and then 45 min after glucose addition. See also Figure 7B.
Additional files
-
Supplementary file 1
A table listing yeast strains used in this study is provided in Supplementary file 1.
- https://doi.org/10.7554/eLife.03307.033