Knockdown of hypothalamic RFRP3 prevents chronic stress-induced infertility and embryo resorption
Figures
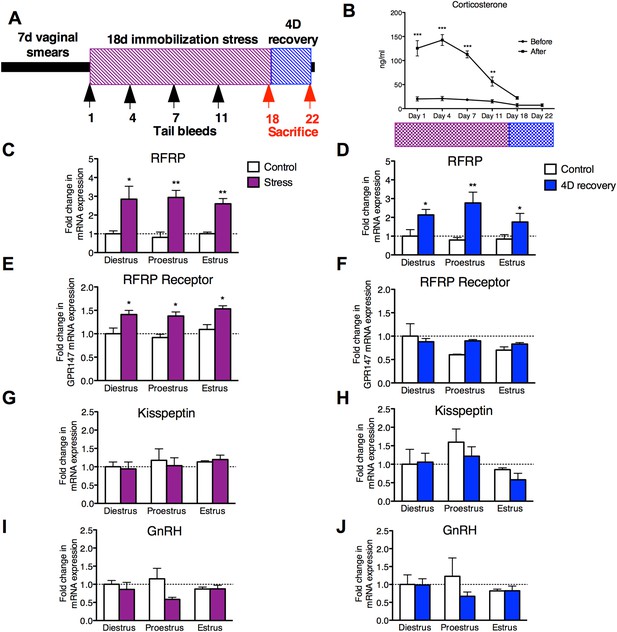
18 days chronic stress leads to an upregulation of RFRP mRNA that persists for at least one estrous cycle in the rat.
(A) Experimental timeline. (B). Corticosterone was measured in serum samples from tail vein blood immediately before and after stress sessions on days 1, 4, 7, 11, and 18, and on day 22, 4 days post-stress cessation (N = 36/group in 1,4,7,11,18 timepoints, N = 18/group on 22). (C, E, G, I) Gene expression changes in the hypothalamus immediately after stress and (D, F, H, J) 4 days after stress. mRNA levels of all (mean ± SEM, N = 6/group) were determined using qRT–PCR relative to the ribosomal reference gene RPLP at day 0 and 4 post-stress cessation. Estrous cycle staging was determined by inspection of daily vaginal smears. *p < 0.05, **p < 0.01, ***p < 0.001. PCR statistics were done by a Kruskal–Wallis one-way ANOVA followed by Dunn's multiple comparison test for post-hoc analysis, CORT statistics analyzed by a repeated two-way ANOVA.
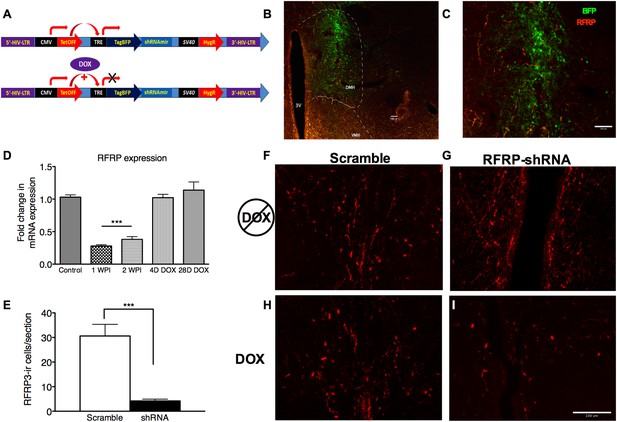
RFRP-shRNA successfully knocks down RFRP expression in the dorsal medial hypothalamus, and expression is recovered upon DOX induction.
(A) Map of RFRP-shRNA viral plasmid. (B) Brain sectioned and stained with an anti-BFP antibody to label virus infection (green) and anti-RFRP 2 weeks post-injection (WPI) to show injection location and (C) spread. Scale bar indicates 100 µm. (D) mRNA levels of RFRP following injection of RFRP-shRNA viral vector were determined using qRT-PCR (WPI = weeks post-injection, mean ± SEM, N = 4). (E) RFRP3-ir cells/section counts in the DMH after 2 weeks post-injection with either scramble or RFRP-shRNA virus. (F–I) Brain sectioned stained with anti-RFRP3 antibody 2 weeks post-injection with scramble or RFRP-shRNA virus and before and after DOX administration. Scale bar indicates 100 µm. *p < 0.05, **p < 0.01, ***p < 0.001. Statistics were done by one-way analysis of variance (ANOVA) and Bonferroni post-hoc tests. For mRNA data, PCR statistics were done by a Kruskal–Wallis one-way ANOVA followed by Dunn's multiple comparison test for post-hoc analysis and statistics for protein counts were a student's t-test.
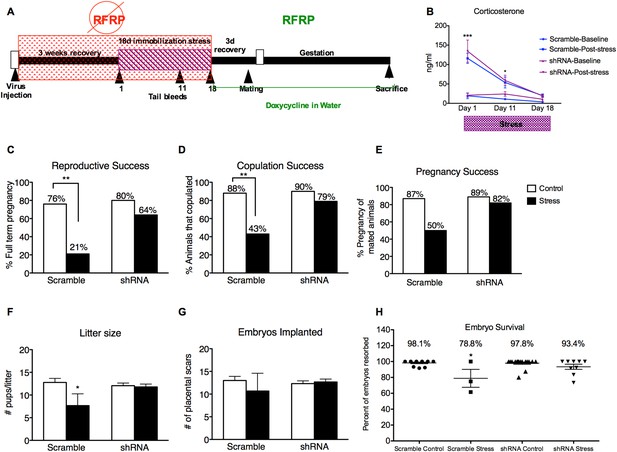
Knocking down RFRP during stress completely prevents stress-induced reproductive dysfunction.
(A) Experimental time line. (B) Corticosterone concentrations were measured in serum samples from tail vein blood immediately before and after stress sessions on days 1, 11, and 18. (C) Total reproductive success was measured as percentage of females that successfully brought a litter to full term (Scramble/control N = 17, Scramble/Stress N = 14, shRNA/control N = 20, shRNA/stress N = 14, g-statistics: G = 5.836, df = 1 p = 0.016, fisher's exact test p = 0.0031). Breaking down total reproductive success, (D) copulation success was measured as percentage of females that exhibited lordosis and allowed a male to achieve intromission within 15 min (g-statistics: G = 2.405, df = 1 p = 0.028, fisher's exact test p = 0.0062), and (E) pregnancy success refers to the percentage of females that got pregnant out of the subgroup that successfully copulated (Scramble/control N = 15, Scramble/Stress N = 6, RFRP-shRNA/control N = 18, RFRP-shRNA/stress N = 11). (F) Litter sizes measured as number of pups born alive immediately after birth (dams-Scramble/control N = 13, Scramble/Stress N = 3, RFRP-shRNA/control N = 16, RFRP-shRNA/stress N = 9). (G) Embryos implanted measured as number of placental scars identified in the dam's uterine horns after birth. (H) Embryo survival was calculated as the number of birthed pups divided by number of maternal placental scars and shown as a percentage (indicative of initial implantation, mean ± SEM) *p < 0.05, **p < 0.01, ***p < 0.001. Reproductive success statistics were done by G-statistics tests followed by Fisher's Exact test, statistics for litter size, placental scars, and embryo resorption were done by a two-way analysis of variance (ANOVA) followed by Bonferroni post-hoc tests and CORT statistics analyzed by a repeated two-way ANOVA.
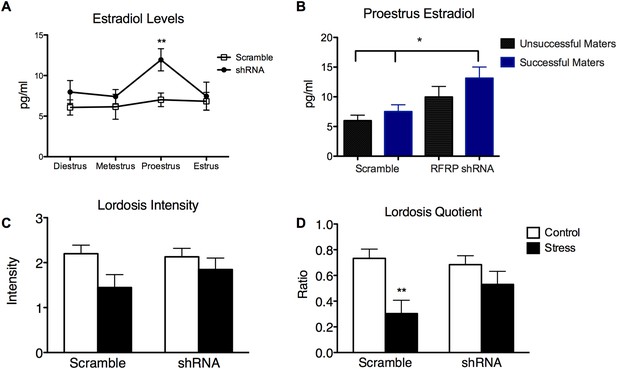
RFRP-shRNA animals had increased plasma estradiol on proestrus during stress and RFRP-shRNA animals that mated had higher circulating estradiol than scrambled animals.
(A) Estradiol levels as measured from tail bleed samples over the cycles of all stressed rats with either scrambled or RFRP-shRNA virus (n = 14). (B) Within proestrus, estradiol measurements were separated by mating success and virus (n = 10, 21, 11, 25 samples for each successive group). (C) Lordosis intensity, or quality of the lordosis pose, scored between 0 and 3 as published in Hardy and Debold (1971). We found a significant main effect of stress (F(1, 61) = 5.15, p = 0.0268), however, no significant differences within groups. (D) Lordosis quotient was calculated as the ratio of male mounts to female lordosis poses of a score of 2 or 3. We found a significant main effect of stress (F(1, 61) = 11.66, p = 0.0011), as well a significant decrease in the scramble stress group. p < 0.05, **p < 0.01, ***p < 0.001. Estradiol, lordosis intensity and quotient statistics were a two-way analysis of variance (ANOVA) followed by Bonferroni post-hoc tests.
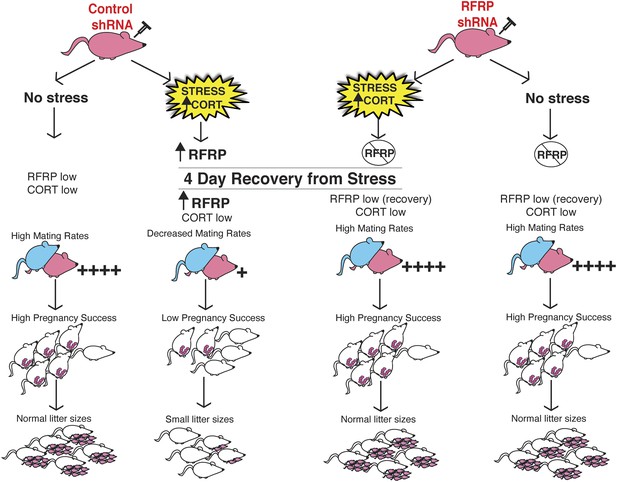
Schematic illustration of experiments.
Female rats (in pink at top) were injected with either an inducible RFRP-shRNA or a scrambled control virus. Each group was furthered separated into a no stress control or subjected to 18 days of immobilization stress. Stressed females exhibited fewer successful copulation events, fewer pregnancies in those that did successfully mate, and increased frequency of embryo resorption. These marked effects of stress on fertility were completely blocked by knockdown of RFRP3.





