Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding
Figures
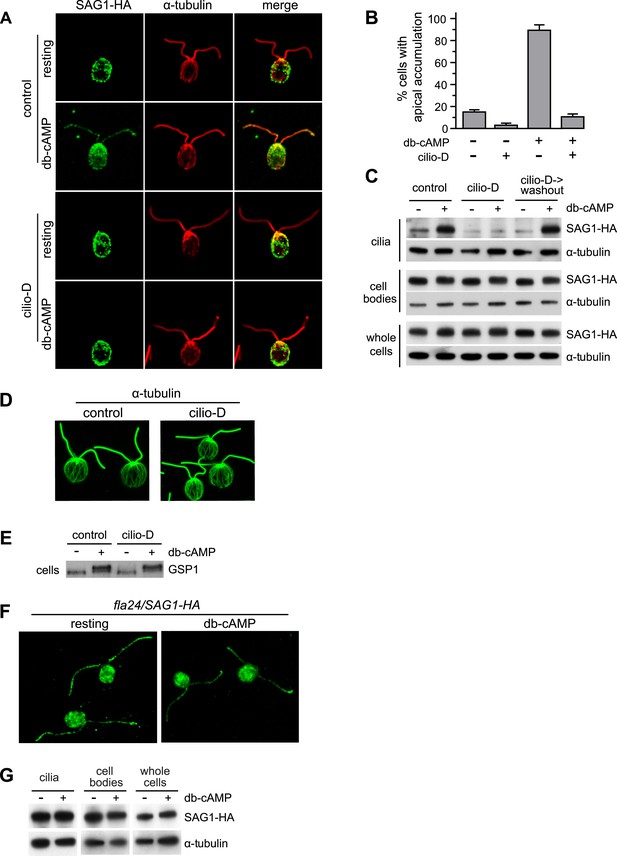
Cytoplasmic dynein 1b is required for the rapid, signaling-induced apical localization and ciliary enrichment of SAG1-C65-HA.
(A) Confocal images of resting gametes in the presence and absence of Cilio-D for 20 min and gametes activated for 5 min in the presence and absence of Cilio-D. (B) Percent of cells showing apical localization of SAG1-HA (see ‘Materials and methods’). Data are from three experiments. 100 cells were scored for each condition. Error bars indicate ± SD. (C) Immunoblots of cilia, cell bodies and whole cells (8 μg protein/lane) of SAG1-HA cells treated as indicated and activated (or not) with db-cAMP for 5 min. (D) Confocal images showing cytoplasmic microtubules in control and Cilio-D treated plus gametes. (E) Immunoblot for GSP1 of plus cells incubated with or without Cilio-D and with or without db-cAMP. (F) Confocal images of resting and db-cAMP activated gametes showing high levels of SAG1-C65-HA in cilia of resting fla24 gametes and lack of redistribution upon activation. (G) Immunoblots of resting and activated whole cells, cell bodies, and cilia (2.5 μg protein/lane) of fla24 gametes.
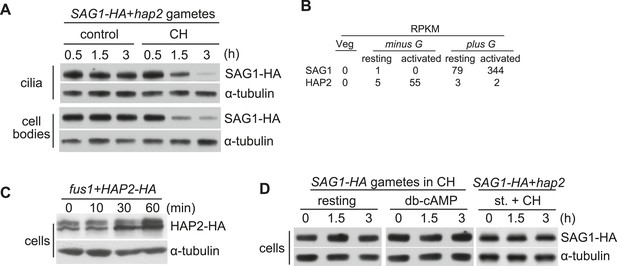
SAG1-C65-HA is lost from cells during adhesion and signaling.
(A) Immunoblot analysis of SAG1-C65-HA in isolated cilia and cell bodies (8 μg protein/lane) from cells activated by mixing with hap2 minus gametes. Cells were pretreated (or not) for 15 min with 10 mg/ml cycloheximide (CH) before mixing. (B) RNA-Seq data from Ning et al. (2013) showing transcript abundance as median reads per kilobase per million mapped reads (RPKM) values of SAG1 and HAP2 transcripts from vegetative cells, resting gametes, and activated gametes of both mating types. (C) Immunoblots using HA and tubulin antibodies of HAP2-HA minus gametes (1.5 × 107 cells/ml) mixed with an equal number of fus1 plus gametes for the indicated times. (D) Immunoblot analysis of SAG1-C65-HA in isolated SAG1-HA resting cells, SAG1-HA cells activated with db-cAMP, and SAG1-HA cells activated by undergoing ciliary adhesion with hap2 minus gametes in the presence of the protein kinase inhibitor staurosporine (1 μM; st.).
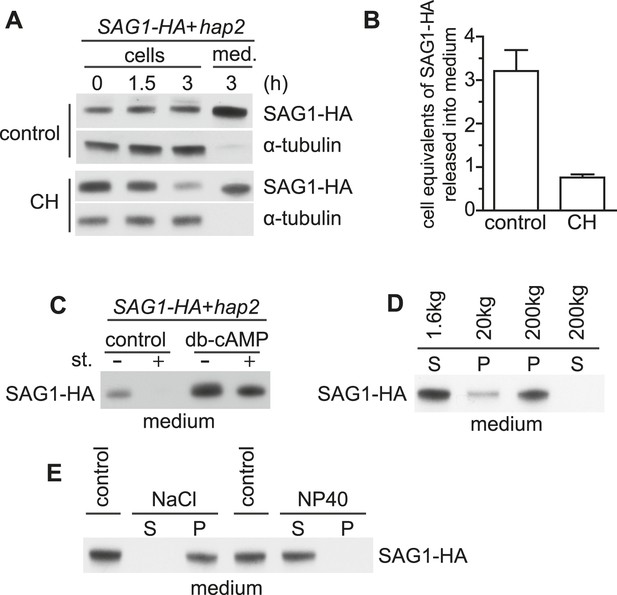
Adhering gametes release SAG1-C65-HA in a membrane-associated form.
(A) Immunoblot analysis of SAG1-C65-HA in cells and medium harvested after undergoing adhesion and signaling with fusion-defective hap2 minus gametes for the indicated times. 1 × 106 cell equivalents were loaded in each lane. (B) Number of cell equivalents of SAG1-HA released into the medium 3 hr after mixing SAG1-C65-HA gametes with hap2 gametes in the presence and absence of CH as assessed by quantitative immunoblotting. Results are averages from the experiment shown in (A) and at least one other independent experiment. Error bars show S.D. (C) Immunoblot analysis of SAG1-C65-HA in the medium collected from SAG1-HA gametes undergoing adhesion with hap2 minus gametes. Before mixing, cells were activated (or not) with db-cAMP and then mixed together in the presence or absence of 1 μM staurosporine (st.) for 3 hr. (D) Immunoblot analysis of SAG1-HA distribution after differential centrifugation of medium from SAG1-C65-HA and hap2 gametes that had been adhering for 3 hr. S, supernatant; P, pellet. (E) Distribution of SAG1-C65-HA after 200,000×g centrifugation of ectosome samples that had been incubated on ice for 20 min in 0.5 M NaCl or 2% NP-40 in HMDEK buffer. S, supernatant; P, pellet.
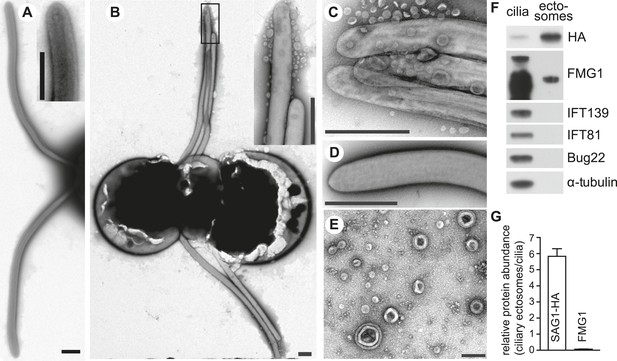
Adhering gametes release SAG1-C65-HA as ciliary ectosomes.
(A) Negative stain transmission electron micrographs of resting wild type plus gametes showing the smooth membranes of the cilia. The inset shows a higher magnification view of the tip of a cilium. (The scale bars shown in this and subsequent TEM images are 1000 nm, with the exception that the bar in Figure 4E is 200 nm.) (B) Wild type plus and hap2 minus gametes were washed into fresh N-free, mixed together for 10–15 min, and prepared for TEM. The adhering cilia contained larger numbers of associated vesicles. The inset shows a higher magnification view of the vesicles at the ciliary tips. (C) High magnification view of adhering cilia of wild type plus and hap2 minus gametes showing vesicles. (D) Vesicles were absent from the cilia of db-cAMP-activated wild type plus gametes. (E) TEM of ciliary ectosomes harvested from the medium of adhering wild type plus and hap2 minus gametes. (F) Immunoblots with the indicated antibodies of equal protein amounts (8 μg/lane) of ciliary ectosomes (right lane) and cilia isolated from a mixture of adhering SAG1-C65-HA plus gametes and hap2 minus gametes (left lane). (G) Relative abundance of SAG1-HA and FMG1 in equal amounts of protein of ciliary ectosomes compared to cilia in a representative experiment. Error bars show S.D. from at least three quantifications of immunoblots.
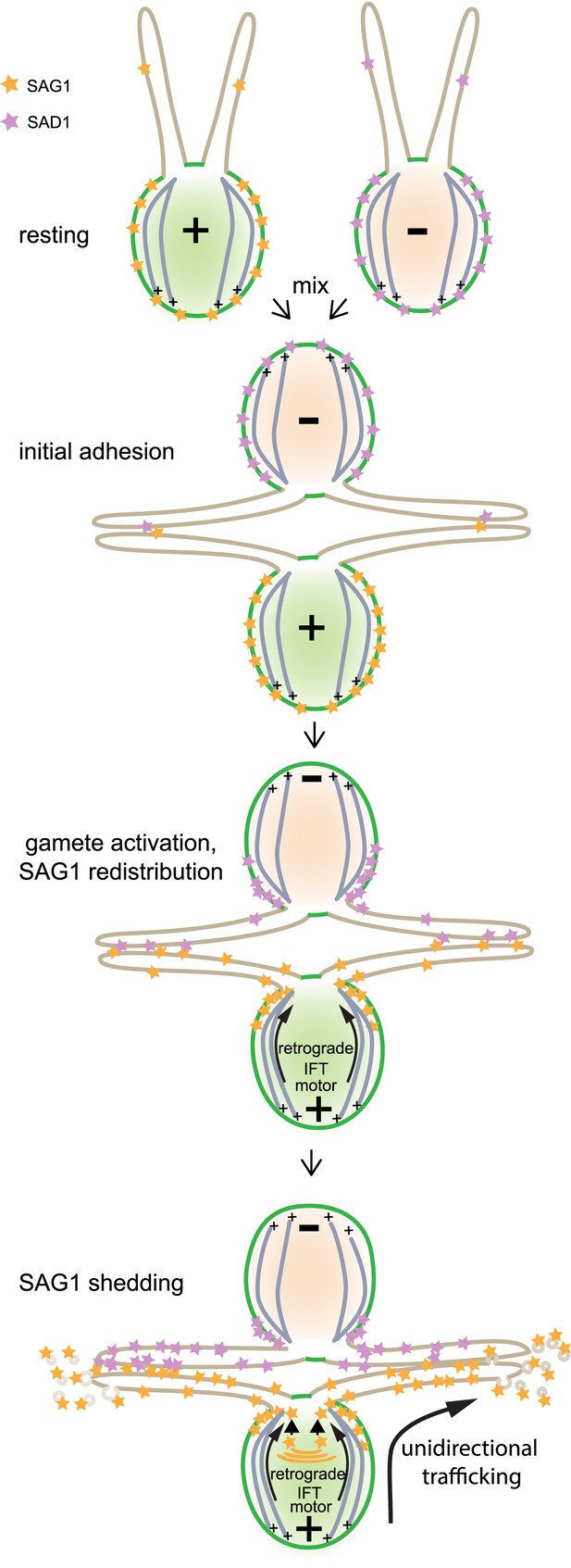
Model illustrating unidirectional trafficking of SAG1 during ciliary adhesion and signaling.
SAG1* in resting cells is present at the cell periphery, with only small amounts in cilia. Initial ciliary adhesion to minus gametes by SAG1 agglutinin–SAD1** agglutinin interactions triggers gamete activation and redistribution of SAG1 to the peri-ciliary region at the apical ends of the cells followed by ciliary entry. The retrograde IFT motor, cytoplasmic dynein 1b, is required for SAG1 movement along the cytoplasmic microtubules that originate from sites near the bases of the cilia. Although the anterograde motor is not required for ciliary entry of SAG1, it remains unknown whether it transports SAG1 once it is in the cilia. During sustained ciliary adhesion and signaling, SAG1 is released into the medium in the form of ciliary ectosomes and is replaced by protein synthesis. *Although we lack information on whether the SAG1 plus agglutinin fragment and SAG1-C65 form a complex, for simplicity they are here depicted as a single unit (orange stars). **For the purpose of illustrating ciliary adhesion, the model depicts SAD1 [violet stars] on minus gametes interacting with SAG1 on plus gametes and undergoing similar redistribution. Whether SAD1 behaves similarly to SAG1 is unknown, and thus SAD1 behavior in the model is purely speculative.
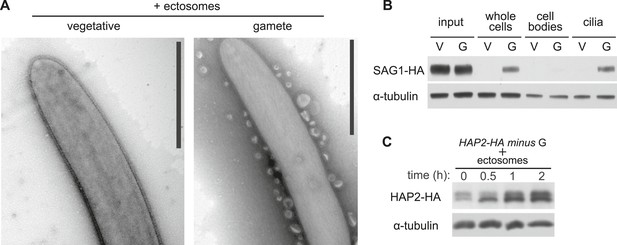
Ciliary ectosomes possess agglutinin receptor binding and signaling activity.
(A) TEM of cilia on minus vegetative cells (left panel) and gametes (right panel) that had been mixed with ciliary ectosomes. Only the cilia on the gametes bound ciliary ectosomes. (B) Immunoblot analysis of binding of SAG1-C65-HA-containing ectosomes to whole cells, cell bodies, and cilia of minus gametes. The initial sample of cells + ectosomes is also shown (input).The lanes contain equal cell equivalents. (C) Cilium-generated signaling was activated when ciliary ectosomes were mixed with HAP2-HA minus gametes, as assessed by activation-induced upregulation of expression of the gamete fusion protein, HAP2.





