Secreted CLCA1 modulates TMEM16A to activate Ca2+-dependent chloride currents in human cells
Figures
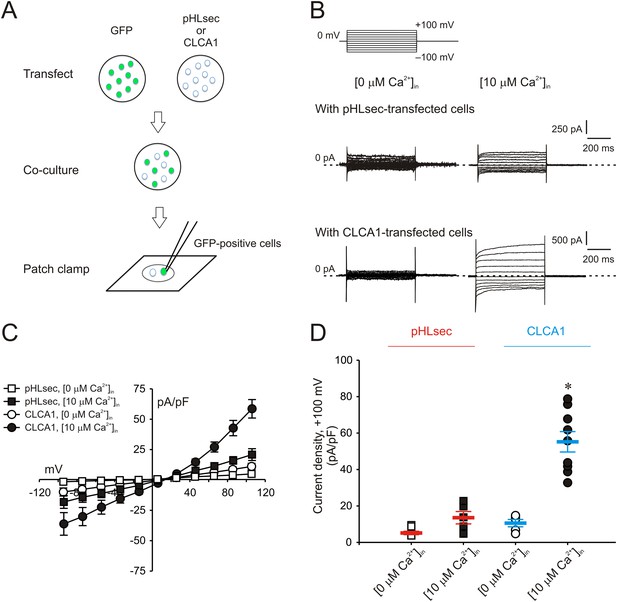
Paracrine activation of calcium-dependent chloride currents in HEK293T cells by CLCA1.
(A) GFP-expressing cells were co-cultured with pHLsec- or CLCA1-transfected cells, and assayed for ICaCC by patch clamp electrophysiology. (B–C) Whole-cell currents measured in GFP-positive cells from experiments as in (A), superfused with standard extracellular solution, and in the absence or presence of 10 μM free Ca2+ in the pipette (respectively, [0 μM Ca2+]in or [10 μM Ca2+]in). (B) Representative current traces. The pulse protocol is shown at the top left. Outward currents are represented by upward deflections, and dotted lines indicate zero current. Membrane capacitance was similar in all cases at ∼25 pF. (C) Current–voltage relationships at the end of the 600-ms voltage steps. Membrane potential values were corrected off-line for the calculated liquid junction potentials, respectively −5.5 mV ([0 μM Ca2+]in) and −6.0 mV ([10 μM Ca2+]in). Data are presented as means ± S.E. (n = 5–9). (D) Current density at +100 mV, from the same experiments as in (C). Symbols represent data from individual patches; bars indicate the means ± S.E. of all experiments. *p < 0.01 (one-way ANOVA, F = 30.3 and p = 1.2 × 10−7; followed by Tukey test).
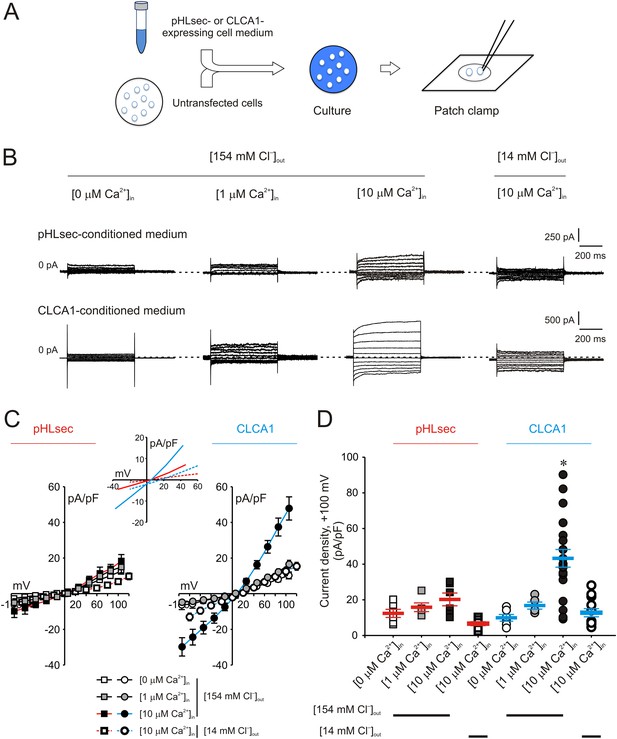
Activation of calcium-dependent chloride currents by secreted CLCA1.
(A) Untransfected cells were cultured in medium from pHLsec- or CLCA1-expressing cells, and assayed by patch clamp electrophysiology. (B–D) Whole-cell currents measured in cells from experiments as in (A), superfused with standard ([154 mM Cl−]out) or reduced Cl− ([14 mM Cl−]out) extracellular solution; and in the absence or presence of 1 μM or 10 μM free Ca2+ in the pipette (respectively, [0 μM Ca2+]in, [1 μM Ca2+]in or [10 μM Ca2+]in). (B) Representative current traces obtained with the same pulse protocol and displayed as in Figure 1B. Membrane capacitance was similar in all cases at ∼25 pF. (C) Current-voltage relationships at the end of the 600-ms voltage steps. Membrane potential values were corrected off-line for the calculated liquid junction potentials, respectively −5.5 mV ([0 μM Ca2+]in) and −6.0 mV ([1 μM Ca2+]in and [10 μM Ca2+]in) for the experiments in [154 mM Cl−]out; and −20 mV for the experiments in [14 mM Cl−]out. Data are presented as means ± S.E. (n = 5–20). Inset, CLCA1-mediated currents right-shifted ∼ +15 mV upon reduction of extracellular Cl−; symbols have been removed for clarity. (D) Current density at +100 mV, from the same experiments as in (C). Symbols represent data from individual patches; bars indicate the means ± S.E. of all experiments. *p < 0.01 (one-way ANOVA, F = 10.4 and p = 2.1 × 10−8; followed by Tukey test).
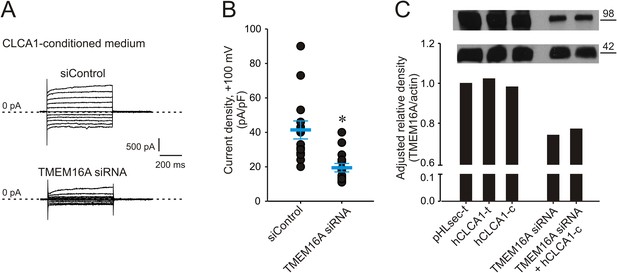
Genetic knockdown of TMEM16A inhibits CLCA1-mediated calcium-dependent chloride currents.
(A–B) HEK293T cells transfected with RNAi negative control (siControl) or TMEM16A siRNA were incubated in CLCA1-conditioned medium and assayed by patch-clamp electrophysiology, in standard extracellular solution ([154 mM Cl−]out) and 10 μM free Ca2+ in the pipette ([10 μM Ca2+]in). (A) Representative current traces obtained with the same pulse protocol and displayed as in Figure 1B. Membrane capacitance was similar in all cases at ∼25 pF. (B) Current density at +100 mV. Symbols represent data from individual patches (n = 14); bars indicate the means ± S.E. of all experiments. *p < 0.01 (unpaired Student's t test). (C) Effect of CLCA1 and/or TMEM16A siRNA treatment on TMEM16A protein expression. Upper panel: top, TMEM16A; and bottom, actin (loading control) Western blot from solubilized HEK293T cells. Lanes are labeled as follows: pHLsec-t, pHLsec transfected cells; CLCA1-t, CLCA1-transfected cells; CLCA1-c, cells treated with CLCA1-conditioned medium; TMEM16A siRNA, cells transfected with TMEM16A siRNA; TMEM16A siRNA + CLCA1-c, cells transfected with TMEM16A siRNA and treated with CLCA1-conditioned medium. Bar graph: quantitation of TMEM16A band intensity normalized to actin band intensity using ImageJ (NIH).
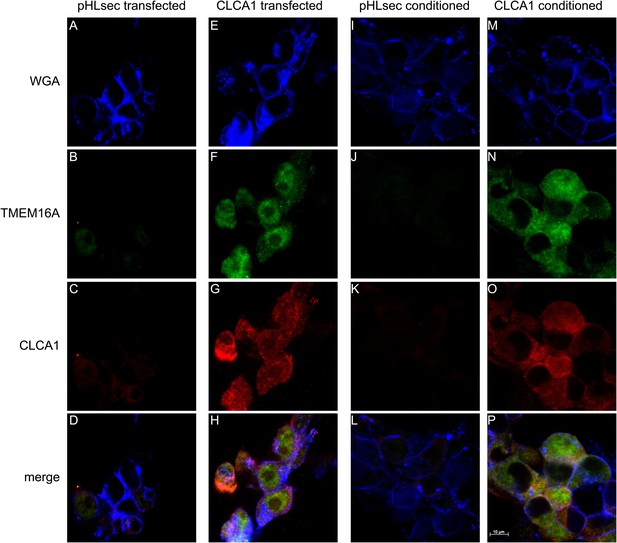
CLCA1 colocalizes with TMEM16A and increases TMEM16A surface expression.
(A–D) Membrane (WGA) or immunostaining of HEK293T cells transfected with pHLsec vector; (E–H), or with CLCA1. Surface TMEM16A is greatly increased by expression of CLCA1. (I–L) Membrane (WGA) or immunostaining of HEK293T cells cultured in conditioned media from cells transfected with pHLsec vector; (M–P) or cells cultured in conditioned media from cells transfected with CLCA1. TMEM16A surface expression is greatly enhanced after cells are exposed to secreted CLCA1.
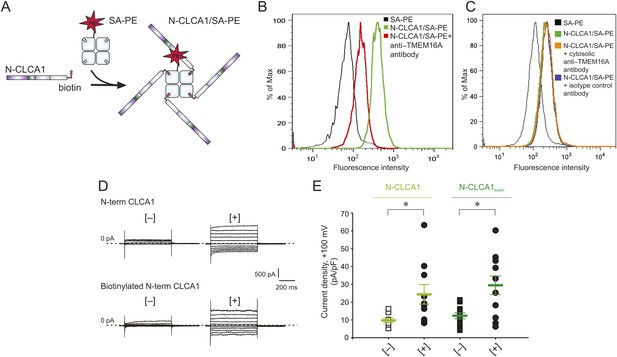
N-CLCA1 engages TMEM16A on the cell surface.
(A) Schematic of CLCA1 N-terminal fragment (N-CLCA1) construct with specific biotinylation site and resultant tetrameric cell-staining reagent created after complexation with SA-PE. (B) Flow cytometry of intact HEK293T cells stained with SA-PE alone (black line), N-CLCA1/SA-PE (green line), or N-CLCA1/SA-PE in the presence of anti-TMEM16A antibody S-20 (red line). (C) Flow cytometry of intact HEK293T cells either stained with SA-PE alone (black line), N-CLCA1/SA-PE (green line), N-CLCA1/SA-PE in the presence of anti-TMEM16A antibody C-5 (raised against an intracellular TMEM16A epitope; orange line), or N-CLCA1/SA-PE in the presence of anti-Aquaporin5 antibody G-19 (blue line). (D–E) Cells were incubated in the absence ([−]) or presence ([+]) of purified N-terminal (N-term) CLCA1 protein before (N-CLCA1) or after biotinylation (N-CLCA1biotin), and assayed by patch-clamp electrophysiology, in standard extracellular solution ([154 mM Cl−]out) and 10 μM free Ca2+ in the pipette ([10 μM Ca2+]in). (D) Representative current traces obtained with the same pulse protocol and displayed as in Figure 1B. Membrane capacitance was similar in all cases at ∼25 pF. (E) Current density at +100 mV. Symbols represent data from individual patches (n = 8–11); bars indicate the means ± S.E. of all experiments. *p < 0.05 (unpaired Student's t test).
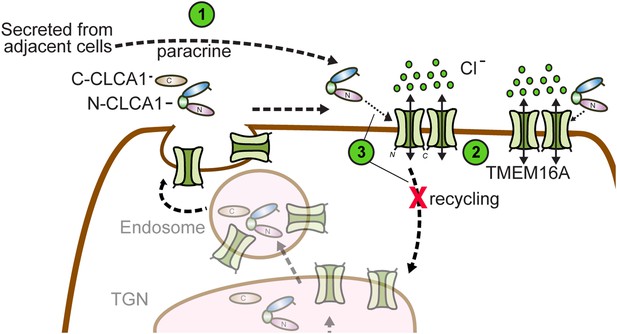
Model for CLCA1 modulation of TMEM16A-mediated calcium-dependent chloride currents.
Following secretion and self-cleavage of CLCA1, the N-terminal fragment (N-CLCA1) acts in paracrine fashion (1). Dimerization appears to regulate surface trafficking of TMEM16A. N-CLCA1 engages TMEM16A on the cell surface (2), stabilizing TMEM16A dimers, preventing internalization (3) and in turn, results in increased TMEM16A surface expression and calcium-dependent chloride current density.





