The HIV-1 envelope protein gp120 is captured and displayed for B cell recognition by SIGN-R1+ lymph node macrophages
Figures
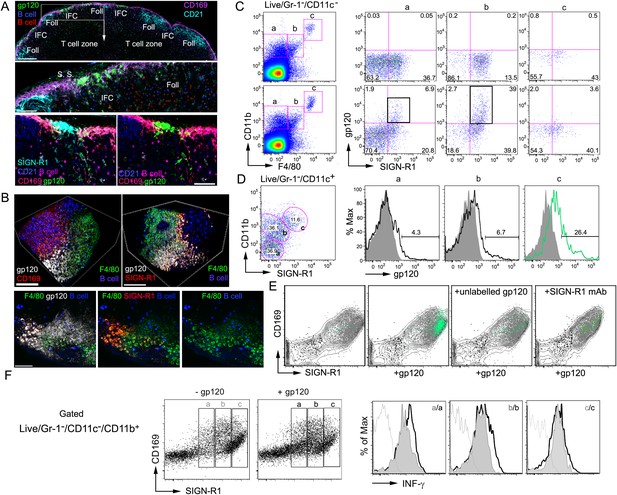
SIGN-R1 positive interfollicular channel (IFC) and cortical medullary junction macrophages rapidly accumulate lymph borne gp120.
(A) Confocal microscopy of thick lymph node (LN) sections prepared from mice that had received adoptively transferred B cells (previous day), injected with fluorescently labeled gp120, and immunostained as indicated. LN section image (sagittal, tiled) shows gp120, green; CD169, pink; CD21/35, cyan; and B cells, red and blue. Scale bar is 200 μm (top). A zoomed image of the white boxed area is shown. Scale bar is 60 μm (middle). Images of an IFC are shown: gp120, green; CD169, red; SIGN-R1, cyan; CD21/35, blue; adoptively transferred B cells, pink; and CD169, red (bottom, left). SIGN-R1 signal removed (bottom, right). Arrows indicate gp120 positive cells. Scale bars is 50 μm. (B) Intravital two-photon laser scanning microscopy (TP-LSM) images of the inguinal LN from a mouse injected with fluorescent gp120 and the indicated antibodies. The top images over the IFC show gp120, white; CD169, red; F4/80, green; and adoptively transferred B cells, blue, (left panel). SIGN-R1 antibody, red, used instead of CD169 (right panel). Scale bars are 100 μm. The bottom images are from the follicular-medullary junction and show gp120, white; SIGN-R1, red; F4/80, green; and adoptively transferred B cells, blue. Scale bar is 50 μm. (C, D) Flow cytometry of LN cells immunostained and gated as indicated using inguinal LN cells from a mouse injected with fluorescent gp120 1.5 hr previously, or not. LiveGr-1−CD11c− gated population plotted for F4/80 vs CD11b. Gates ‘a’, ‘b’, and ‘c’ as indicated were re-plotted to show SIGN-R1 vs gp120 in right three plots (top 2 rows). (C). LiveGr-1−CD11c+ population is shown plotted for SIGN-R1 vs CD11b. Histogram of indicated three populations (a, b, and c) plotted as gp120 signal (black line) vs % of maximum intensity compare to gp120 negative control (shaded). Numbers are % gp120 positive cell population in gate (D). (E) In vitro binding by LN cells incubated with fluorescent gp120, or not, and in the presence of non-labeled gp120 or non-labeled SIGN-R1 antibody (different epitope) and then analyzed by flow cytometry. LiveGr-1−CD11c−CD11b+ cells were analyzed for gp120 vs SIGN-R1 (not shown) and the CD11b+ cells, gray contour; the SIGN-R1+gp120+ cells, green dots; SIGN-R1−gp120− cells, black dots; and SIGN-R1+gp120− cells, gray dots, were plotted to show CD169 vs SIGN-R1. (F) Interferon-γ intracellular flow cytometry of cells prepared from the inguinal LNs of mice administered gp120 near the tail base, or not, 3 hr prior to collection. LiveGr-1−CD11c−CD11b+ cells were analyzed for SIGN-R1 vs CD169 and separated into three populations (left panels). The levels of intracellular interferon-γ are shown as histograms of maximum intensity in cells from the gp120 non-exposed (gray) and gp120 injected mice (white, outlined by black lines). Unstained control is delineated by a gray line.
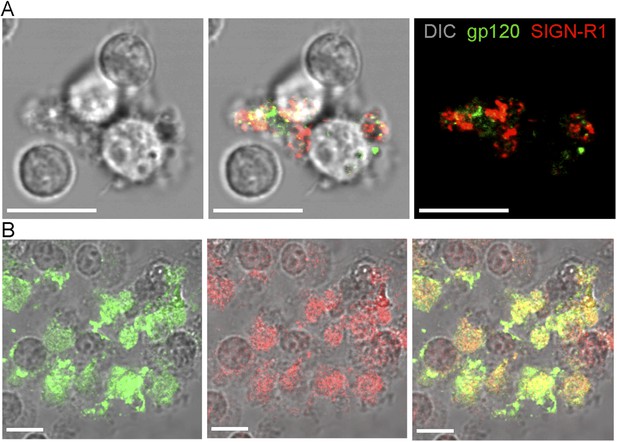
Sorted SIGN-R1+ macrophages capture gp120.
(A) Confocal microscopy image of FACS sorted SIGN-R1+ macrophage 2 hr after sorting. Differential interference contrast (DIC) visualized the cell body and nucleus and was used as a background (left). gp120 (green) and SIGN-R1 (red) signals were overlapped with DIC (middle). gp120 (green) and SIGN-R1 (red) signals were visualized without DIC (right). Scale bars are 10 μm. (B) Confocal microscopy image of FACS sorted SIGN-R1+ macrophage, which were cultured with 20 ng/ml of M-CSF for 7 days. Cells were fixed with 4% paraformaldehyde for 2 hr and overlaid with fluorescent gp120 (green). The cells were washed and immunostained with SIGN-R1 antibody (red). The fluorescent gp120 signal was overlapped with DIC (left). SIGN-R1 signal was overlapped with DIC (middle). Merged signal (yellow) was overlapped with DIC (right). Scale bars are 10 μm.
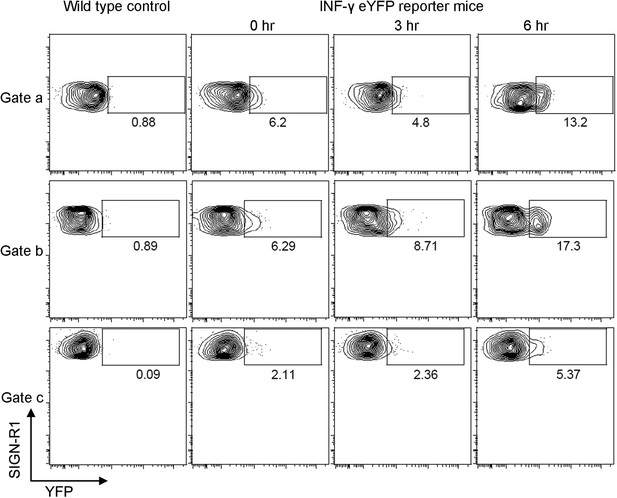
The injection of gp120 triggers transcription of interferon- γ in SIGN-R1+ macrophages as assessed by using a interferon-γ-eYFP reporter mouse.
Flow cytometric analysis of cells prepared from the inguinal LNs of interferon-γ-eYFP reporter mice administered gp120 near the tail base at 0, 3 and 6 hr prior to collection. eYFP vs SIGN-R1 expression in cell located in gates a, b and c are shown via a contour plot. WT mice were used as a negative control for eYFP expression in each of the gates.
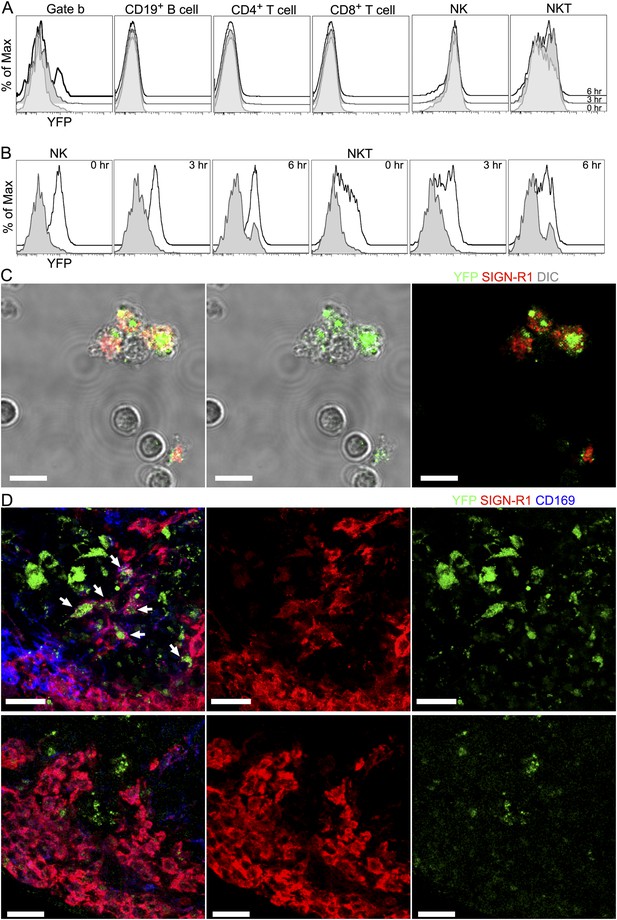
The injection of gp120 triggers transcription of interferon- γ in SIGN-R1+ macrophages.
(A) Flow cytometric analysis of various cellular populations prepared from the inguinal LNs of interferon-γ-eYFP reporter mice administered gp120 near the tail base, 0 (shaded), 3 (shaded, darker) and 6 (black line) hs prior to collection. Histograms of indicated populations were plotted as eYFP signal vs % of maximum intensity. (B) Histogram of NK cells (black line, first to third graph from left) and NKT cells (black line, forth to sixth graph from left) were re-plotted with histogram of ‘Gate b’ to compare eYFP signal at indicated time points. (C) Confocal microscopy image of FACS sorted SIGN-R1+ macrophage at 0.5 hr after sorting. Differential interference contrast (DIC) (gray) visualized cell body as a background (left). eYFP (green) and SIGN-R1 (red) signals were overlapped with DIC (left). eYFP (green) signals were visualized with DIC (middle). eYFP (green) and SIGN-R1 (red) signals were visualized without DIC (right). Scale bars are 10 μm. (D) Confocal microscopy of a thick LN section from the draining LN of a mouse previously injected with gp120 6 hr previously (upper panels) and a thick section of a cervical LN (far from the site of gp120 injection) obtained at the same time point (lower panels). Sections were immunostained for SIGN-R1 (red) and CD169 (blue). Arrows in panel (upper, left) indicate eYFP (green) expressed in SIGN-R1+ macrophages. eYFP, SIGN-R1 and CD169 signals were overlapped in the left panels. SIGN-R1 only and eYFP only signals were shown in middle and right panels, respectively. Scale bar is 30 μm.
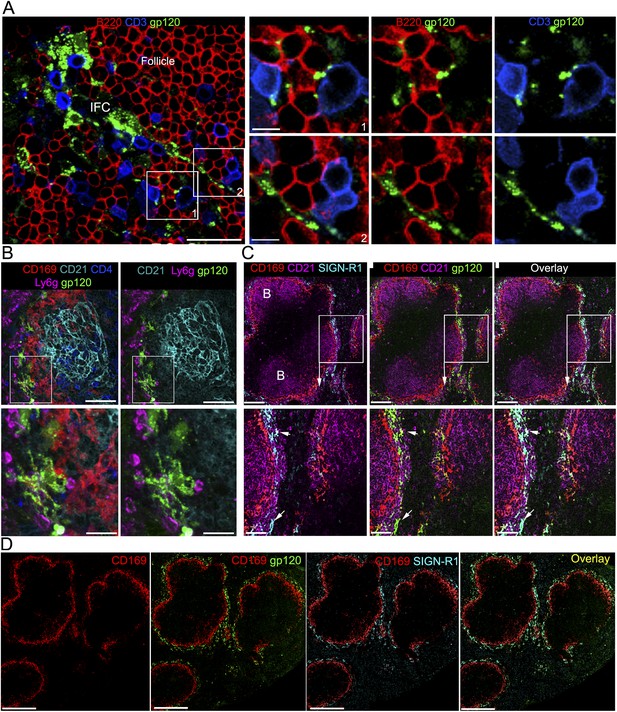
IFC cell processes bearing gp120 directly contact B cells and a subset of splenic marginal zone cells also bind gp120.
(A) Confocal microscopy of a thick LN section from a mouse previously injected with fluorescent gp120 and immunostained for B220 and CD3. Scale bar is 30 μm. Boxed areas in left image were enlarged and shown in the right panels. Scale bars are 10 μm. (B) Confocal microscopy of a thick splenic section from a mouse previously injected intravenously with fluorescent gp120 and immunostained with the indicated markers. Zoomed images shown below. Scale bars are 100 μm, above, and 30 μm, below. (C) Confocal microscopy of a thick splenic section overlaid with fluorescent gp120 and immunostained for the indicated markers. Zoomed images are shown below. Scale bars are 100 μm, above, and 40 μm, below. (D) Tiled confocal microscopy images of a spleen section immunostained with CD169 (red) and SIGN-R1 (cyan) and overlaid with fluorescent gp120 (green). As indicated the images show CD169 alone, CD169 and SIGN-R1; CD169 and gp120; and overlay of all three. Scale bar is 300 μm.
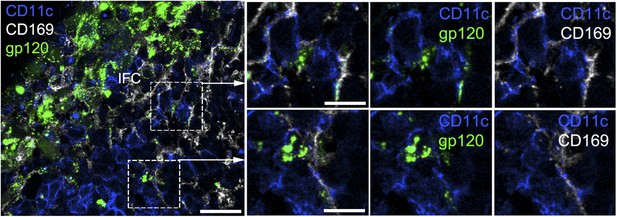
The IFC network macrophages contact CD11c positive cells.
Confocal microscopy of a thick LN section from a mouse previously injected with fluorescent gp120 and immunostained for CD169 and CD11c. The image is centered over an IFC. Scale bar is 30 μm. Boxed areas in left image were enlarged and shown in the right panels. Scale bars are 10 μm.
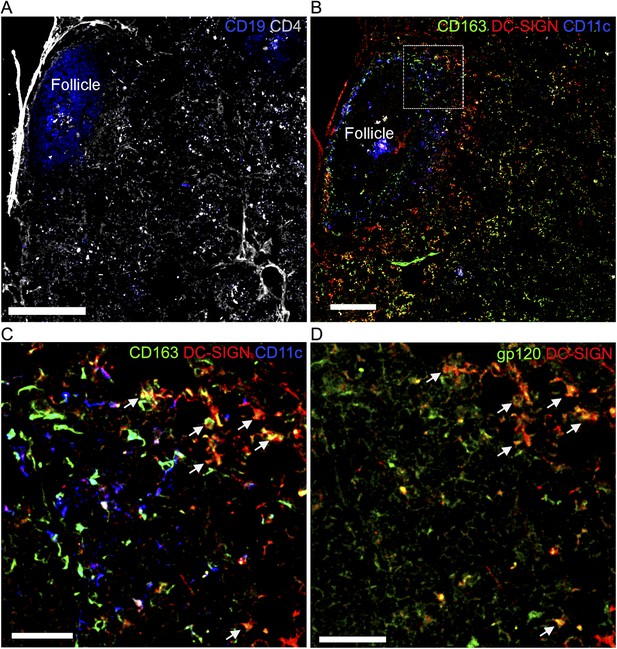
Overlay of gp120 visualizes DC-SIGN+/CD163+ macrophages in a human LN section.
Confocal microscopy of human frozen LN sections overlaid with fluorescent gp120 and immunostained for the indicated markers. (A) Section was stained with CD19 (blue) and CD4 (gray). Scale bar is 400 μm. (B) Adjacent section from (A) section was stained with CD163 (green), DC-specific ICAM-3-grabbing non-integrin (DC-SIGN) (red) and CD11c (blue). Scale bar is 200 μm. (C) Zoomed image of the white box in (B). Arrows indicated overlap (yellow) of CD163+ cells (green) and DC-SIGN+ cells (red). Scale bar is 100 μm. (D) Same area with (C). Arrow indicated overlap (yellow) of gp120+ cells (green) and DC-SIGN+ cells (red). Scale bar is 100 μm.
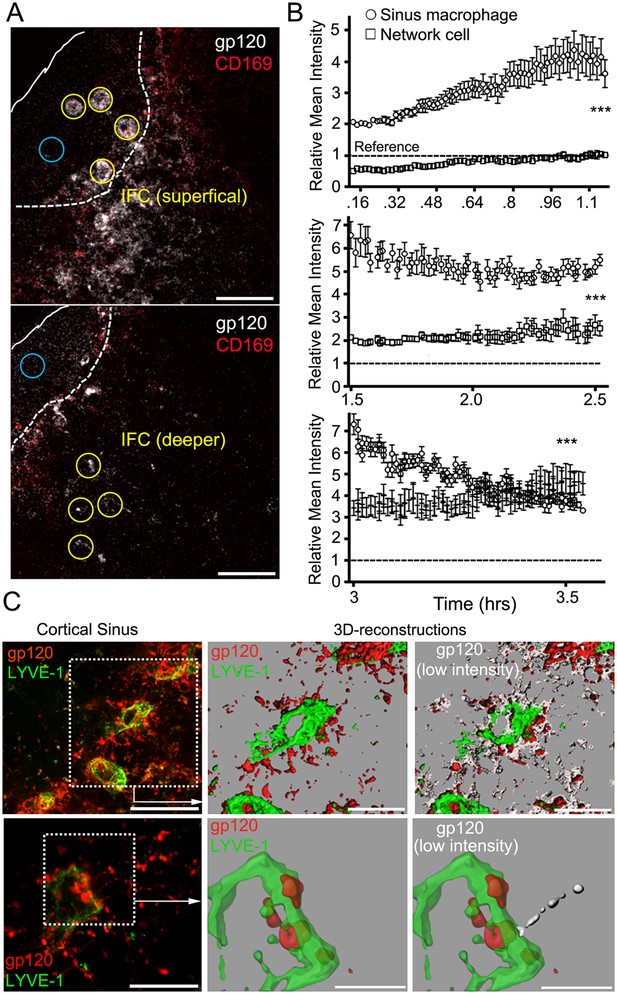
The IFC network of macrophages extends from the subcapsular sinus to the cortical sinus.
(A) Intravital TP-LSM images of the inguinal LN 8 min after injection of fluorescent gp120 and 30 min after CD169. Yellow circles indicate sinus macrophages (upper panel) or network cells in the IFC (lower panel). Blue circles indicate subcapsular sinus lumen. Distance between two slices is 30 μm. Scale bars are 50 μm. (B) The gp120 signal associated with SIGN-R1+ SM (○) or deeper in the IFC (⃞) was quantitated over time following gp120 injection: 8 min-1 hr 14 min, upper; 1 hr 30 min–2 hr 30 min, middle; and 3 hr–3 hr 32 min, bottom panel. Calculated slopes (○, ⃞) are 1.59 ± 0.046 and 0.45 ± 0.013, upper; −1.04 ± 0.16 and 0.63 ± 0.11; middle, and −5.61 ± 0.23 and 2.35 ± 0.33; bottom panel. In the same panel the slopes differed by a p value <0.0001. The reference signal of gp120 in subcapsular sinus lumen is shown with dotted lines. Error bars, ±SEM (B). (C) Intravital TP-LSM images from deep in the IFC following injection of fluorescent gp120 and LYVE-1 antibody. The left upper panel show an image from a 30 μm z-projection. Scale bar is 100 μm. Dotted box was reconstructed using the LYVE-1 signal, green, and gp120 signal, red (upper middle panel) and modified by adding low intensity gp120 signal, white (upper right panel). Scale bars are 50 μm. The left lower panel shows a higher power 20 μm z-projection image. Scale bar is 50 μm. Dotted box was reconstructed using the LYVE-1 signal, green, and gp120 signal, red (lower middle panel) and modified by adding the low intensity gp120 signal, white (lower right panel). Scales bars are 20 μm.
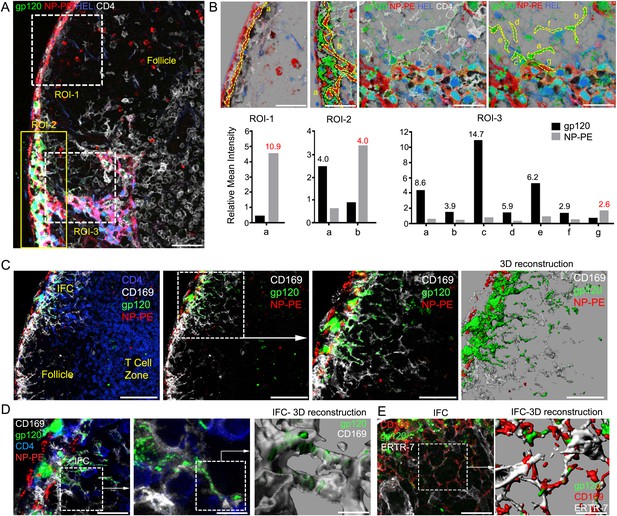
IFC network macrophages do not uptake hen egg lysozyme (HEL) or nitrophenylacetyl (NP)-phycoerythrin (PE).
(A, B) Confocal microscopy of a thick LN section immunostained for CD4, white, following injection of NP-PE, red, fluorescent gp120, green, and fluorescent HEL, blue, near the inguinal LN. Three regions of interest are shown over LN follicle region of interest (ROI-1), superficial IFC (ROI-2), and deep IFC (ROI-3). Scale bar is 40 μm (A). In part B each ROI is further subdivided as indicated by letters to delineate specific cells or groups of cells. The fluorescent intensity of NP-PE or gp120 in each of these regions was quantitated and is indicated. Numbers in graphs indicate fold difference. (C) Confocal microscopy image of a thick LN section from a mouse previously injected with fluorescent gp120, green, plus NP-PE, red, and immunostained for CD169, white, and CD4, blue. CD4 is excluded in the 2nd–4th panels. Electronically zoomed image of boxed area is shown in 3rd panel. A 3-D reconstruction of the 3rd panel image is shown in the 4th panel. Scale bars from left to right are 100, 100, 50, and 50 μm. (D) Confocal microscopy image of a thick LN section immunostained for CD4 and CD169 prepared from a mouse injected near the inguinal LN with fluorescent gp120 and NP-PE. The middle image is an electronically zoomed image of the region in the left panel. A portion of the middle image was used to perform a 3-D reconstruction of the imaging data shown in the right panel. The scale bars from left to right are 25, 10, and 5 μm. (E) Confocal microscopy image of a LN section immunostained for ERTR-7, white, and CD169, red, from a mouse previously injected with fluorescent gp120, green, focusing on the IFC. The indicated portion of left panel was used for the 3-D reconstruction shown in the right panel. The scale bars from left to right are 30 and 15 μm.
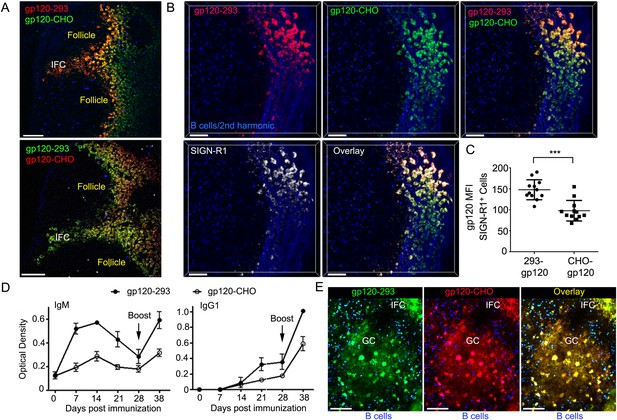
IFC network macrophages differentially uptake two different R66M gp120 preparations.
(A) Intravital TP-LSM image of the inguinal lymph following the injection of differentially labeled R66M gp120 expressed in either 293F or CHO-S cells. Scale bars are 100 μm. (B) A z-projection (50 μm) of intravital TP-LSM images of the inguinal LN following the injection of differentially labeled R66M gp120 expressed in either 293F cells, red, or CHO-S cells, green, and SIGN-R1 antibody, white. Adoptively transferred B cells (blue) and the 2nd harmonic signal delineated the LN follicle and capsule. The various signals shown are indicated. Scale bars are 50 μm. (C) Level of gp120 binding to SIGN-R1+ cells was quantitated. The amount of 293 gp120 and CHO-gp120 bound was determined using Imaris. ***p < 0.001. (D) Results from ELISA assays to analyze gp120 specific antibodies present in the sera of mice at various days following immunization with R66M gp120 expressed in either 293F or CHO-S cells. Error bars, ±SEM. (E) A z-projection (50 μm) of intravital TP-LSM images of the inguinal LN following the local injection of R66M gp120 expressed in 293F cells, green, or CHO-S cells, red. The mice had been immunized with 293-gp120 and boosted 4 weeks later. 3 weeks after the boost the mice were injected near the inguinal LN with labeled gp120s. The day prior to the injection naive B cells (blue) were adoptively transferred. The LN follicle images are from 4 hr after gp120 injection. Scale bar are 100 μm.
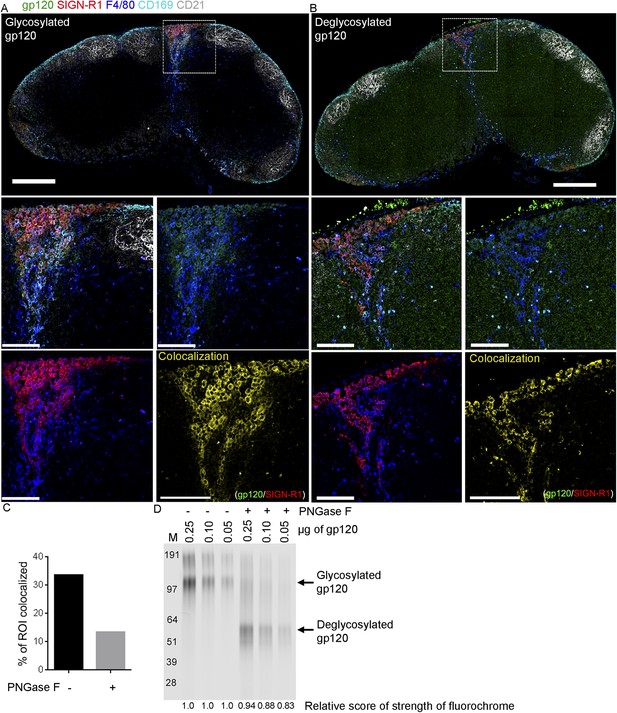
Deglycosylated gp120 loses its binding specificity to SIGN-R1+ macrophages.
Confocal microscopy of thick LN sections overlaid with fluorescent gp120, which is treated with PNGase F or not, and immunostained for the indicated markers. (A) LN section image (sagittal, tiled) shows control gp120, green; CD169, cyan; CD21/35, gray; F4/80, blue and SIGN-R1, red. Scale bar is 200 μm (top). (B) LN section image (sagittal, tiled) shows deglycosylated gp120, green; CD169, cyan; CD21/35, gray; F4/80, blue and SIGN-R1, red. Scale bar is 200 μm (top). Zoomed images of the white boxed area in (A) and (B) were shown in middle and bottom panels. Image in middle left shows gp120, green; CD169, cyan; CD21/35, gray; F4/80, blue and SIGN-R1, red. Image in middle right shows gp120, green and CD169, cyan. Image in bottom left shows CD169 and SIGN-R1, red. Image in bottom right shows the generated channel (yellow) of colocalization between gp120 and SIGN-R1. Scale bars are 100 μm. (C) Percentage of ROI colocalized area in bottom left panel in (A) and (B) were compared in graph. (D) The strength of fluorochrome after PNGase F treatment was measured by Odyssey CLx Infrared Imaging System. The numbers on top of each lanes indicated the amount of loaded gp120. The numbers on bottom of each lanes indicated the relative score of strength of fluorochrome on gp120.
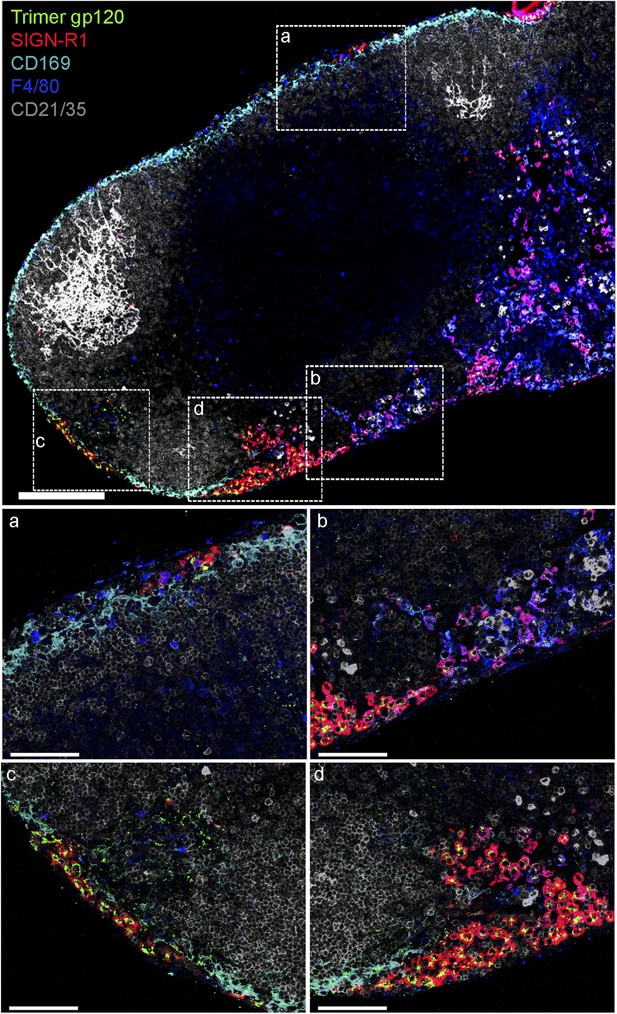
SIGN-R1+ IFC and cortical medullary junction macrophages rapidly accumulate lymph borne soluble trimeric gp120.
Confocal microscopy of thick LN sections prepared from mice that had been injected with biotinylated soluble trimeric gp120. Biotinylated gp120 was detected by AlexaFluor 488 conjugated streptavidin. In the LN section image (sagittal, tiled) trimeric gp120, green; CD169, cyan; CD21/35, gray; F4/80, blue and SIGN-R1, red, are shown. Scale bar is 200 μm (top). Zoomed images of the white boxed areas are shown in the middle and bottom panels. Scale bars are 100 μm.
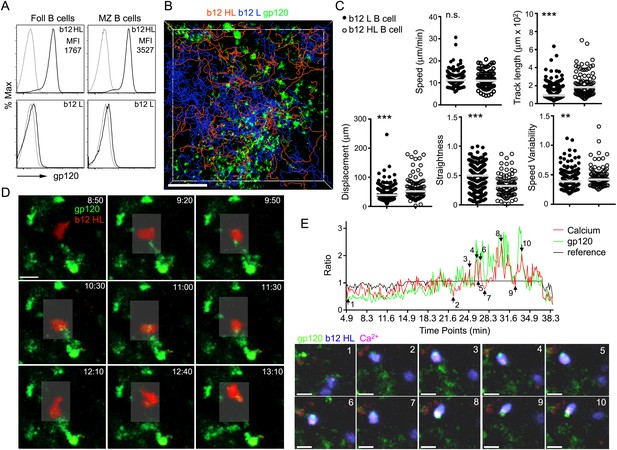
Recently arrived LN B cells that express the b12 antigen receptor can extract gp120 from IFC network cells.
(A) Flow cytometry to evaluate the binding of labeled R66M gp120 to follicular and marginal zone B cells from either b12 HL or b12 L B cells. Light gray line is background fluorescence. MFI-mean fluorescence intensity. (B) Tracks of b12L and b12 HL B cells in the IFC after adoptive transfer to a mouse previously injected with fluorescent gp120. The tracks are superimposed on the IFC network delineated by gp120. Scale bar is 100 μm. (C) Comparison of the motility parameters generated from the analysis of b12 L and b12 HL B cells adoptively transferred into mice previously injected with gp120. Statistics calculated using unpaired t-test, **p < 0.01, ***p < 0.001. (D) Time laps images of a fluorescently labeled b12 HL B cell, red, approaching and departing from a cell in the IFC network that has accumulated gp120, green. Scale bar is 10 μm. Time stamps in top right, min:s. (E) Intravital TP-LSM imaging of b12 HL B cell labeled with eFluor 450, blue, and Calcium Orange, red, as it approaches and interacts with gp120 expressing cells, green, in the IFC. In graph, signal intensity ratio was plotted as a function of time. The ratio was calculated by dividing the intensity of an individual point by the average intensity of all the time points. Reference ratio calculated using the eFluor 450 b12 HL B cell signal. Arrows from 1 to 10 correspond to the numbered time lapse images shown below. Scale bar is 10 μm.
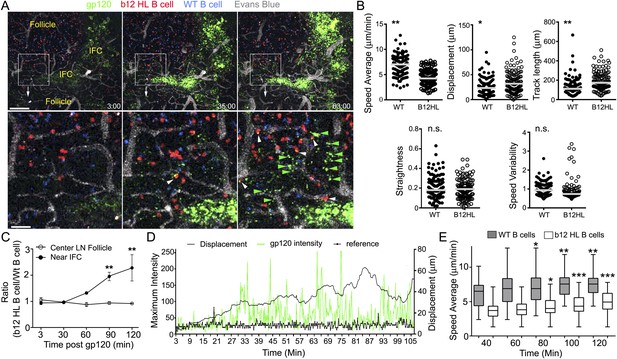
LN follicle B cells that express the b12 antigen receptor can extract gp120 from IFC network cells.
(A) Intravital TP-LSM images of b12 HL, red, and WT B, blue, cells in the inguinal LN at 3, 35, and 63 min following injection of fluorescent gp120, green, near the inguinal LN. Blood vessels were visualized by intravenous injection of Evans blue, white. Scale bar is 100 μm. Bellow each image is an electronic zoomed image from the indicated area. White arrowheads indicate B cells that have accumulated gp120 and green arrowheads gp120 in the LN follicle. Scale bar is 25 μm. (B) Motility parameters. Analyses of b12 HL and wild type (WT) tracks are shown. Statistics are by unpaired t-test *p < 0.01, **p < 0.001. (C) The ratio between the number of WT and b12 HL B cells at various times points following antigen injection near the IFC or in the center of follicle. Error bar, ±SEM. **p < 0.002. (D) Tracking a b12 HL B cell located in the LN follicle following injection of gp120. Displacement and gp120 signal overlying the cell tracked over time. Graph shows the displacement, black line, from the origin and gp120 signal, green peaks, for each individual time point. The reference, thick black, is the fluorescent signal in another channel. (E) The average speed of b12 HL and WT B cells near the IFC channel increases over time following gp120 injection. WT and b12 HL B cells were tracked over 20 min intervals. The time shown below is the endpoint of the tracking interval. Average speed is shown as a box- and-whisker blot. The results from the later intervals were compared to the initial interval for each cell type by unpaired t-test. *p < 0.5; **p < 0.003; ***p < 0.00002.
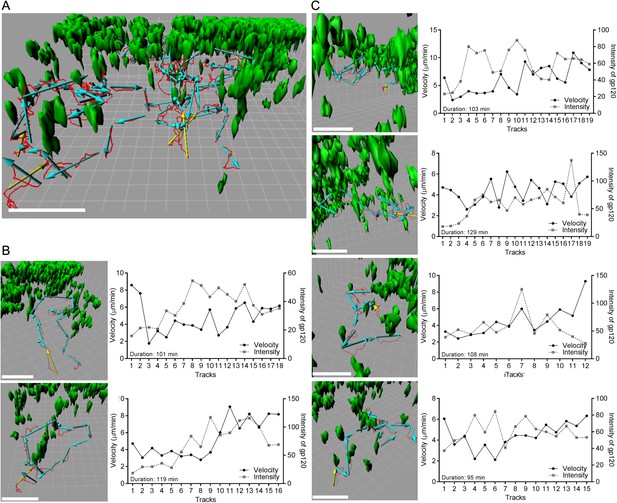
Individual b12 HL B cell tracks from b12 HL B cells located in the LN follicle near the IFC channel following injection of gp120.
(A) Six tracks, red lines, and displacements, yellow or cyan arrows, of b12 HL B cells were superimposed on 3-D reconstruction image of gp120 loaded IFC network cells, green. 3-D reconstruction images were generated with 50 μm z-stack volume image. Displacement arrows were generated with fragments of the original track, which disconnected at time points that showed typical turning or discontinues movement from the original single track. Yellow arrows in each track indicate the starting point. Scale bar is 50 μm. Grid spacing in 3-D view is 10 μm. (B, C) Each track in (a) was visualized from a different angle to see typical tracked cell pattern (approach, survey, move away). Scale bar is 50 μm. Grid spacing in 3-D view is 10 μm. Each individual track is shown as a single track plot of velocity (solid line) and gp120 intensity (dotted line). Duration is total time span of the original single tracks in a 2 hr intravital TPLSM imaging.
Videos
Intravital two-photon laser scanning microscopy (TP-LSM) images of the interfollicular channel (IFC) network that extends from the subcapsular sinus to the cortical sinus.
Images from two focal planes separated by 30 μm and located over the IFC channel of a mouse inguinal lymph node (LN). The mouse had previously been injected with fluorescent CD169 antibody, red, which delineates the subcapsular sinus macrophages (SSMs). The images were acquired over an hour (8 min–1 hr 8 min post fluorescent gp120, white, injection into the tail base). The white lines delineate the subcapsular sinus. Scale bar is 50 μm. Time counter shows hr:min:s.
Intravital TP-LSM images of the gp120 loaded cellular network in the IFC of the inguinal LN.
An image sequence of a 30 μm z-projection was acquired from a LysM-EGFP mouse, which had previously received by adoptive transfer both B cells, blue, and CD4 T cells, purple. Host endogenous neutrophils/monocytes, strong green signal, and stromal cells, weak green signal, can be seen on the basis of their expression of LysM-EGFP. Images were acquired for an hr from 1.5–2.5 hr after fluorescent gp120, white, injection near the tail base. GFP positive cells can be seen flowing in blood vessels in the IFC. CD169 antibody, red, delineated the SSMs. Second harmonic signal, blue, from collagen delineated LN capsule. Scale bar is 100 μm. Time counter shows hr:min:s.
Intravital TP-LSM images of the dynamic interaction of SIGN-R1+ gp120+ macrophages and a lymphocyte.
An image sequence of a 20 μm z-projection was acquired from the inguinal LN following nearby injection of fluorescent gp120, red, and SIGN-R1, green, antibody (left). Track and displacement (yellow arrow) of lymphocytes (gray spot) superimposed with 3D-reconstructed images of cell process is gray (right). Scale bars are 20 μm and 10 μm. Time counter shows hr:min:s.
Intravital TP-LSM images of a newly arriving b12 B cell extracting gp120 from IFC network cells.
An image sequence of a 20 μm z-projection was acquired from the inguinal LN of a mouse, which had fluorescent gp120 (green) delineated IFC network cells. Fluorescently labeled b12 HL B cells (red) were adoptively transferred an hour after gp120 injection. Scale bar is 30 μm. Time counter shows hr:min:s.
Intravital TP-LSM images of in vivo calcium response of a b12 B cell engaging a gp120 bearing IFC cell.
An image sequence of a 25 μm z-projection was acquired from the inguinal LN of a mouse, which has fluorescent gp120 (green) delineated IFC network cells. b12 HL B cells (blue) were labeled with calcium orange (red). Shaded circle indicates the interaction between b12 HL B cells and gp120 loaded IFC network cells. Images were acquired for 40 min beginning 41 min after fluorescent gp120 injection into tail base. Scale bar is 15 μm. Time counter shows hr:min:s.
Intravital TP-LSM images of resident b12 B cells that extract gp120 from the IFC network cells.
An image sequence of a 20 μm z-projection was acquired from inguinal LN of mouse, which had received by adoptive transfer the previous day b12 HL B cells, red, and wild type B cells, blue. Evans blue delineated the blood vessels, gray. Fluorescent gp120, green, was injected near the tail base and the image sequence was acquired over the next 2 hr. Scale bar is 50 μm. Time counter shows hr:min:s.





