TGFβ-dependent expression of PD-1 and PD-L1 controls CD8+ T cell anergy in transplant tolerance
Figures
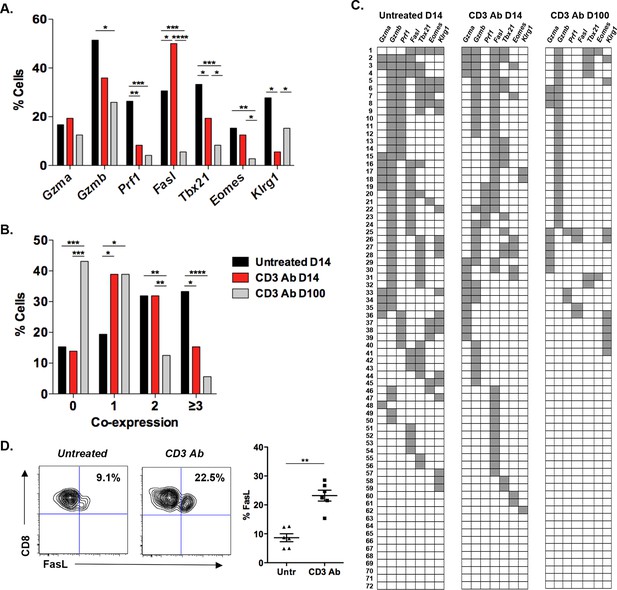
Coexpression of effector genes in graft-infiltrating CD8+ T cells after CD3 antibody therapy.
C57BL/6 mice were transplanted under the kidney capsule with BALB/c pancreatic islets and treated or not with CD3 Abs on day +7 post-transplant. Individual CD8+ T cells (n = 72) present within the graft were FACS sorted on day +14 or day +100 post-transplant and subjected to multiplex gene expression analysis. (A) Proportion of CD8+ T cells among the 72 cells tested that expressed Gzma, Gzmb, Prf1, Fasl, Tbx21, Eomes and Klrg1 mRNA in each group. (B) Polyfunctionality distribution of intragraft CD8+ T cells. (C) Coexpression of inflammatory and cytotoxic molecules by individual graft infiltrating CD8+ T cells. Each row represents one individual cell that is numbered. Each column represents a different gene. For better visualization of coexpression patterns, individual cells were ordered by the degree of gene coexpression. (D) FasL expression by intragraft CD8+ T cells 14 days after transplant and CD3 Ab therapy. (Figures 1A and 1B: χ2 test, *p<0.03, **p<0.01, ***p<0.0003, ****p<0.0001).
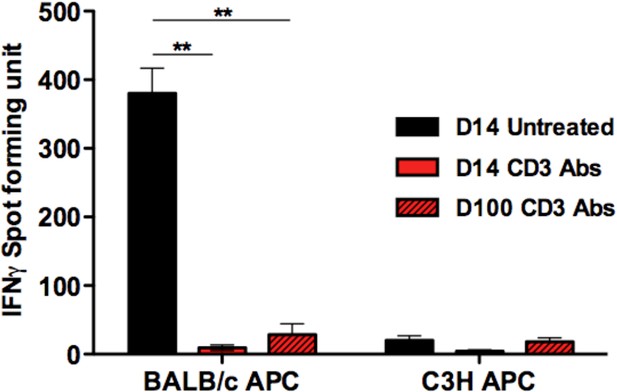
IFNγ responses by intragraft T cells after CD3 Ab therapy.
https://doi.org/10.7554/eLife.08133.004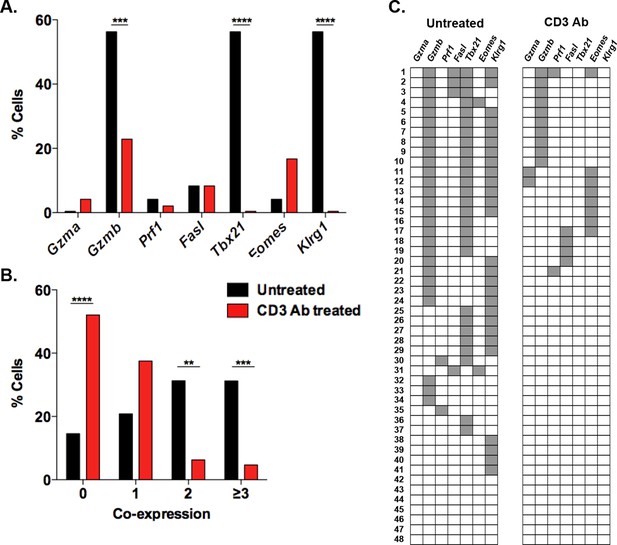
Multiplex gene expression analysis on individual splenic CD8+ T cells after CD3 Ab therapy.
https://doi.org/10.7554/eLife.08133.005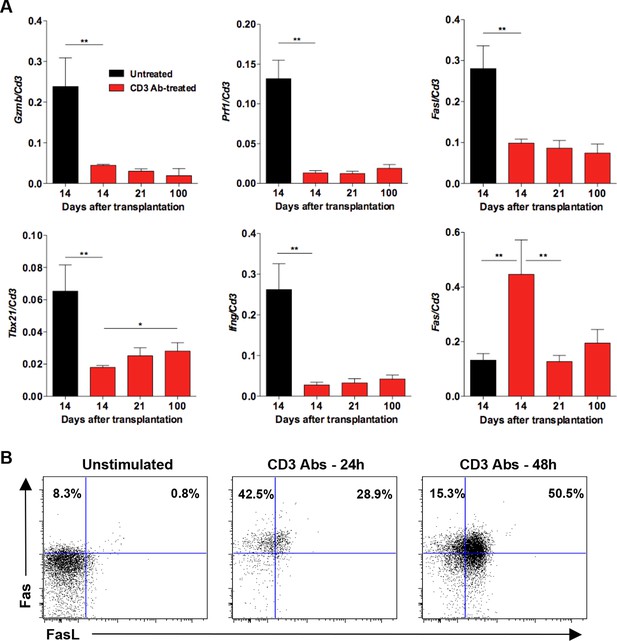
Expression of inflammatory, cytotoxic and apoptotic markers by CD8+ T cells.
https://doi.org/10.7554/eLife.08133.006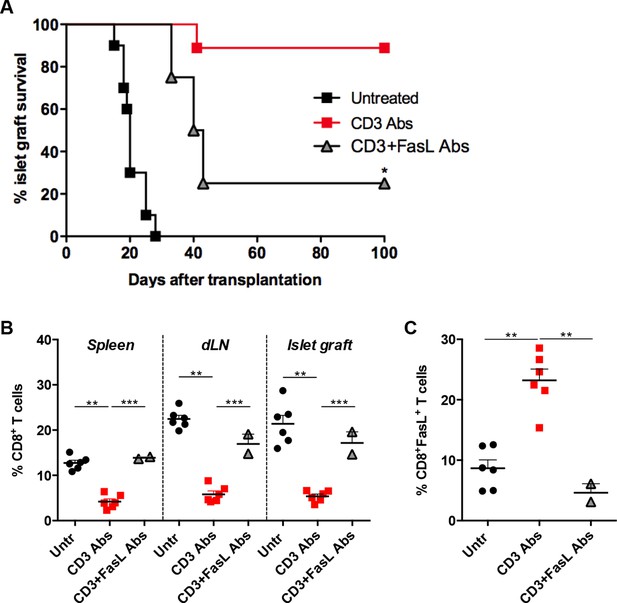
FasL blockade reversed CD3 antibody-induced transplant tolerance.
(A) Graft survival of BALB/c islets was measured in C57BL/6 mice treated at day 7 with CD3 antibodies (50 μg, 5 days) alone (n = 10) or combined with neutralizing antibodies against FasLigand injected at the dose of 500 μg i.p. on day 6, 7 and 8 post-transplant (n = 4) (*p<0.02 between anti-CD3 and anti-CD3+anti-FasL Ab-treated mice). (B) Additional untreated (n = 6), CD3 Ab (n = 6) or CD3+FasL Ab (n = 2)-treated mice were sacrificed on day +14 post-transplant and proportion of CD8+ T cells was analyzed in the spleen, renal draining lymph nodes (dLN) and the islet allografts (**p<0.003, ***p<0.0006). (C) Expression of FasL by graft infiltrating CD8+ T cells isolated on day +14 from untreated (n = 6), CD3 Abs (n = 6) or CD3+FasL Abs (n = 2)-treated recipients (**p<0.002).
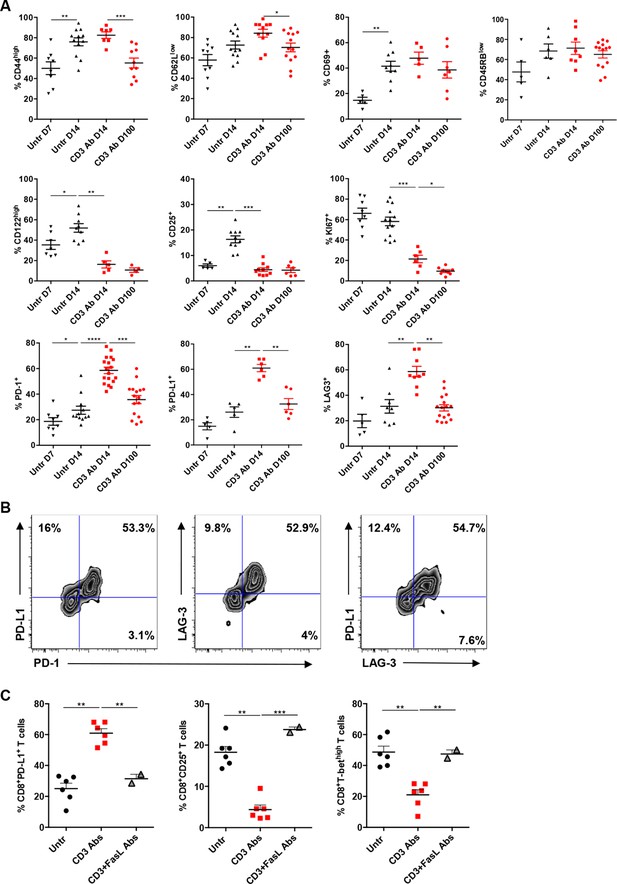
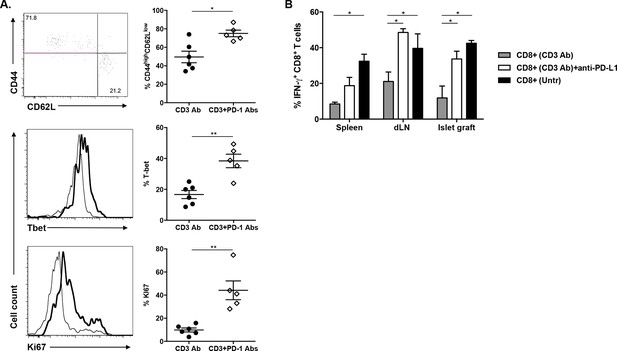
Phenotypic and functional characteristics of tolerant CD8+ T cells.
Pancreatic islet allografts were recovered from C57BL/6 mice after CD3 Ab treatment administered at day +7 post-transplant. (A) Expression of CD44, CD62, CD69, CD45RB, CD122, Ki67, CD25, PD-1, PD-L1 and LAG-3 (6–16/group) was evaluated on CD8+ T cells on day +7, day +14 and day +100 post-transplant (*p<0.05, **p<0.01, ***p<0.001). (B) Co-expression of PD-1/PDL-1, PD-1/LAG-3 and PD-L1/LAG-3 on graft-infiltrating CD8+ T cells recovered from CD3 Ab-treated mice on day +14 post-transplant. (C) Expression of PD-L1, CD25 and T-bet by graft infiltrating CD8+ T cells isolated on day14 from untreated, CD3 Abs or CD3FasL Abs-treated recipients (n = 2–6/group) (**p<0.005).
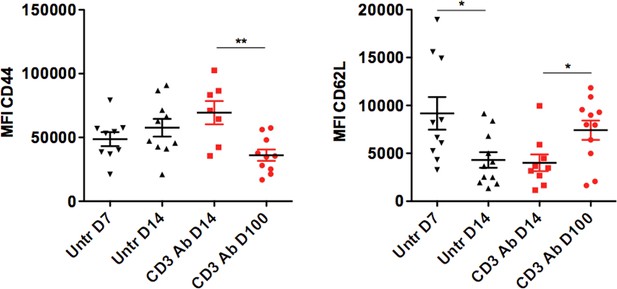
Mean fluorescence intensity of CD44 and CD62L expressed by intragraft CD8+ T cells after CD3 Ab therapy.
https://doi.org/10.7554/eLife.08133.009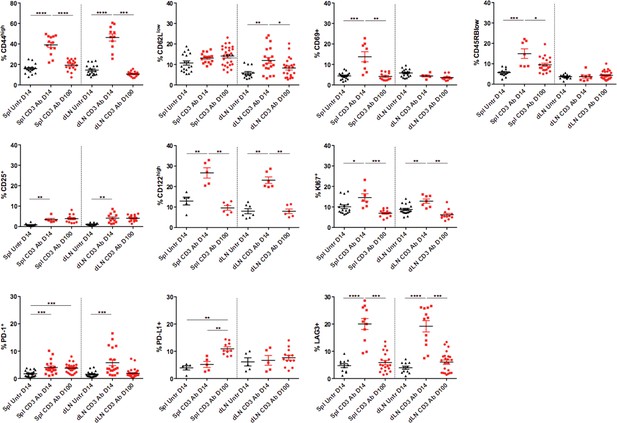
Phenotype of peripheral CD8+ T cells after CD3 Ab therapy.
https://doi.org/10.7554/eLife.08133.010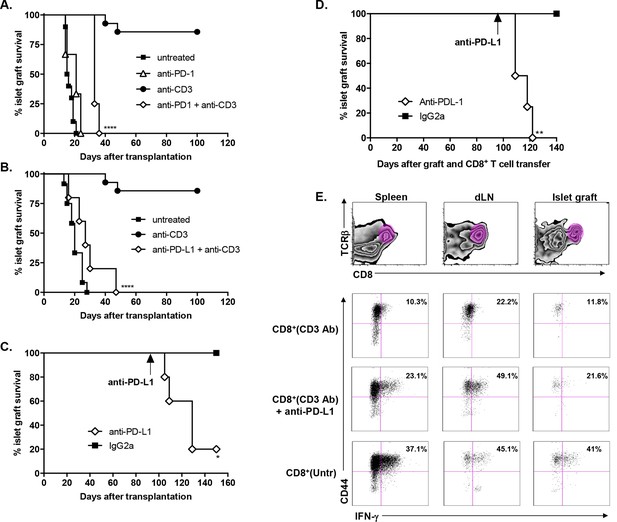
Transplant tolerance and CD8+ T cell anergy rely on the PD-1/PDL-1 pathway.
Graft survival of BALB/c islets was measured in C57BL/6 mice treated at day 7 with a combination of anti-CD3 F(ab’)2 and neutralizing antibodies against PD-1 (panel A, n = 5) or PD-L1 (panel B, n = 5) injected at the dose of 500 μg i.p. every other day for a total of 5 injections. (****p<0.0001 between anti-CD3 and anti-CD3+anti-PD-1/anti-PD-L1 Ab-treated mice). (C) C57BL/6 mice showing long-term islet graft acceptance after CD3 Ab therapy were treated on day +100 post-transplant with anti-PD-L1 antibodies or isotype control IgG2a (n = 5). Graft rejection occurred 2–3 weeks later (*p<0.03). (D) CD8+ T lymphocytes were purified from the spleen of CD3 Ab-treated tolerant C57BL/6 mice and were transferred into C57BL/6 Rag-/- mice (3x106/recipient). Recipient mice were grafted with pancreatic islets from BALB/c on day 0 and graft survival was monitored. On day +100 post-transplant, anti-PD-L1 antibodies or isotype control IgG2a were injected (n = 5) (**p<0.007). (E) Tolerant CD8+ T cells were detected in the spleen, draining lymph nodes and islet allograft of C57BL/6 Rag-/- recipients. IFNγ production and CD44 expression were compared to the ones of CD8+ T cells recovered after treatment with anti-PD-L1 antibodies or of CD8+ T cells issued from untreated C57BL-6 mice and rejecting the islet graft.
Administration of anti-PD-1 or anti-PD-L1 Abs reversed CD3 Ab-induced unresponsiveness of CD8+ T cells.
https://doi.org/10.7554/eLife.08133.012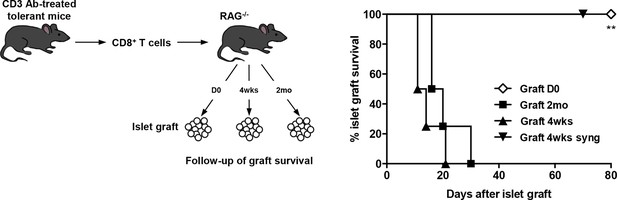
CD8+ T cell anergy depends on the presence of the antigens.
CD8+ T lymphocytes were purified from CD3 Ab-treated tolerant C57BL/6 mice on day +100 post-transplant and were adoptively transferred into C57BL/6 Rag-/- mice (3x106/recipient, day 0). C57BL/6 Rag-/- recipients were transplanted with pancreatic islets from BALB/c donors either on day 0 (D0, n = 4) or 4 weeks (4wks, n = 4) or 2 months (2mo, n = 4) after cell transfer (**p<0.007). Syngeneic islets from C57BL/6 donors were grafted 4 weeks after cell transfer and were used as controls.
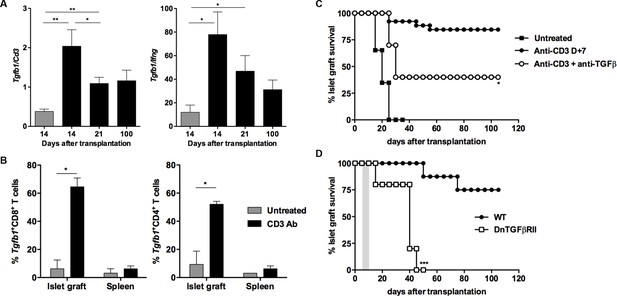
CD3 antibody-induced transplant tolerance depends on in situ TGFβ production and signaling in T cells.
(A) Expression of Tgfb1 mRNA and evaluation of the Tgfb1/Ifng ratio in pancreatic islet allografts recovered at day 14 from untreated mice or day 14, 21 and 100 after transplantation from CD3 Ab-treated recipients. Data are shown as mean ± SEM of 5–9 individual samples (*p<0.05, **p<0.01). (B) Single cell PCR: individual CD4+ (n = 48) and CD8+ (n = 60) T cells were sorted on day +14 post-transplant from the spleen or islet allografts recovered from C57BL/6 mice treated or not with CD3 antibodies. Expression of Tgfb1 mRNA was measured in each cell. Results show the proportion of CD4+ and CD8+ T cells positive for Tgfb expression (*p<0.05). (C) Graft survival of BALB/c islets in wild-type C57BL/6 mice treated at day +7 with anti-CD3 F(ab’)2 and neutralizing TGFβ antibodies (1 mg/injection, twice a week for 3 weeks) (n = 4 to 8 per group, *p<0.05). (D) Abrogation of tolerance in DnTGFβRII C57BL/6 mice transplanted with BALB/c pancreatic islets and treated with CD3 antibodies on day7 post-transplant (n = 5 per group, ***p = 0.0002).
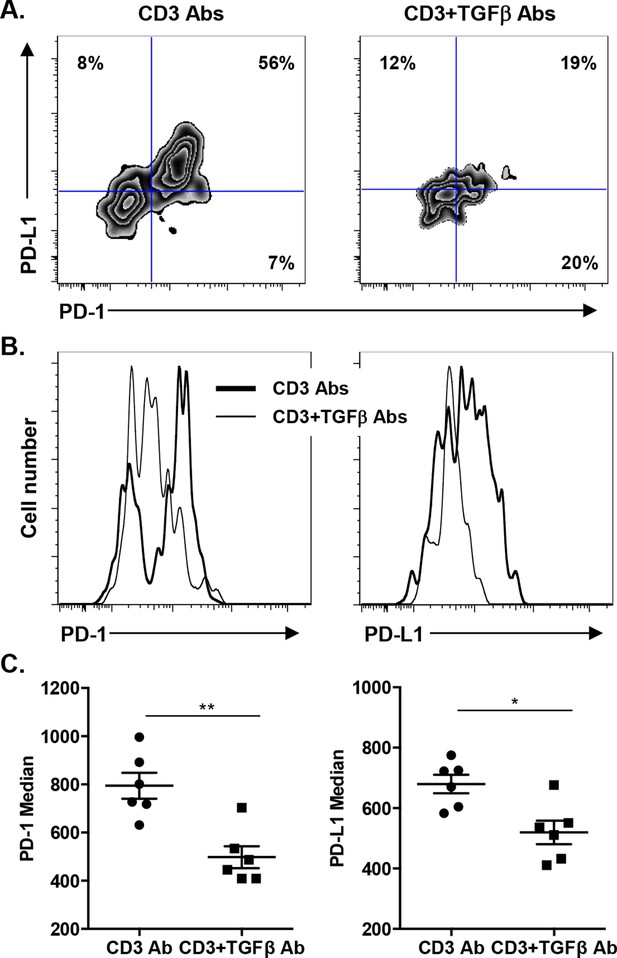
Induction of PD-1 and PD-L1 expression on intragraft CD8+ T cells is regulated by TGFβ.
C57BL/6 mice were transplanted with BALB/c pancreatic islets and treated at day 7 with anti-CD3 F(ab’)2 with or without neutralizing TGFβ antibodies. Mice were sacrificed on day 14 post-transplant and PD-1 and PD-L1 expressions were analyzed on graft-infiltrating CD8+ T cells. (A) Co-expression of PD-1 and PD-L1 on CD8+ T cells. (B) Histograms representing PD-1 and PD-L1 expression. (C) Median fluorescence intensity of PD-1 and PD-L1 (*p<0.02, **p<0.005).
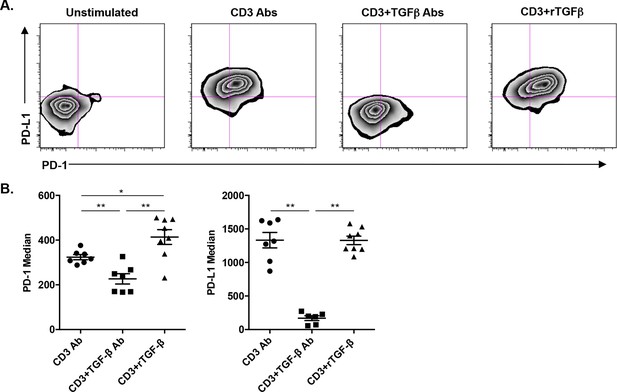
TGFβ modulates PD-1/PD-L1 expression on CD8+ T cells.
https://doi.org/10.7554/eLife.08133.016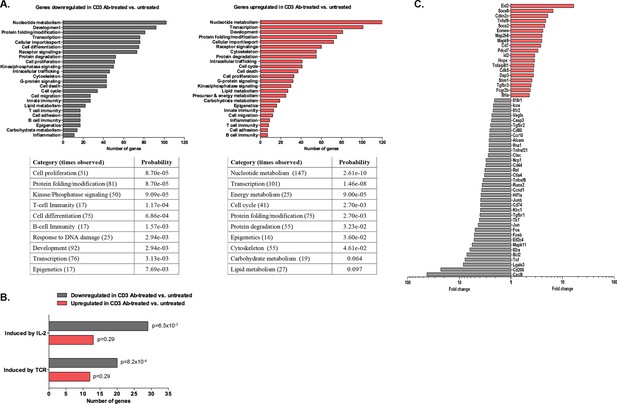
Transcriptomic analysis of tolerant intragraft CD8+ T cells after CD3 Ab therapy.
Five hundred graft-infiltrating CD8+ T lymphocytes were sorted from untreated or CD3 Ab-treated C57BL/6 mice, on day +14 and day +100, respectively (n = 8 per group). Agilent Whole Mouse Genome Microarrays were performed after amplification of RNAs. Functional grouping analysis used annotations derived from Gene Ontology (fold-change >2, p<0.05). (A) Bar chart showing the frequency of representative categories downregulated (left panel) or upregulated (right panel) in tolerant CD8+ T cells as compared to CD8+ T cells from untreated recipient mice. Below, statistically enriched categories were indicated by their adjusted p-value (only the top 10 categories, Fisher’s exact test with Benjamini-Hochberg correction for multiple testing). (B) Bar chart showing the frequency of target pathways induced by IL-2 ou TCR signaling. (C) Selection of immune genes that were downregulated (grey bars) or upregulated (red bars) in tolerant CD8+ T cells as compared to CD8+ T cells from untreated recipient mice (fold-change >2, p<0.05).
-
Figure 8—source data 1
Genes upregulated in anergic CD8+ T cells from CD3 Ab-treated recipient mice.
- https://doi.org/10.7554/eLife.08133.018
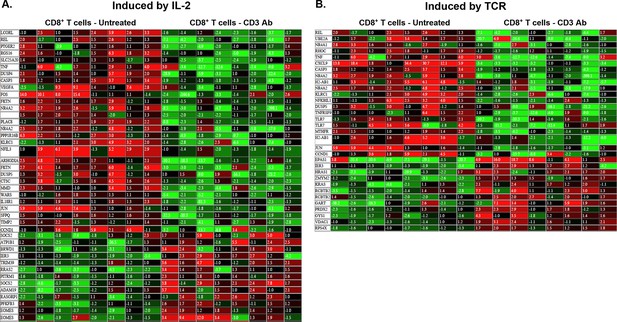
Heatmap of genes induced by IL-2 (A) or TCR (B) that are differentially expressed (>twofold, p<0.05) in CD8+ T cells recovered from pancreatic islet allografts of CD3 antibody-treated tolerant mice (day +100 post-transplant) or of untreated mice (day +14 post-transplant).
https://doi.org/10.7554/eLife.08133.019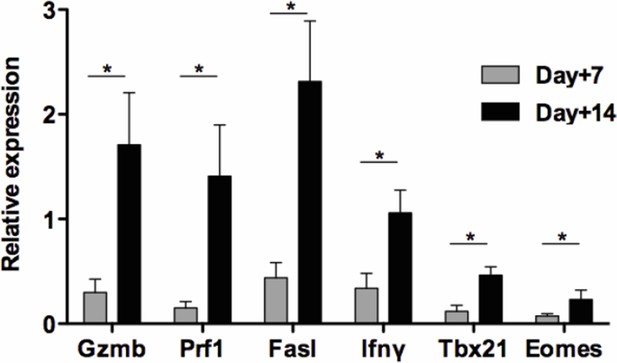
Kinetics of intragraft effector gene expression in untreated recipients.
Pancreatic islets from BALB/c mice were transplanted under the kidney capsule of C57BL/6 recipients. Allografts were recovered on day 7 and 14 post-transplant and expression Gzmb, Prf1, Fasl Ifnγ, Tbx21 and Eomes mRNA was analyzed by RT-qPCR (n = 4-5) (*p<0.05).
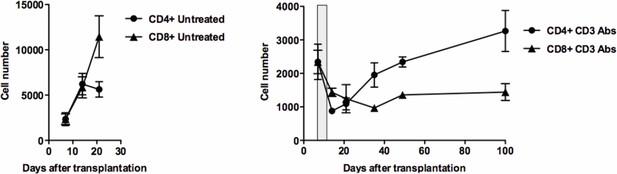
Intragraft T cell counts.Pancreatic islet allografts were recovered from untreated (left) or CD3 antibody-treated (right) mice and CD4+ and CD8+ T cells were counted at different time-points post-transplant.
CD3 antibody treatment was applied on day +7 post-transplant for 5 days (gray area).





