Passive accumulation of alkaloids in inconspicuously colored frogs refines the evolutionary paradigm of acquired chemical defenses
Figures
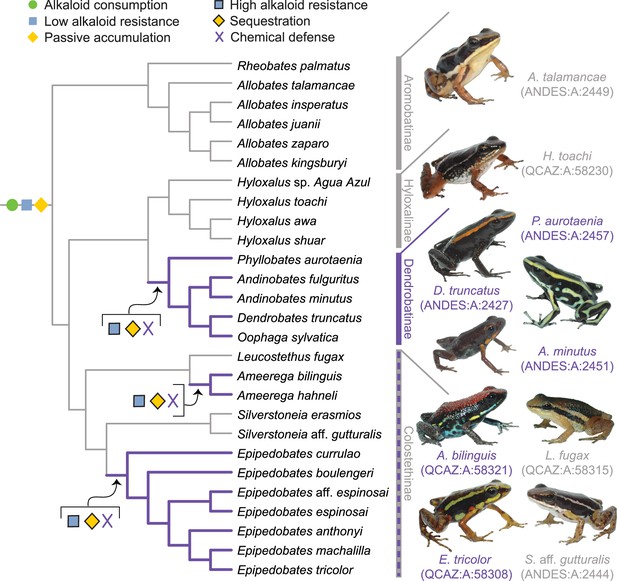
A new evolutionary model of toxin sequestration in Dendrobatidae.
We propose that alkaloid consumption, some level of alkaloid resistance, and passive accumulation were present in the most recent common ancestor of Dendrobatidae; enhanced resistance and sequestration mechanisms then arose later, resulting in the chemical defense phenotype. Our model places less emphasis on dietary changes compared to prior studies, and more strongly emphasizes novel molecular mechanisms (e.g. binding proteins and target-site insensitivity; Alvarez-Buylla et al., 2023; Tarvin et al., 2017; Tarvin et al., 2016). Purple lines indicate lineages with chemical defense. Gray lines indicate lineages that putatively lack chemical defense. All images of frogs were taken by RDT and are identified by their museum number.
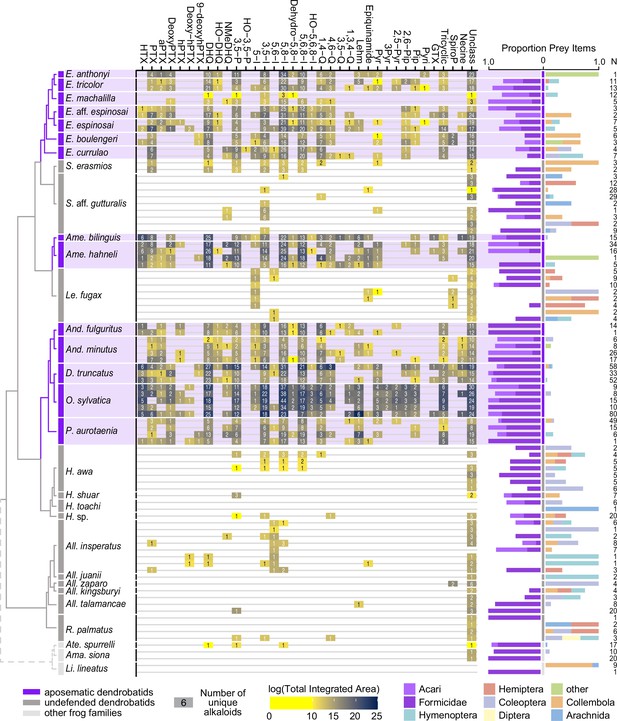
From left to right: an ultrametric tree showing phylogenetic relationships inferred previously (Wan et al., 2023) among sampled species with the three defended poison frog clades highlighted in purple, the undefended clades in dark gray, and non-dendrobatids in light gray (Bufonidae: Amazophrynella siona and Atelopus aff. spurrelli; Leptodactylidae: Lithodytes lineatus).
Tile color indicates the log of the total quantity of alkaloids in each class as measured by the sum of integrated areas of alkaloids of that class from GC-MS data per individual. The number in each tile indicates the number of alkaloids (including isomers) detected in each individual for each class. On the right are prey items recovered from the stomach of each individual, colored by arthropod group and scaled to 1 (total number of prey identified are shown under N). Note the large proportion of ants (Formicidae, dark purple) and mites (Acari, light purple) in many of the individuals compared to other prey types. See Supplementary file 1 for raw diet data and Supplementary file 4 for full alkaloid data. Poison-frog genera names are abbreviated as follows: All., Allobates; Ame., Ameerega; And., Andinobates; D., Dendrobates; E., Epipedobates; H., Hyloxalus; Le., Leucostethus; O., Oophaga; P., Phyllobates; R., Rheobates; S., Silverstoneia; Alkaloid class abbreviations are based on Daly et al., 2009; Daly et al., 2005 and are as follows: HTX, histrionicotoxins; PTX, pumiliotoxins; PTXB, pumiliotoxin B; aPTX, allopumiliotoxins; DeoxyPTX, deoxypumiliotoxins; hPTX, homopumiliotoxins; deoxy-hPTX, deoxy-homopumiliotoxins; DHQ, decahydroquinolines; NMeDHQ, N-methyldecahydroquinolines; HO-DHQ, hydroxy-decahydroquinolines; 3,5 P, 3,5-disubstituted pyrrolizidines; HO-3,5-P, hydroxy-3,5-disubstituted pyrrolizidines; 5-I, 5-substituted indolizidines; 3,5-I, 3,5-disubstituted indolizidines; 5,6-I, 5,6-disubstituted indolizidines; 5,8-I, 5,8-disubstituted indolizidines; Dehydro-5,8-I, dehydro-5,8-indolizidines; 5,6,8-I, 5,6,8-trisubstituted indolizidines; HO-5,6,8-I, hydroxy-5,6,8-trisubstituted indolizidines; 1,4-Q, 1,4-disubstituted quinolizidines; 4,6-Q, 4,6-disubstituted quinolizidines; 3,5-Q, 3,5-disubstituted quinolizidines; 1,3,4-Q, 1,3,4-trisubstituted quinolizidines; Lehm, lehmizidines; Epiquinamide, epiquinamide; 2-Pyr, 2-substituted pyrrolidine; 3-Pyr, 3-substituted pyrrolidine; 2,5-Pyr, 2,5-disubstituted pyrrolidines; Pyr, pyrrolizidine of indeterminate substitution; 2,6-Pip, 2,6-disubstituted piperidines; Pip, other piperidines; Pyri, pyridines (including epibatidine); GTX, gephyrotoxins; Tricyclic, coccinelline-like tricyclics; SpiroP, spiropyrrolizidines; Necine, unspecified necine base; Unclass, unclassified alkaloids without known structures.

Hypothesized physiological processes that interact to determine the defense phenotype: toxin intake, toxin elimination (Elim.), and toxin sequestration (Seq.).
A new paradigm: the passive-accumulation hypothesis for definitions. Although toxin intake sets a maximum for the total possible amount of toxin accumulation (Acc.), it cannot fully explain the defensive phenotype. We hypothesize that an undefended “no accumulation” phenotype is characterized by the absence of any ability to sequester toxins in combination with a high rate of elimination, resulting in 0 toxin accumulation (dashed gray lines); this phenotype is a likely ancestral state for many animals. In contrast, we hypothesize that an undefended passive-accumulation phenotype is characterized by lower elimination than the no accumulation phenotype, leading to a low amount of toxin accumulation (yellow lines). We hypothesize that a defended sequestration phenotype evolves from an intermediate passive-accumulation phenotype through the addition of novel sequestration mechanisms, and possibly even lower elimination rates, that result in high toxin accumulation and the defended phenotype (purple lines).
Tables
Range and median of alkaloid quantity (estimated by the sum of integrated areas) and alkaloid diversity (number of different compounds) by species from the GC-MS assessment.
The presumed chemical defense phenotype for poison frogs is given according to Santos and Cannatella, 2011. Purple rows highlight defended species. *From a UHPLC-HESI-MS/MS dataset for which alkaloids were not quantified. Note that the UHPLC-HESI-MS/MS and GC-MS assays differed in both instrument and analytical pipeline, so ‘Alkaloid Number’ values from the two assay types should not be compared to each other directly.
| Family | Subfamily | Species | Phenotype | Sample Size (frogs) | Log (Total Integrated Area) | Alkaloid Number | ||
|---|---|---|---|---|---|---|---|---|
| Range | Median | Range | Median | |||||
| Dendrobatidae | Aromobatinae | Rheobates palmatus | undefended | 4 | 13.07–14.24 | 14.00 | 1–4 | 1.5 |
| Dendrobatidae | Aromobatinae | Allobates insperatus | undefended | 8 | 13.47–15.44 | 14.99 | 1–9 | 5.0 |
| Dendrobatidae | Aromobatinae | Allobates juanii | undefended | 1 | 14.10 | 14.10 | 1 | 1.0 |
| Dendrobatidae | Aromobatinae | Allobates kingsburyi | undefended | 1 | 13.63 | 13.63 | 2 | 2.0 |
| Dendrobatidae | Aromobatinae | Allobates talamancae | undefended | 3 | 14.89–16.27 | 15.09 | 2–4 | 3.0 |
| Dendrobatidae | Aromobatinae | Allobates zaparo | undefended | 1 | 16.78 | 16.78 | 8 | 8.0 |
| Dendrobatidae | Colostethinae | Leucostethus fugax | undefended | 8 | 12.57–15.33 | 14.00 | 3–8 | 4.5 |
| Dendrobatidae | Colostethinae | Ameerega bilinguis | defended | 1 | 21.97 | 21.97 | 133 | 133.0 |
| Dendrobatidae | Colostethinae | Ameerega hahneli | defended | 4 | 20.21–22.29 | 21.68 | 85–140 | 128.5 |
| Dendrobatidae | Colostethinae | Silverstoneia flotator* | undefended | 12 | NA | NA | 0–1 | 0.0 |
| Dendrobatidae | Colostethinae | Silverstoneia aff. gutturalis | undefended | 9 | 11.80–17.33 | 15.40 | 1–10 | 3.0 |
| Dendrobatidae | Colostethinae | Silverstoneia erasmios | undefended | 2 | 14.70–16.11 | 15.41 | 15–15 | 15.0 |
| Dendrobatidae | Colostethinae | Epipedobates aff. espinosai | defended | 2 | 18.44–20.20 | 19.32 | 83–131 | 107.0 |
| Dendrobatidae | Colostethinae | Epipedobates anthonyi | defended | 1 | 20.54 | 20.54 | 127 | 127.0 |
| Dendrobatidae | Colostethinae | Epipedobates boulengeri | defended | 2 | 18.87–19.39 | 19.13 | 77–94 | 85.5 |
| Dendrobatidae | Colostethinae | Epipedobates currulao | defended | 2 | 19.49–19.68 | 19.59 | 99–105 | 102.5 |
| Dendrobatidae | Colostethinae | Epipedobates espinosai | defended | 2 | 18.82–21.33 | 20.08 | 85–146 | 115.5 |
| Dendrobatidae | Colostethinae | Epipedobates machalilla | defended | 2 | 12.98–15.67 | 14.32 | 8–38 | 23.0 |
| Dendrobatidae | Colostethinae | Epipedobates tricolor | defended | 2 | 18.36–19.07 | 18.72 | 91–114 | 102.5 |
| Dendrobatidae | Hyloxalinae | Hyloxalus awa | undefended | 7 | 0.00–16.05 | 13.58 | 0–12 | 3.0 |
| Dendrobatidae | Hyloxalinae | Hyloxalus shuar | undefended | 1 | 14.92 | 14.92 | 5 | 5.0 |
| Dendrobatidae | Hyloxalinae | Hyloxalus sp. Agua Azul | undefended | 1 | 14.30 | 14.30 | 8 | 8.0 |
| Dendrobatidae | Hyloxalinae | Hyloxalus toachi | undefended | 2 | 0.00–0.00 | 0.00 | 0–0 | 0.0 |
| Dendrobatidae | Dendrobatinae | Phyllobates aurotaenia | defended | 4 | 17.72–21.08 | 18.88 | 48–118 | 67.5 |
| Dendrobatidae | Dendrobatinae | Dendrobates truncatus | defended | 3 | 20.05–23.95 | 20.42 | 111–172 | 115.0 |
| Dendrobatidae | Dendrobatinae | Oophaga sylvatica | defended | 5 | 22.86–24.85 | 23.76 | 152–189 | 175.0 |
| Dendrobatidae | Dendrobatinae | Andinobates fulguritus | defended | 2 | 20.09–20.51 | 20.30 | 80–85 | 82.5 |
| Dendrobatidae | Dendrobatinae | Andinobates minutus | defended | 4 | 16.57–18.77 | 18.07 | 34–80 | 66.0 |
| Bufonidae | Amazophrynella siona | NA | 2 | 14.12–14.40 | 14.26 | 1–1 | 1.0 | |
| Bufonidae | Atelopus aff. spurrelli | NA | 1 | 11.58 | 11.58 | 4 | 4.0 | |
| Eleutherodactylidae | Eleutherodactylus cystignathoides* | NA | 3 | NA | NA | 0–0 | 0.0 | |
| Leptodactylidae | Leptodactylinae | Lithodytes lineatus | NA | 2 | 0.00–0.00 | 0.00 | 0–0 | 0.0 |
Additional files
-
Supplementary file 1
Stomach content data for every individual.
- https://cdn.elifesciences.org/articles/100011/elife-100011-supp1-v1.xlsx
-
Supplementary file 2
A summary of data available on alkaloid detection in undefended lineages of poison frogs.
- https://cdn.elifesciences.org/articles/100011/elife-100011-supp2-v1.docx
-
Supplementary file 3
Collection localities, specimen numbers, size, sex, and summary of alkaloid quantities and diversity for each individual.
- https://cdn.elifesciences.org/articles/100011/elife-100011-supp3-v1.docx
-
Supplementary file 4
Alkaloid-level data for every individual analyzed by GC-MS.
- https://cdn.elifesciences.org/articles/100011/elife-100011-supp4-v1.csv
-
Supplementary file 5
S5a A feature table including information on Silverstoneia flotator and Eleutherodactylus cystignathoides skin alkaloids; S5b identifying information for samples corresponding to run numbers listed in Table S5a columns.
- https://cdn.elifesciences.org/articles/100011/elife-100011-supp5-v1.xlsx
-
Supplementary file 6
List of the subset of classes and most specific classes of compounds in Silverstoneia flotator annotated as alkaloids (“Alkaloid Pathway” of NPClassifier) at >99% probability, their presence/absence in Eleutherodactylus cystignathoides, whether the compound is from one of the classes of lipophilic alkaloids listed in the Daly database, and whether the molecular formula for the metabolite is found in the Daly database.
- https://cdn.elifesciences.org/articles/100011/elife-100011-supp6-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/100011/elife-100011-mdarchecklist1-v1.docx





