Prominin 1 and Tweety Homology 1 both induce extracellular vesicle formation
Figures
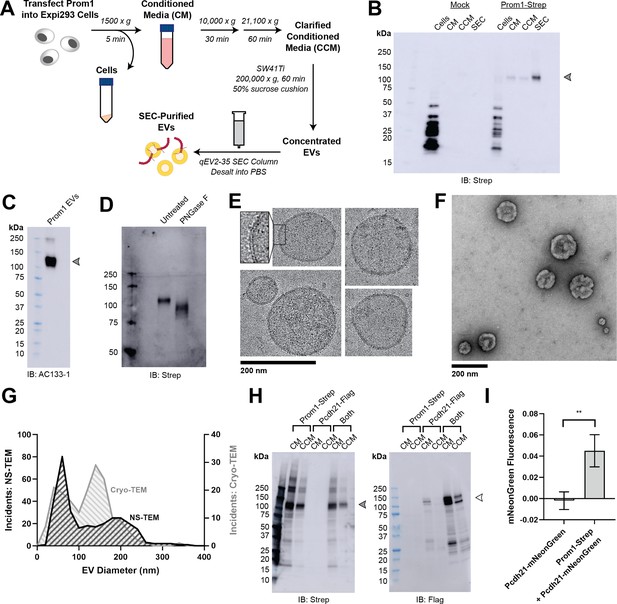
Reconstitution and purification of Prom1 EVs.
(A) Extracellular vesicle (EV) expression and purification graphic protocol. (B) Anti-Strep immunoblot of cell pellet, conditioned media (CM), clarified conditioned media (CCM), or size-exclusion chromatography (SEC)-purified EVs from mock-transfected or Prom1-Strep-transfected Expi293 cells. Arrowhead indicates the expected molecular weight of Prominin 1 (Prom1). (C) AC133-1 immunoblot of SEC-purified Prom1 EVs. Arrowhead indicates the expected molecular weight of Prom1. (D) Anti-Strep immunoblot of Prom1 EVs treated with or without PNGase F to remove N-glycan moieties. (E) Cryo-transmission electron microscopy (cryo-TEM) images of purified Prom1 EVs. Inset image is magnified to emphasize membrane bilayer density. Images are lowpass filtered to 5 Å to enhance contrast. (F) Negative-stain transmission electron microscopy (NS-TEM) image of purified Prom1 EVs. (G) Measured diameters of Prom1 EVs from cryo-TEM or NS-TEM images (n = 322 and n = 176 for NS-TEM and cryo-TEM measurements, respectively). (H) Anti-Strep (Prom1) and anti-Flag (Pcdh21) immunoblots of CM and CCM from cells transfected with Prom1-Strep, Pcdh21-Flag, or both. Note that Pcdh21 is only detected in CCM when co-expressed with Prom1. Filled and empty arrows indicate expected molecular weights of glycosylated Prom1-Strep and Pcdh21-Flag, respectively. (I) Comparative fluorescence measurements of Pcdh21-mNeonGreen co-immunoprecipitated with or without Prom1-Strep (n = 3, **p < 0.01 by Student’s two-tailed unpaired t test).
-
Figure 1—source data 1
Raw source gel and blot images.
- https://cdn.elifesciences.org/articles/100061/elife-100061-fig1-data1-v2.zip
-
Figure 1—source data 2
Labelled source gel and blot images.
- https://cdn.elifesciences.org/articles/100061/elife-100061-fig1-data2-v2.zip
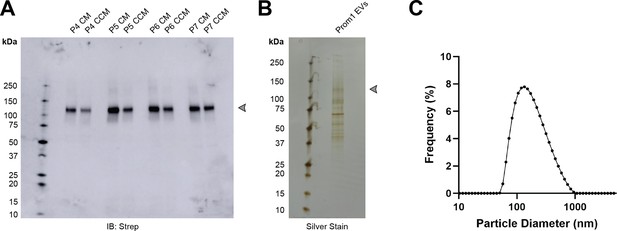
Prom1 induces formation of extracellular vesicles.
(A) Anti-Strep immunoblot of conditioned media (CM) or clarified conditioned media (CCM) from a stable polyclonal Expi293 cell line expressing lentiviral-transduced Prom1-Strep. Arrowhead indicates the expected position of glycosylated Prom1-Strep. P4, P5, P6, and P7 indicate the passage number of the suspension cell culture. (B) Total protein stain of purified Prom1-Strep EVs. Arrowhead indicates the expected position of glycosylated Prom1-Strep. (C) Dynamic light scattering (solution size) measurement of purified Prom1 EVs.
-
Figure 1—figure supplement 1—source data 1
Raw source gel and blot images.
- https://cdn.elifesciences.org/articles/100061/elife-100061-fig1-figsupp1-data1-v2.zip
-
Figure 1—figure supplement 1—source data 2
Labelled source gel and blot images.
- https://cdn.elifesciences.org/articles/100061/elife-100061-fig1-figsupp1-data2-v2.zip
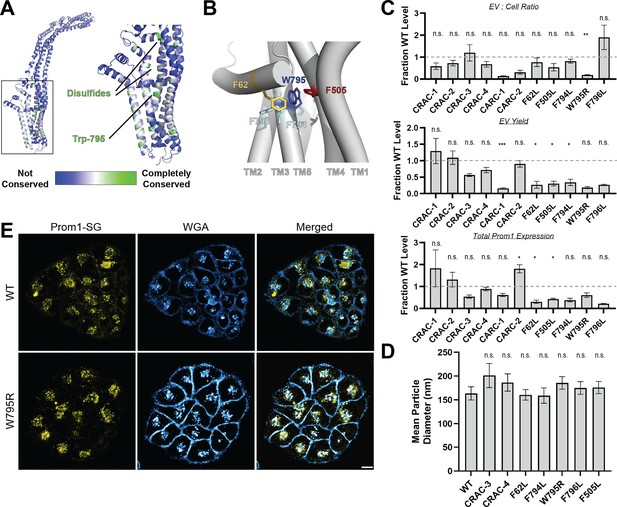
Mutations in the Prom1 transmembrane domain impair EV formation.
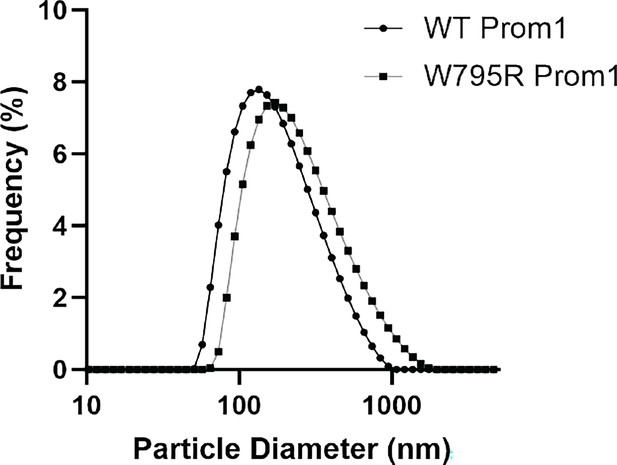
(A) AlphaFold2 model of human Prominin 1 (Prom1; Jumper et al., 2021) with residues color coded by level of conservation across a multiple-sequence alignment of metazoan prominin sequences. (B) Possible network of interactions between Trp-795 and several adjacent aromatic residues in human Prom1. (C) Quantification of anti-Strep immunoblots of cellular and clarified conditioned media pools of Prom1-Strep mutants relative to wild-type Prom1-Strep (n = 3, n.s.p > 0.0045, *p < 0.0045, **p < 0.0009, ***p < 0.00009 by Student’s two-tailed unpaired t test with significance thresholds adjusted by Bonferroni correction). Raw blot images from which measurements are derived are included in Figure 2—figure supplement 4. (D) Mean particle diameter of purified Prom1-Strep EVs measured by dynamic light scattering. Error bars indicate standard deviation (SD) (n = 5, n.s.p > 0.007, *p < 0.007 by Student’s two-tailed unpaired t test with significance thresholds adjusted by Bonferroni correction). (E) Confocal fluorescence microscopy images of HeLa cells stably expressing WT (top) or W795R (bottom) Prom1-StayGold (yellow), stained with wheat germ agglutinin (WGA) (blue). Scale bar is 10 μm. Line scan traces across cell junctions are included in Figure 2—figure supplement 5.
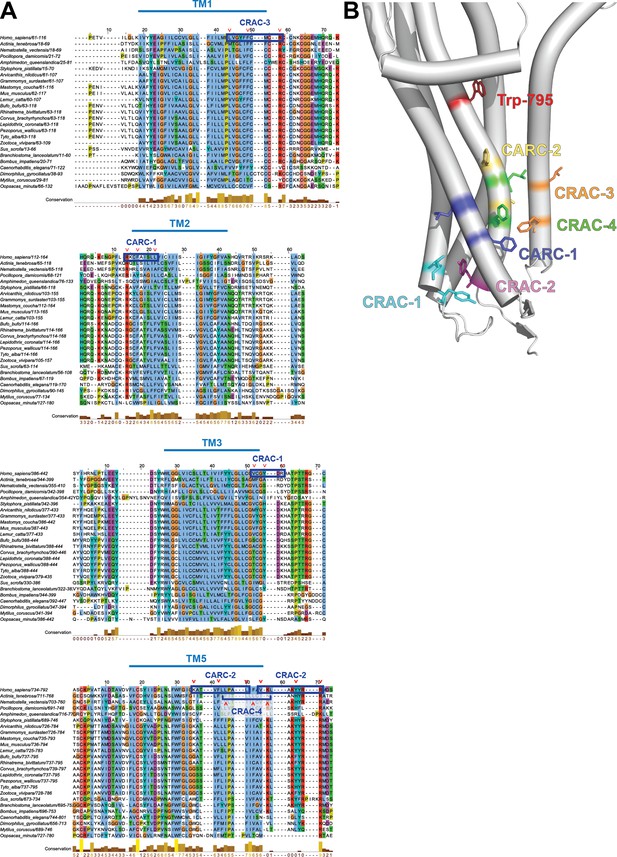
Putative cholesterol-binding motifs in the Prom1 transmembrane domain.
(A) Multiple sequence alignment of metazoan Prom1 focused on transmembrane domains 1, 2, 3, and 5, with human CRAC and CARC domains highlighted. Red carats indicate key charged, aromatic, or hydrophobic residues that define the CRAC and CARC domains. Alignment visualized using Jalview (Clamp et al., 2004). (B) Trp-795, CRAC-1, CRAC-2, CRAC-3, CRAC-4, CARC-1, and CARC-2 mutation sites superposed onto an AlphaFold2 model (Jumper et al., 2021) of the transmembrane domain of Prom1.
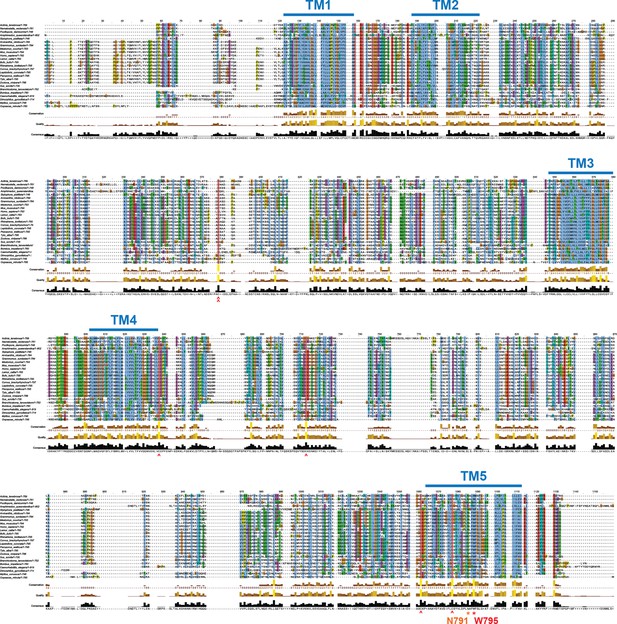
Conservation analysis of metazoan Prom1 sequences.
Multiple-sequence alignment of prominin sequences from metazoa with the five transmembrane segments indicated. Red asterisk indicates perfectly conserved non-cysteine residues. Orange asterisk indicates less-than-perfectly conserved non-cysteine residues of interest. Red carat indicates cysteines predicted to form internal disulfides. Double red carat indicates cysteines that are not predicted to form internal disulfides. Alignment visualized using Jalview (Clamp et al., 2004).
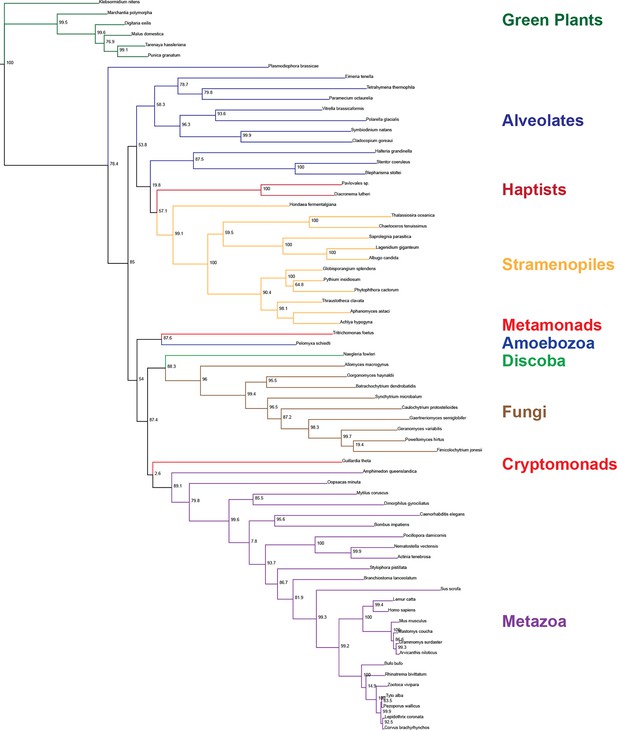
Phylogenetic analysis of Prom1 sequences.
Inferred phylogenetic relationships between putative prominin homologs identified across eukaryotes. Node labels indicate aLRT branch supports (Anisimova and Gascuel, 2006). Tree visualized using IcyTree (Vaughan, 2017).
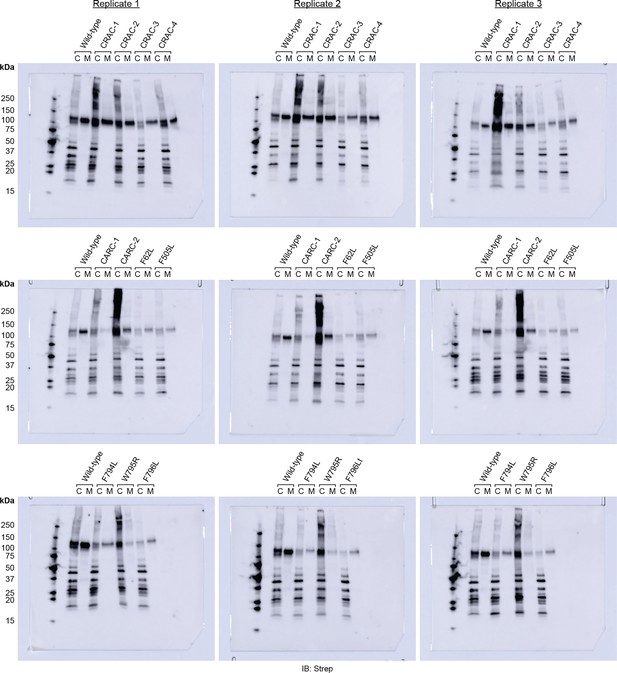
Blot images used for EV quantification in Figure 1C.
Anti-Strep immunoblots used for quantification of EV production by Prom1 mutants in Figure 2C. Lanes are labeled “C” for cells and “M” for clarified conditioned media.
-
Figure 2—figure supplement 4—source data 1
Raw source gel and blot images.
- https://cdn.elifesciences.org/articles/100061/elife-100061-fig2-figsupp4-data1-v2.zip
-
Figure 2—figure supplement 4—source data 2
Labelled source gel and blot images.
- https://cdn.elifesciences.org/articles/100061/elife-100061-fig2-figsupp4-data2-v2.zip
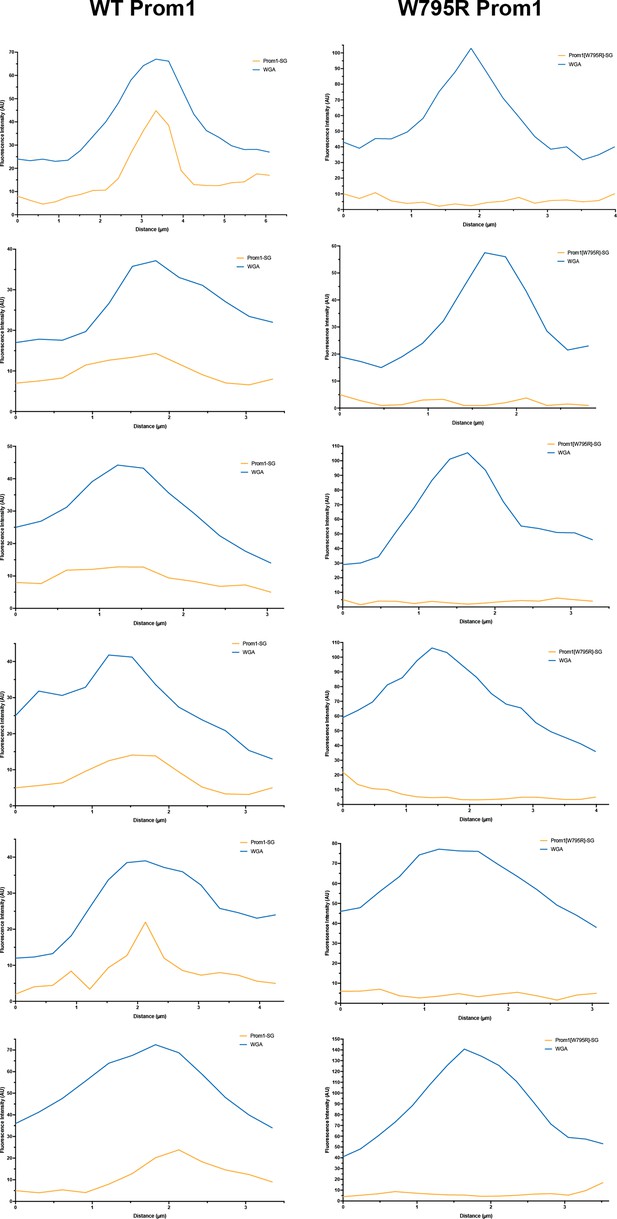
Line scan traces of Prom1 signal in cell lines.
Line scan traces across cell junctions (n = 6) for plasma membrane (WGA) (blue) or Prom1-mStayGold fluorescence signal for WT (left panels) or W795R (right panels) Prom1 (yellow).
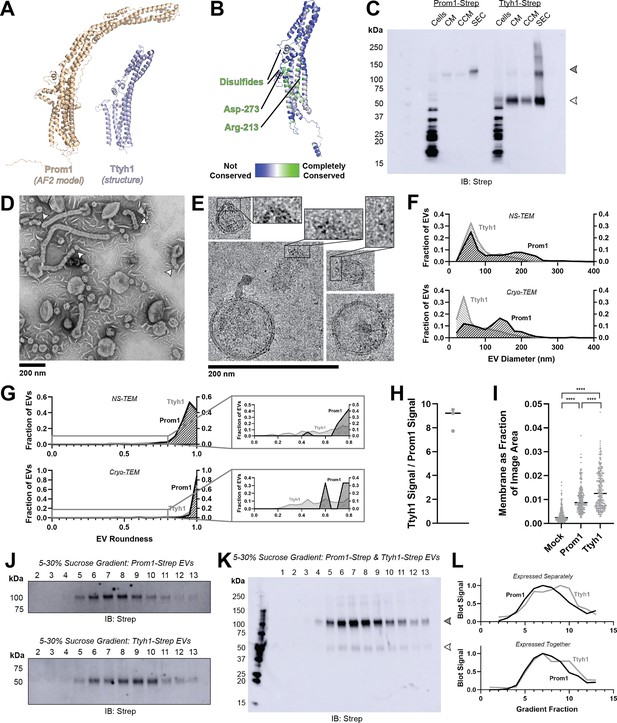
Prominin homolog Ttyh1 produces EVs.
(A) Comparison of a Ttyh1 subunit from cryo-transmission electron microscopy (cryo-TEM) structure 7P5J (Sukalskaia et al., 2021) with an AlphaFold2-predicted Prominin 1 (Prom1) monomer (Jumper et al., 2021). (B) Residue-level conservation among metazoan Tweety Homology (Ttyh) proteins plotted onto a subunit of human Ttyh1. No residue analogous to Prom1 Trp-795 is present in Ttyh. (C) Anti-Strep immunoblot comparing Prom1- and Ttyh1-Strep extracellular vesicles (EVs) throughout different stages of purification. Filled and empty arrowheads indicate the expected positions of Prom1 and Ttyh1, respectively. Doublet and higher bands in Ttyh1 lanes are products of on-gel disulfide crosslinking in concentrated samples. (D) Representative negative-stain transmission electron microscopy (NS-TEM) images of Ttyh1-Strep EVs. White arrowheads indicate possible sites of EV fission. (E) Representative cryo-TEM images of Ttyh1-Strep EVs. Magnified insets show bilayer density at highly curved membrane segments. Images are lowpass filtered to 5 Å to enhance contrast. (F) Comparison of Prom1 or Ttyh1 EV diameter in NS-TEM or cryo-TEM images (n = 322, n = 1357, n = 176, and n = 2224 for Prom1 NS-TEM, Ttyh1 NS-TEM, Prom1 cryo-TEM, and Ttyh1 cryo-TEM measurements, respectively). (G) Comparison of Prom1 or Ttyh1 EV roundness in NS-TEM or cryo-TEM images. Secondary plots only include EVs with roundness ≤0.8 (n = 322, n = 1357, n = 122, and n = 1546 for Prom1 NS-TEM, Ttyh1 NS-TEM, Prom1 cryo-TEM, and Ttyh1 cryo-TEM measurements, respectively). (H) Quantification of Prom1 and Ttyh1 protein levels in EVs from anti-Strep Western blots (n = 3). Immunoblots used for quantification are included in Figure 3—figure supplement 1A. (I) Quantification of total EV membrane area from NS-TEM micrographs (n = 236, ****p < 0.0001 by unpaired Mann–Whitney test). Representative micrographs used for quantification are included in Figure 3—figure supplement 1. (J) Anti-Strep immunoblots of fractions from sucrose gradient equilibrium sedimentation of Prom1-Strep EVs (top) or Ttyh1-Strep EVs (bottom). (K) Anti-Strep immunoblots of fractions from sucrose gradient equilibrium sedimentation of EVs from cells co-expressing Prom1-Strep and Ttyh1-Strep. Filled and empty arrowheads indicate the expected positions of Prom1 and Ttyh1, respectively. (L) Quantification of immunoblots in panels K (top) and L (bottom).
-
Figure 3—source data 1
Raw source gel and blot images.
- https://cdn.elifesciences.org/articles/100061/elife-100061-fig3-data1-v2.zip
-
Figure 3—source data 2
Labelled source gel and blot images.
- https://cdn.elifesciences.org/articles/100061/elife-100061-fig3-data2-v2.zip
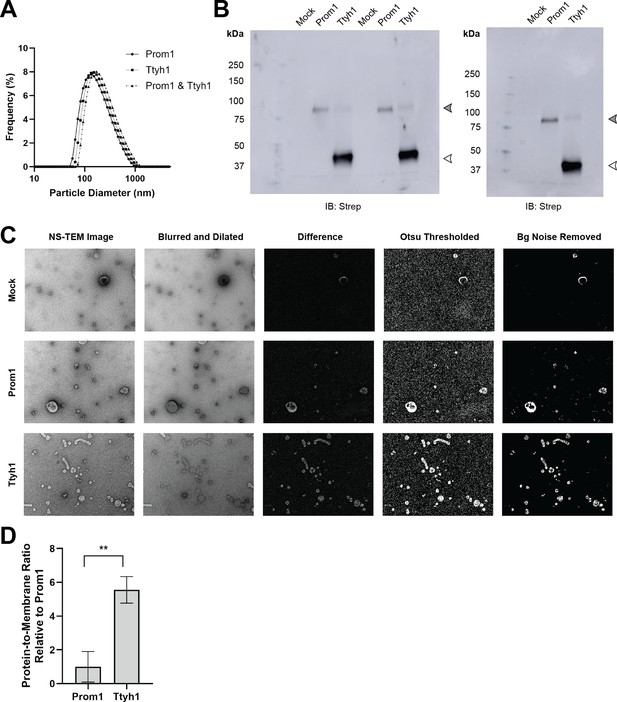
Characterization of Prom1 and Ttyh1 EVs.
(A) Dynamic light scattering measurement of EV diameter for purified Prom1-Strep, Ttyh1-Strep, or Prom1-Strep + Ttyh1-Strep co-expression EVs. (B) Anti-Strep immunoblots used for relative quantification of Prom1 and Ttyh1 levels in purified EVs. Filled and empty arrowheads indicate the expected positions of Prom1 and Ttyh1, respectively. (C) Representative NS-TEM micrographs and image processing intermediates used for membrane area quantification. (D) Quantification of relative protein-to-membrane ratio for Prom1 and Ttyh1 EVs using data from Figure 3I, J. Error bars indicate S.D. (n = 3, ** p < 0.01 by Student’s two-tailed unpaired t test).
-
Figure 3—figure supplement 1—source data 1
Raw source gel and blot images.
- https://cdn.elifesciences.org/articles/100061/elife-100061-fig3-figsupp1-data1-v2.zip
-
Figure 3—figure supplement 1—source data 2
Labelled source gel and blot images.
- https://cdn.elifesciences.org/articles/100061/elife-100061-fig3-figsupp1-data2-v2.zip
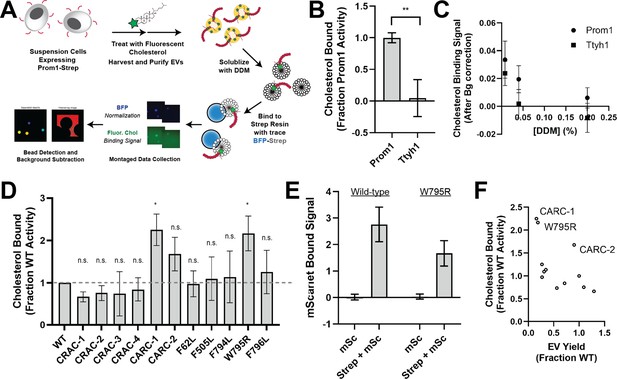
Ttyh1 binds cholesterol less stably than Prom1.
(A) Cholesterol co-immunoprecipitation (chol-IP) assay graphic protocol. (B) Comparison of relative cholesterol bound by Prom1-Strep or Ttyh1-Strep (n = 3, **p < 0.01 by Student’s two-tailed unpaired t test). (C) Fluorescent cholesterol co-immunopurified by Prom1-Strep or Ttyh1-Strep at different concentrations of n-dodecyl-β-D-maltoside (DDM) detergent. Error bars indicate standard deviation (SD) (n = 3). (D) BODIPY-cholesterol-binding measurements for WT and mutant variants of Prominin 1 (Prom1). Error bars indicate SD (n = 3, n.s.p > 0.0045, *p < 0.0045 by Student’s two-tailed unpaired t test with significance thresholds adjusted by Bonferroni correction). (E) Red fluorescence signal from anti-Strep immunopurification of DDM-solubilized extracellular vesicles (EVs) from cells expressing Prom1-mScarlet (mSc) or both Prom1-mScarlet and Prom1-Strep (Strep + mSc). Error bars indicate SD (n = 3). (F) Comparison between EV yield (Figure 2C) and chol-IP fluorescent cholesterol binding (D), with notable outliers labeled.
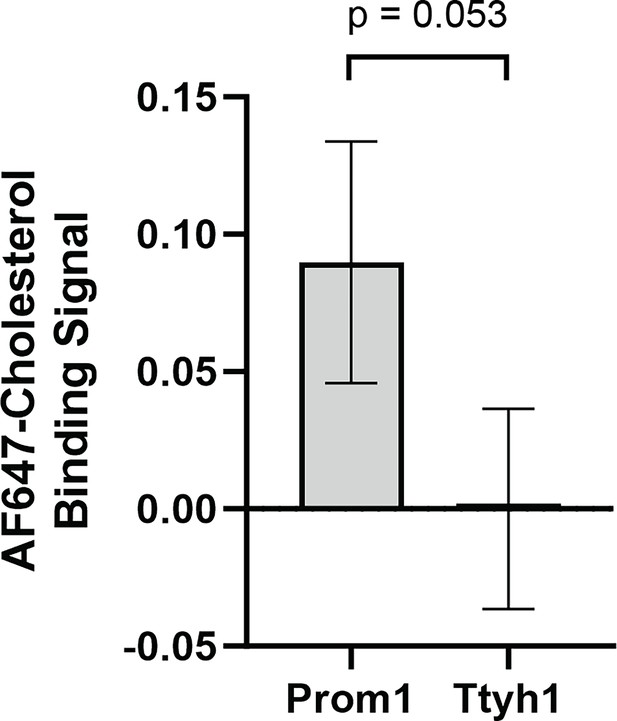
Fluorescent cholesterol binding by Prom1 and Ttyh1.
Cholesterol co-immunopurification (Chol-IP) measurement of AlexaFluor647-cholesterol binding in Prom1-Strep or Ttyh1-Strep EVs. Error bars indicate S.D. (n = 3, p = 0.053 by Student’s two-tailed unpaired t test).
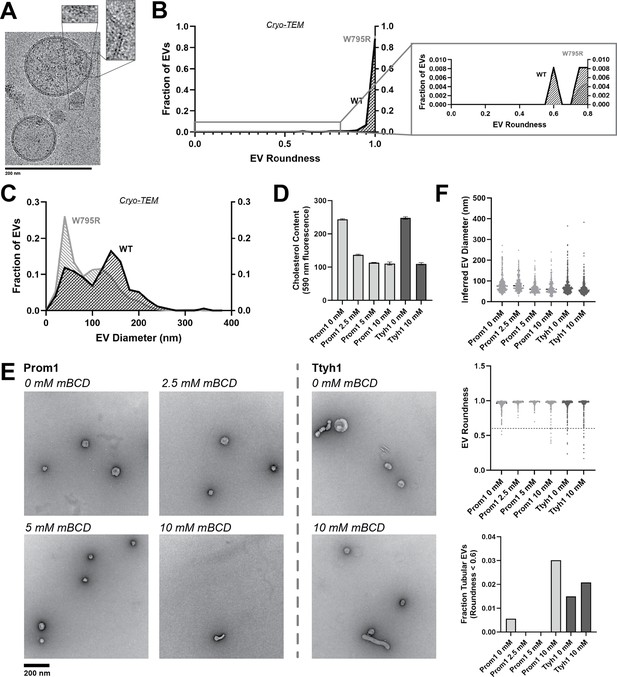
Cholesterol binding stability inversely correlates with Prom1 EV membrane bending.
(A) Representative cryo-transmission electron microscopy (cryo-TEM) images of W795R Prom1-Strep extracellular vesicles (EVs). Magnified insets show bilayer density. Images are lowpass filtered to 5 Å to enhance contrast. (B) Comparison of WT or W795R EV roundness in cryo-TEM images. Secondary plot only includes EVs with roundness ≤0.8 (n = 176 and n = 1211 for WT and W795R Prominin 1 (Prom1) cryo-TEM measurements, respectively.) (C) Comparison of WT or W795R Prom1 EV diameter in cryo-TEM images (n = 122 and n = 821 for WT and W795R Prom1 cryo-TEM measurements, respectively). (D) Cholesterol content of Prom1- or Ttyh1-purified EV samples after treatment with methyl-beta cyclodextrin (mBCD). Error bars indicate standard deviation (SD) (n = 3). (E) Representative negative-stain transmission electron microscopy (NS-TEM) images of purified mBCD-treated EVs. (F) Quantification of EV diameter (top) and roundness (middle) from NS-TEM images, as well as quantification of tubulated EVs (roundness <0.6) (bottom).
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/100061/elife-100061-mdarchecklist1-v2.docx
-
Supplementary file 1
Prom1 mutants used in this study.
- https://cdn.elifesciences.org/articles/100061/elife-100061-supp1-v2.docx