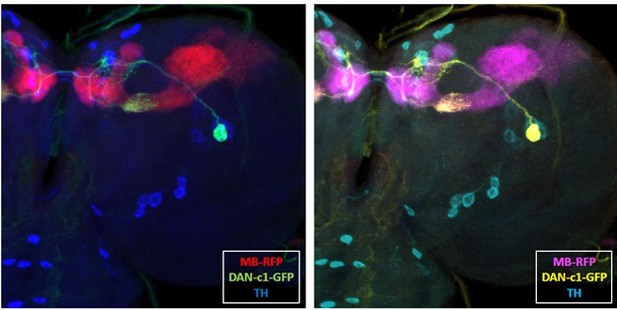
A pair of dopaminergic neurons DAN-c1 mediate Drosophila larval aversive olfactory learning through D2-like receptors
Figures
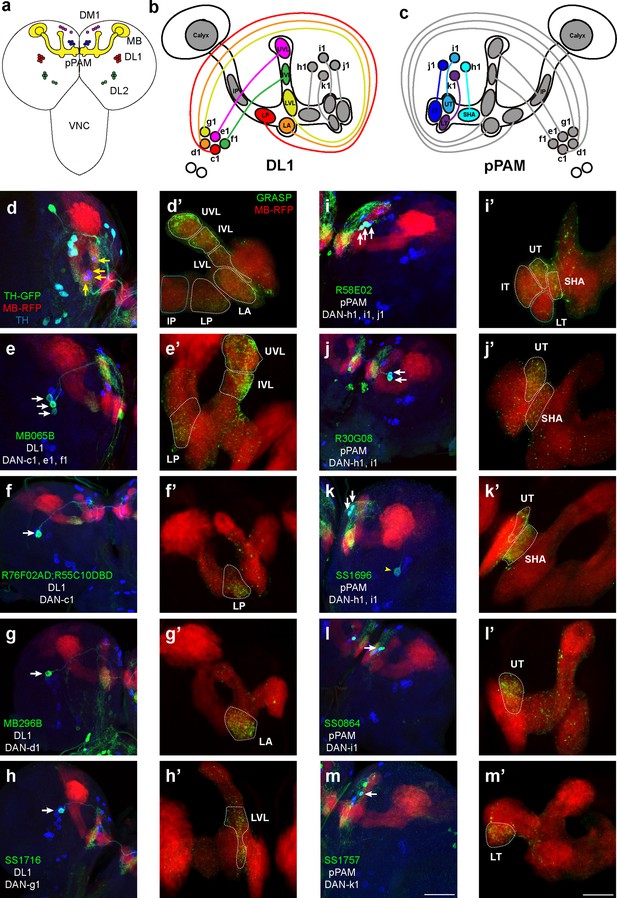
Identification of driver strains for a pair of dopaminergic neurons (DANs) in the Drosophila larval brain.
(a) A schematic diagram shows the DAN clusters and the mushroom body (MB) in the third-instar larval brain. (b and c) Schematic diagrams show the innervation patterns from distinct DANs to different compartments in the MB. All 11 MB compartments are shown; note that there is no synapse formed from DANs to the calyx and intermediate peduncle (IP). DANs in DL1 (b) and in pPAM (c). (d–m) Drivers covering DANs in the DL1 cluster (d–h). Drivers covering DANs in the pPAM cluster (i–m). The first column shows drivers covering distinct DANs. Neurons under the drivers are labeled by GFP, the MB is labeled by RFP, and DANs are marked by tyrosine hydroxylase (TH) antibody. The second column (d’–m’) shows the GFP reconstitution across synaptic partners (GRASP) signals from DANs under the driver to the corresponding compartments in the MB. The green channel represents the GRASP signals, and RFP marks the morphology of the MB. In the first column, white arrows mark the DANs under the driver strains. Yellow arrows in (d) show the pPAM neurons not labeled by TH-GAL4 driver strain. The yellow arrowhead in (k) showed the DL1 neuron not innervating the MB. Scale bars: 50 µm for the first column, and 20 µm for the second column. Abbreviations: DL, dorsolateral; DM, dorsomedial; IP, intermediate peduncle; IT, intermediate toe; IVL, intermediate vertical lobe; LA, lateral appendix; LP, lower peduncle; LT, lower toe; LVL, lower vertical lobe; pPAM, primary protocerebral anterior medial; SHA, shaft; UT, upper toe; UVL, upper vertical lobe. Note GFP expression patterns in the entire larval central nervous system (CNS) by GAL4 driver strains used in this study can be found in Figure 1—figure supplement 1. N numbers for each strain can be found in Supplementary file 2.
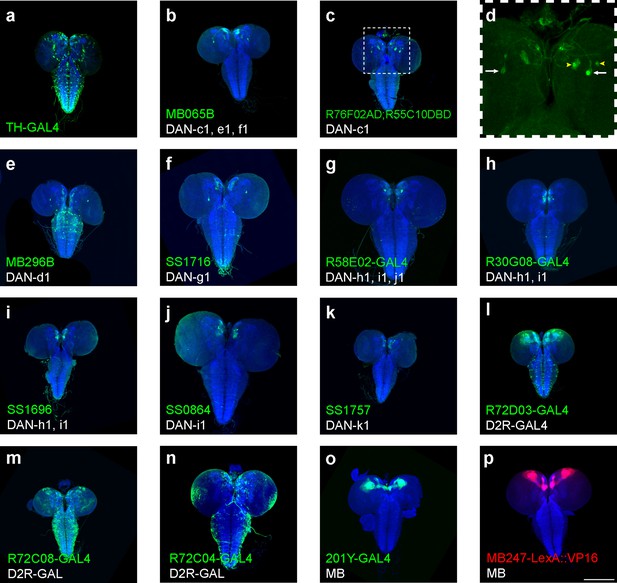
GFP expression patterns in the larval central nervous system (CNS) by GAL4 driver strains used in this study.
(a–o) Representative pictures show the patterns of distinct GAL4 driver strains crossed with a UAS-GFP strain. (p) A representative picture shows the pattern of MB247-LexA driver (MB247-LexA::VP16) crossed with a LexAop-RFP strain (LexAop-rCD2::RFP). Blue channel represents neuropils marked by nc82 antibody. Driver strains in (a–k) were used in Figure 1d–m. Driver strains in (l–n) are strains used in Figure 3—figure supplement 1. 201Y-Gal4 (o) is the driver strain used in Figure 6. Square region is enlarged in (d) to show the soma and neurites. White arrows label the soma of DAN-c1, while yellow arrowheads label other neurons. Summary of analysis can be found in Supplementary file 3. Scale bar: 200 µm. Note: N numbers can be found in Supplementary file 2. In Figure 1 and Figure 1—figure supplement 1 , we mainly showed the strains labeling distinct pairs of dopaminergic neurons. The labeling patterns of the remaining 47 strains screened are summarized in Table 1, whose brain images are available and can be provided upon request.
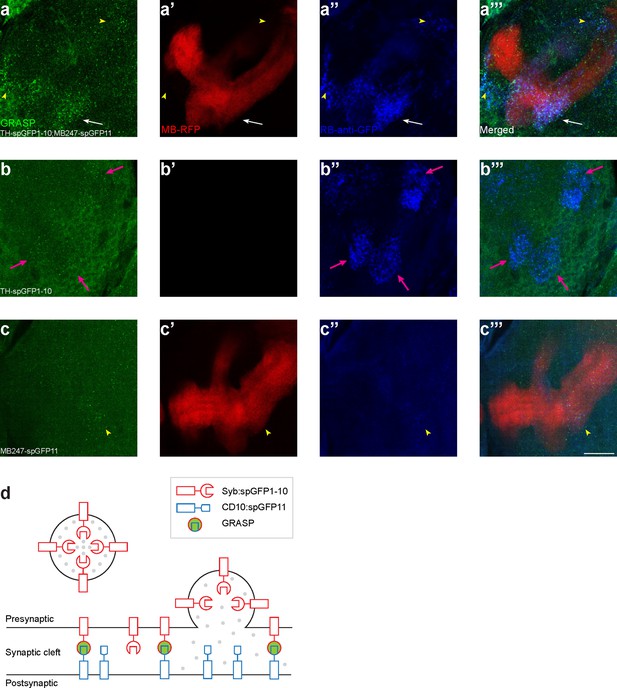
Controls for GFP reconstitution across synaptic partners (GRASP) experiments.
(a–c) The GRASP signals were not observed in control groups. The first column shows the mouse anti-GFP staining (green) for GRASP signals, the second column shows mushroom body (MB) lobes by RFP (red), and the third column shows the rabbit anti-GFP staining (blue) which only recognizes the spGFP1-10. (a) Both GRASP (mouse antibody, green) and spGFP1-10 (rabbit antibody, blue) can be recognized in the lower peduncle (LP) compartment (white arrows), in a larval brain expressing spGFP1-10 under TH-Gal4 and spGFP11 under MB247-LexA. Some strong spGFP1-10 signals outside of MB are also colocalized with GRASP signals (yellow arrowheads). (b) GRASP signals are hardly observed in a larval brain only expressing spGFP1-10 under TH-Gal4 (no MB247-LexA in this brain), while anti-spGFP1-10 signals are still strong (magenta arrows). (c) Neither GRASP nor spGFP1-10 can be recognized in a larval brain only expressing spGFP11 under MB247-LexA (cyan arrowheads). Scale bars: 20 µm. (d) A schematic diagram shows the mechanism of GRASP. Modified from Macpherson et al., 2015. Note: N numbers can be found in Supplementary file 2.
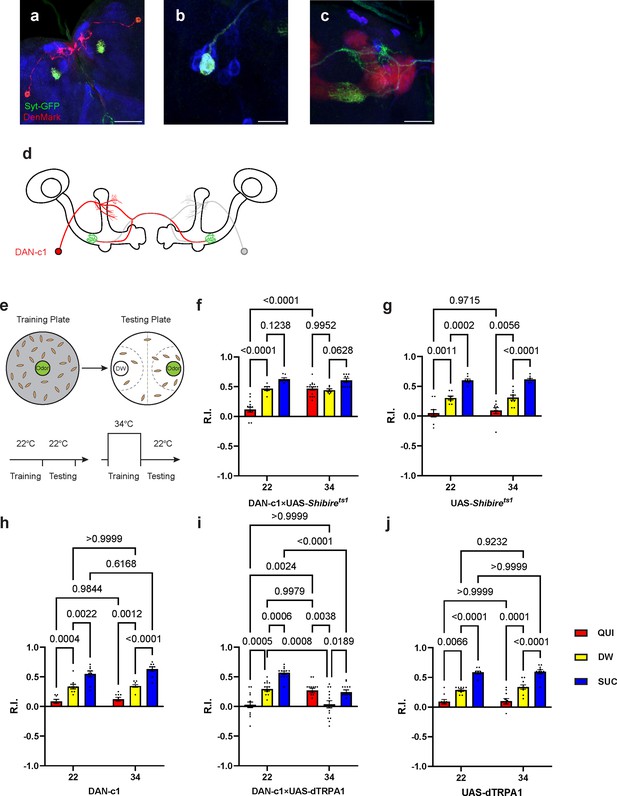
Dopamine release from DAN-c1 mediates larval aversive learning.
(a–d) R76F02-AD;R55C10-DBD driver is used as it covers one dopaminergic neuron, DAN-c1, in each brain hemisphere. (a) Dendrites and axons of DAN-c1 are labeled by DenMark and sytGFP, correspondingly. (b and c) Soma and neurites from Figure 1f with higher magnification. DAN-c1 is labeled with GFP, the mushroom body (MB) with RFP, and dopaminergic neurons (DANs) with TH antibody (blue color). Only one DAN soma is identified (b), axons from DAN-c1 innervate the lower peduncle (LP) of the MB (c). A schematic diagram (d) shows the innervation patterns of DAN-c1. Modified from Eichler et al., 2017 and Saumweber et al., 2018. (e) A schematic paradigm for larval olfactory learning (top) and two different training paradigms for thermogenetics (bottom). (f–h) Blocking dopamine release from DAN-c1 during learning using shibirets1 strain at 34°C impairs larval aversive learning. (h–j) Activation of DAN-c1 with dTRPA1 at 34°C induces aversive learning. QUI, quinine; DW, distilled water; SUC, sucrose. Data are shown as mean ± SEM. Two-way ANOVA, Tukey’s multiple comparison test. For N numbers, interaction p-values, row factor p-values, and column factor p-values, see Supplementary file 4. Scale bars: 50 µm (a), 20 µm (b and c). Note: N numbers of immunostaining for each strain can be found in Supplementary file 2.
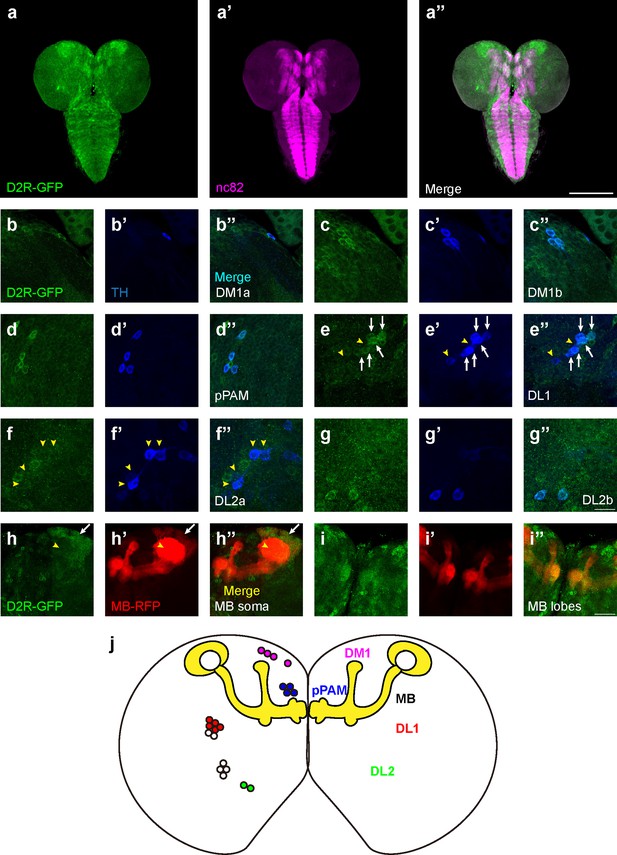
D2-like receptors (D2Rs) are expressed in dopaminergic neurons (DANs) and mushroom body (MB) in Drosophila larval brains.
(a) The expression pattern of D2R in a general view. D2R is shown with tagged GFP (D2R-GFP). Magenta represents neuropils marked by nc82 antibody (a’). (b–g) D2Rs (presynaptic) are found in most DANs: DM1a (b) and DM1b (c), pPAM (d), DL1 (e), DL2a (f), and DL2b (g) clusters. D2Rs are expressed in parts of DL1 neurons (white arrows in e) but not in DL2a neurons (yellow arrowheads in f). (h–i) D2Rs (postsynaptic) are found in the soma of MB neurons (white arrows in h), and MB lobes and peduncles (i), but not in calyx (yellow arrowheads in h). (j) A schematic diagram shows the expression pattern of pre- and postsynaptic D2R in DANs and MB (yellow) in the Drosophila larval brain, respectively. Scale bars: 200 µm (a); 50 µm (b–g); 20 µm (h, i). Abbreviations: DL, dorsolateral; DM, dorsomedial; pPAM, primary protocerebral anterior medial. Note: N numbers can be found in Supplementary file 2.
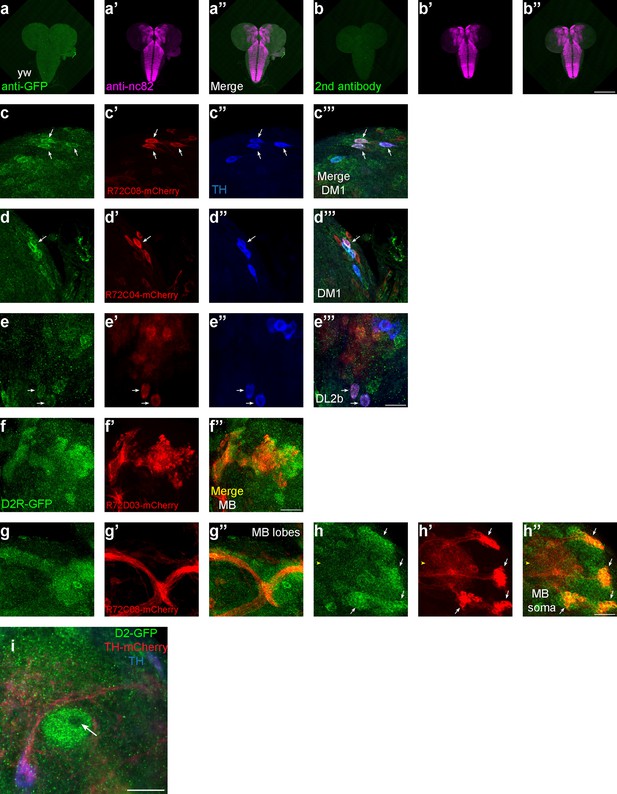
D2R-GAL4 strains support the expression pattern of D2-like receptor (D2R).
(a–b) Control staining for D2R-tagged GFP in Figure 3. The green channel represents GFP signals, whereas magenta represents neuropils marked by nc82 antibody. GFP staining in WT (a). Secondary antibody only staining for the D2R-tagged GFP strain (b). (c–e) D2R-GAL4 strains support the expression of D2R in some dopaminergic neurons (DANs). The green channel represents GFP signals, red represents mCherry signals under D2R-GAL4 drivers, and blue marks DANs with TH antibodies. R72C08 labels three DM1 neurons (c). R72C04 labels one DM1 (d) and two DL2b neurons (e). (f–h) D2R-GAL4 strains support the expression of D2R in mushroom body neurons (MBNs). The green channel represents GFP signals, and red represents mCherry signals under D2R-GAL4 drivers. R72D03 marks some MBNs in mushroom body (f). R72C08 labels some MBNs in axons (g), dendrites, and soma (h). (i) D2R is absent in the core of mushroom body lobes, from a transection view of the lower peduncle. Scale bars: 200 µm (a and b); 20 µm (c–e, g–i); 50 µm (f). Note: N numbers can be found in Supplementary file 2.
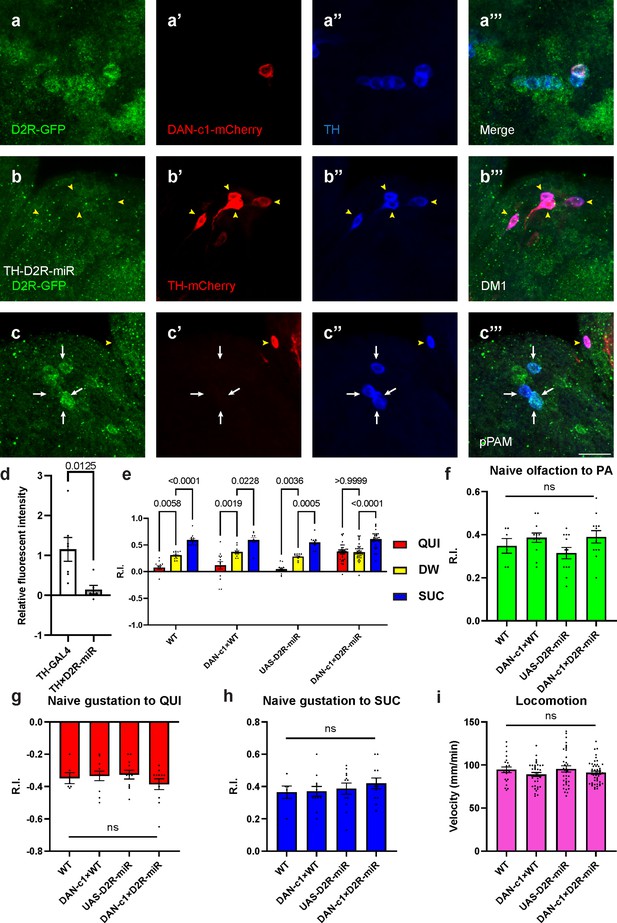
Presynaptic D2-like receptor (D2R) in DAN-c1 is necessary for larval aversive learning.
(a) D2R is expressed in DAN-c1. The expression pattern of D2R is shown with tagged GFP, DAN-c1 is marked by mCherry, and all dopaminergic neurons (DANs) are marked with TH antibody (blue). (b–c) Knockdown of D2R by D2R-miR reduces fluorescent intensities of D2R-tagged GFP (D2R-GFP). The TH-GAL4 driver is used to express mCherry. The intensity of D2R-tagged GFP is reduced in the DM1 cluster (yellow arrowheads in b), which is still intact in primary protocerebral anterior medial (pPAM) neurons (white arrows in c). (d) D2R-GFP fluorescent intensity is quantified by standardizing the values in DM1 with those in the pPAM. Data are shown as mean ± SEM. For the TH-GAL4 group, N=7 brains; for the TH×D2R-miR group, N=6 brains. Unpaired t-test, p=0.0125. (e) Knockdown of D2R in DAN-c1 by D2R-miR impairs larval aversive learning. QUI quinine; DW, distilled water; SUC, sucrose. Data are shown as mean ± SEM. Two-way ANOVA, Tukey’s multiple comparison test, p<0.0001 for interaction p-values. For N numbers, see Supplementary file 5. (f–i) D2R knockdown in DAN-c1 does not affect naïve sensory and motor functions. Data are shown as mean ± SEM. One-way ANOVA, Tukey’s multiple comparison test. For N numbers, see Supplementary file 5. Scale bar: 20 µm. Note: Figure 4—figure supplement 2 and Figure 4—figure supplement 3 show additional information on naïve sensory and motor functions in larvae related to D2R-miR experiments. N numbers of immunostaining for each strain can be found in Supplementary file 2.
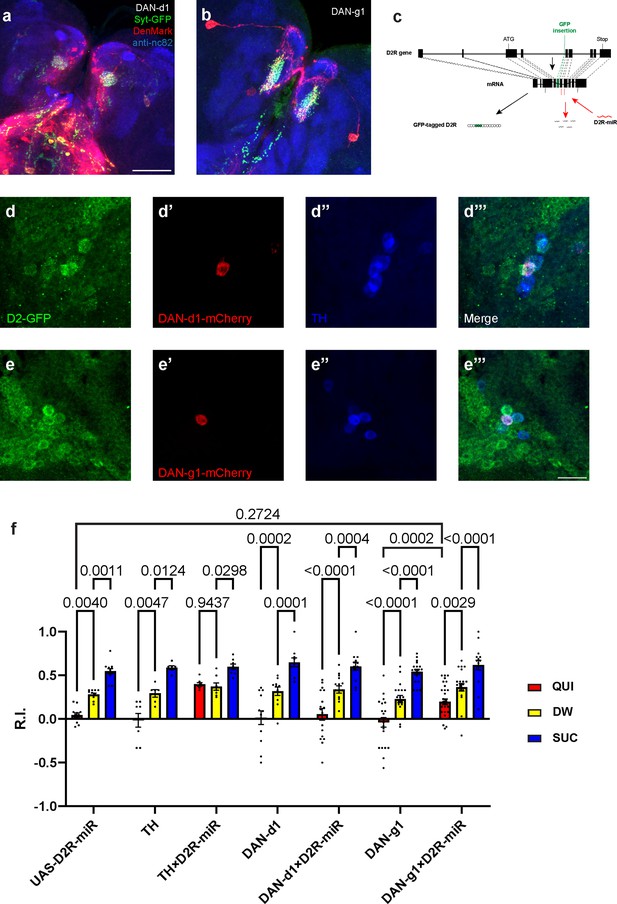
D2-like receptors (D2Rs) in DAN-d1 and DAN-g1 are not necessary for aversive learning.
(a–b) Dendrites and axons of DAN-d1 (a) and g1 (b) are labeled by DenMark (red) and sytGFP (green), correspondingly. (c) The gene structure of D2R, the insertion site of GFP, and the D2R-microRNA targeting sequence are shown in a schematic diagram. Modified from Xie et al., 2018. (d–e) D2Rs are expressed in DAN-d1 (d) and DAN-g1 (e). The expression pattern of D2R is shown with tagged-GFP, DAN-g1 and d1 are marked by mCherry, and dopaminergic neurons (DANs) are labeled by TH antibody (blue). (f) Knockdown of D2R in neither DAN-g1 nor DAN-d1 impairs aversive learning. Data are shown as mean ± SEM. UAS-D2R-miR data is from Figure 4e. Two-way ANOVA, Dunnett’s multiple comparison test. p=0.0544 for interaction, p<0.0001 for row factor (genotype), and p<0.0001 for column factor (US). For N numbers, see Supplementary file 5. Scale bars: 50 µm (a and b), 20 µm (d and e). Note: N numbers of immunostaining for each strain can be found in Supplementary file 2.
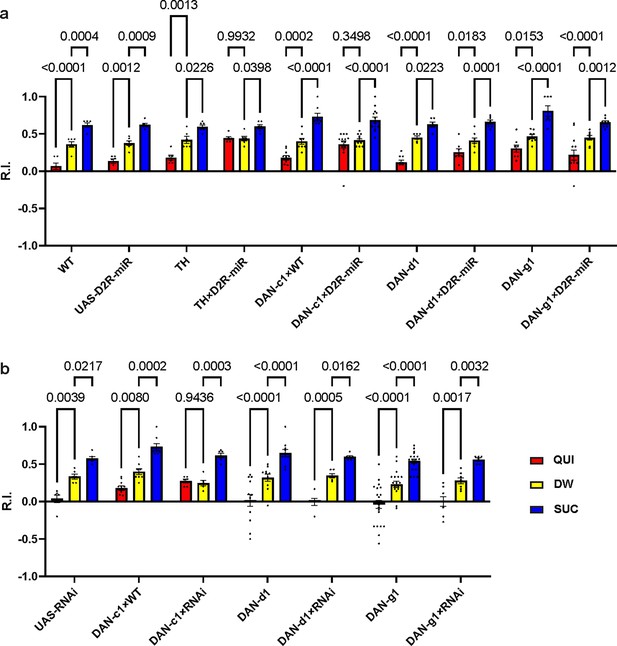
Knockdown D2-like receptor (D2R) in DAN-c1 with an RNAi strain also impairs aversive learning.
(a) Knockdown of D2R with D2R-miR in DAN-c1 impairs larval aversive learning when using propionic acid as the odorant. (b) Knockdown of D2R in DAN-c1 with an RNAi strain also impairs larval aversive learning when using pentyl acetate as the odorant, while knockdown of D2R in either DAN-d1 or g1 does not affect learning. Data are shown as mean ± SEM. Two-way ANOVA, Dunnett’s multiple comparison test. In D2R-miR experiments (a), p=0.0009 for interaction; in D2R-RNAi experiments (b), p=0.3802 for interaction, p=0.0001 for row factor (genotype), and p<0.0001 for column factor (US). For N numbers, see Supplementary file 5.
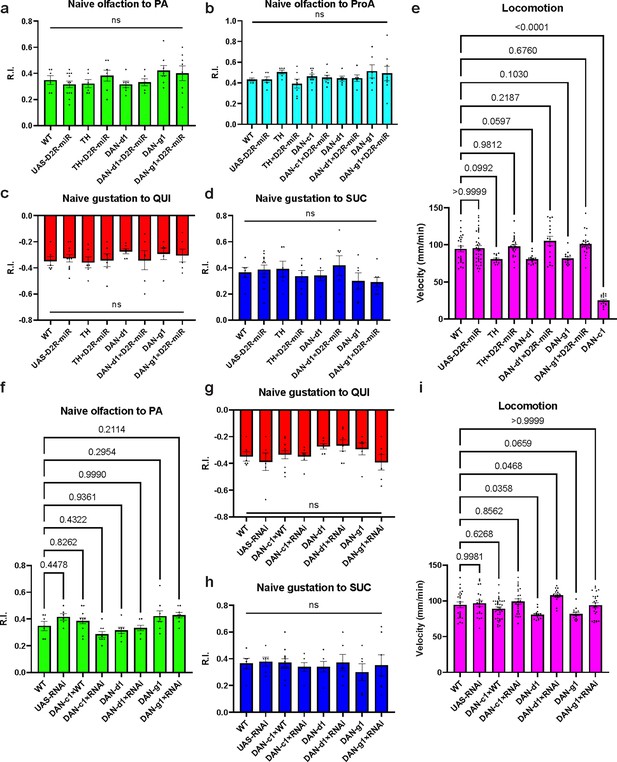
Naïve sensory and motor functions of Drosophila larvae.
(a–e) The naïve sensory and motor functions in larvae related to D2-like receptor (D2R)-miR experiments. (a) No significant difference exists between strains in naïve olfactory tests toward pentyl acetate (PA). (b) No significant difference exists between strains in naïve olfactory tests toward propionic acid (ProA). (c) No significant difference exists between strains in naïve gustatory tests toward quinine (QUI). (d) No significant difference exists between strains in naïve gustatory tests toward sucrose (SUC). (e) No significant difference is found between strains and WT, except DAN-c1. So, larvae from DAN-c1×WT are used for all behavioral assays (refer to Figure 4i). (f–i) The naïve sensory and motor functions in larvae related to RNAi experiments. (f) No significant difference exists between strains and WT. (g) No significant difference exists between strains in naïve gustatory tests toward QUI. (h) No significant difference exists between strains in naïve gustatory tests toward SUC. (i) There is a significant difference between strains in larval locomotion speed, and there is a significant difference between DAN-d1, DAN-d1×RNAi, and WT, but these differences do not affect the learning ability of larvae. Data are shown as mean ± SEM. One-way ANOVA, Dunnett’s multiple comparison test. For N numbers, see Supplementary file 5.
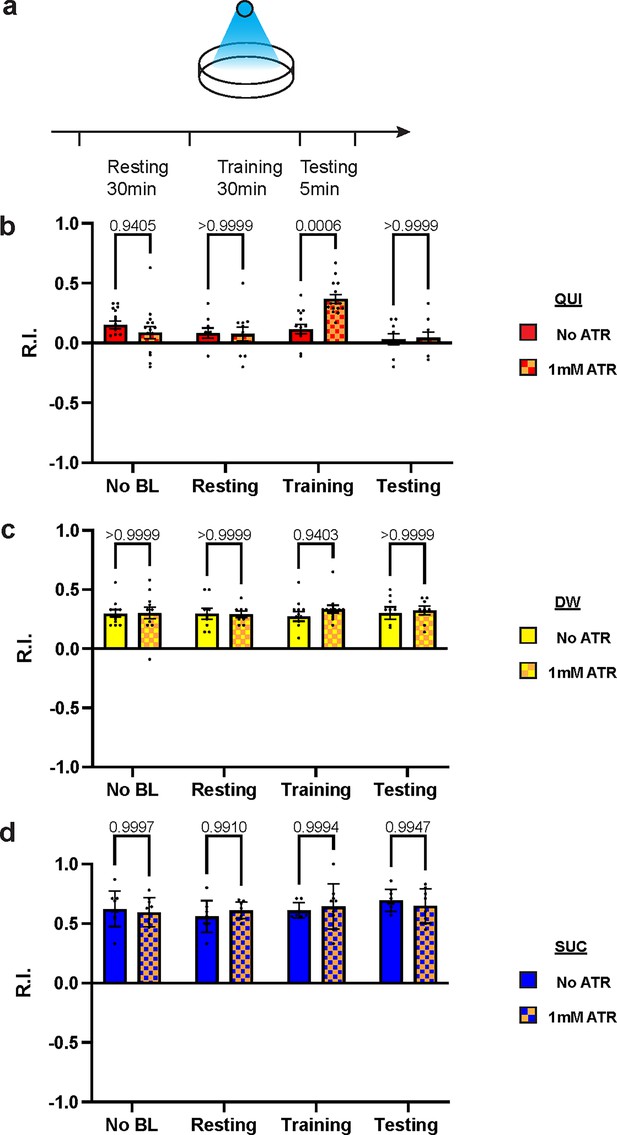
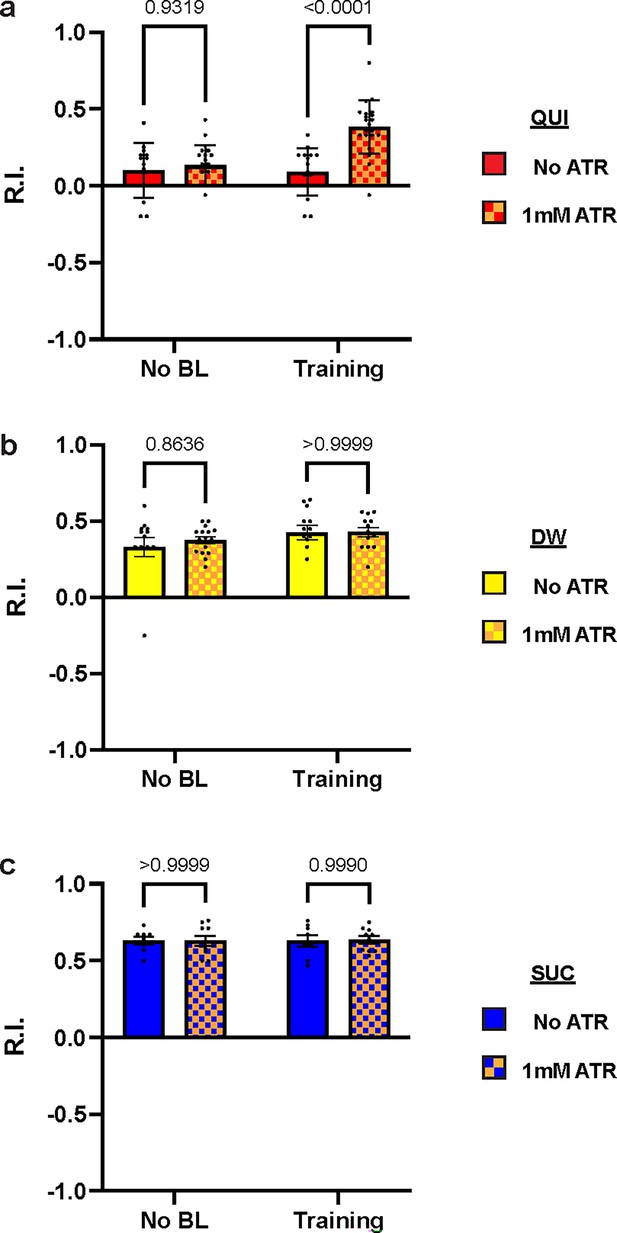
Over-excitation of DAN-c1 impairs larval aversive learning.
(a) A schematic diagram of optogenetic manipulations of neuronal excitability in DAN-c1 during distinct stages in learning. (b–d) Activation of DAN-c1 during training impairs larval aversive learning (b), while keeping appetitive learning intact (d). Unconditioned stimuli used were quinine (QUI) (b) and sucrose (SUC) (d). No learning behaviors are observed in the control distilled water (DW) groups (c). Third-instar larvae with ChR2 expression in DAN-c1 are used. ATR, all-trans-retinal. Data are shown as mean ± SEM. Two-way ANOVA, Tukey’s multiple comparison test. In QUI group (b), p=0.0009 for interaction; in DW group (c), p=0.8367 for interaction, p=0.9750 for row factor (training stages), and p=0.4872 for column factor (whether with ATR); in SUC group (d), p=0.6247 for interaction, p=0.2550 for row factor (training stages), and p=0.9437 for column factor (whether with ATR). For N numbers, see Supplementary file 6.
Over-excitation of dopaminergic neurons (DANs) during learning impairs larval aversive learning.
Third-instar larvae with ChR2 expression in most DANs (TH-Gal4) were used. Activation of DANs during training impairs larval aversive learning (a), while keeping appetitive learning intact (c). No learning behaviors are observed in the control DW groups (b). Data are shown as mean ± SEM. Two-way ANOVA, Tukey’s multiple comparison test. In the QUI group (a), p=0.0011 for interaction; in the DW group (b), p=0.6126 for interaction, p=0.0850 for row factor (training stages), and p=0.5748 for column factor (whether with ATR); in the SUC group (c), p=0.8910 for interaction, p=0.9239 for row factor (training stages), and p=0.9503 for column factor (whether with ATR). For N numbers, see Supplementary file 6.
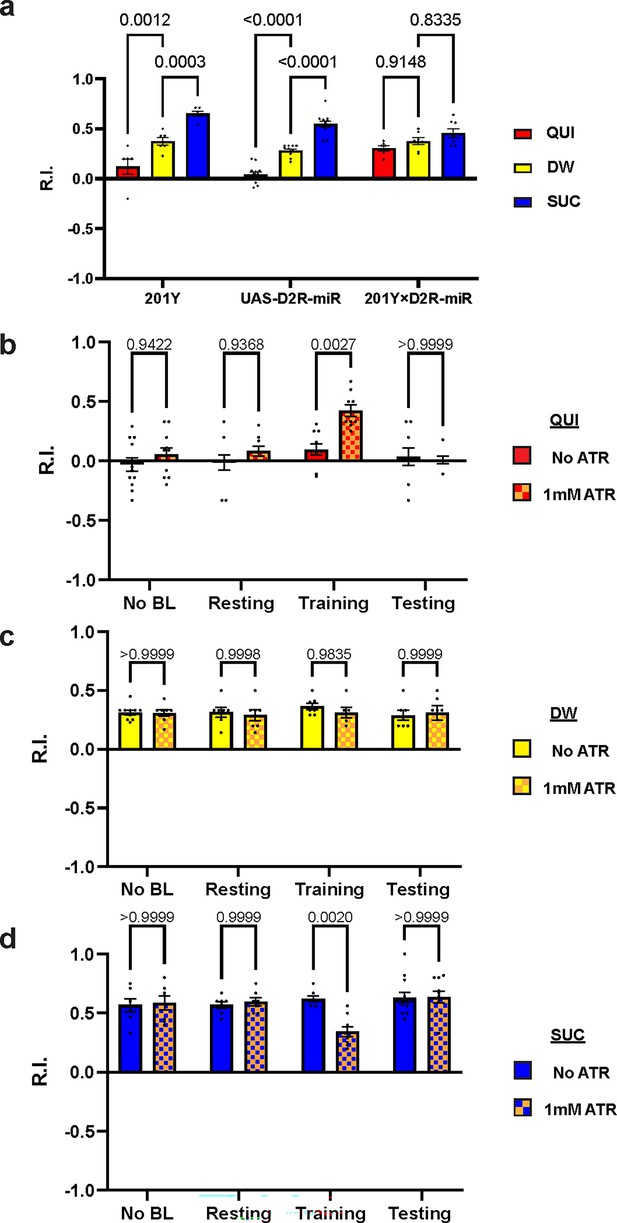
D2-like receptor (D2R) in mushroom body is necessary for both aversive and appetitive learning.
(a) Knockdown of D2R in mushroom body neurons (MBNs) impairs larval aversive and appetitive learning. (b–d) Activation of MBNs during training impairs both larval aversive and appetitive learning. Unconditioned stimuli used were quinine (QUI) (b) and sucrose (SUC) (d). No learning behaviors are observed in the control distilled water (DW) groups (c). Third-instar larvae with ChR2 expression in MBNs (201Y-GAL4) are used. ATR, all-trans retinal. Data are shown as mean ± SEM. Two-way ANOVA, Tukey’s multiple comparison test. In D2R knockdown experiments (a), p<0.0001 for interaction. In the optogenetic QUI group (b), p=0.0259 for interaction; in the DW group (c), p=0.8077 for interaction, p=0.7623 for row factor (training stages), and p=0.5846 for column factor (whether with ATR); in the SUC group (d), p=0.0035 for interaction. For N numbers, see Supplementary file 5 and 6.
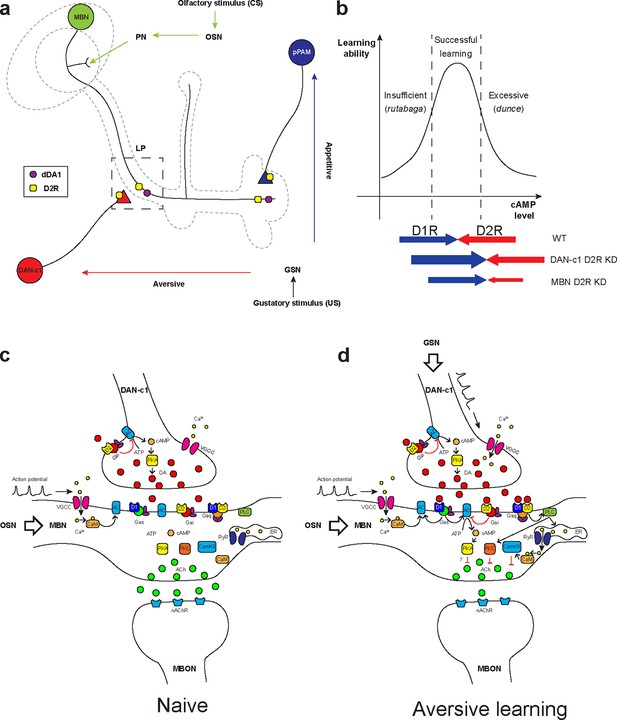
Roles of D2-like receptors (D2Rs) in dopaminergic (DANs) and mushroom body (MBNs) neurons during larval olfactory learning.
(a) A schematic diagram shows the roles of D2R in DANs and MBNs in larval olfactory associative learning. During learning, olfactory stimuli (conditioned stimulus [CS]) are received by olfactory sensory neurons (OSNs) and transmitted to MBNs (green) via projection neurons (PNs). Distinct gustatory stimuli (unconditioned stimulus [US]) are received by gustatory sensory neurons (GSNs) and transferred to different DANs. Aversive stimuli are sent to DAN-c1 (red) in the DL1 cluster which connects the lower peduncle compartment (LP, square in dashed line), while appetitive stimuli are received by pPAM neurons (blue) innervating the medial lobe (ML). D2Rs (yellow square) are expressed in DAN-c1 and pPAM as autoreceptors, regulating dopamine release. Both D2R and dDA1 (magenta circle) are expressed in the MBNs. (b) A hypothetical curve showing the relationship between learning ability and cAMP level in the mushroom body. Insufficient cAMP (rutabaga mutant) cannot induce learning, while excessive cAMP (dunce mutant) also impairs learning. Only the appropriate level of cAMP regulated by the opposing actions of D1R (dDA1) and D2R leads to successful learning in wild-type larvae (WT). Knockdown of D2R in DAN-c1 causes excessive dopamine release, elevating cAMP and resulting in impaired learning. D2R knockdown in MBNs relieves the inhibition effect of D2R, resulting in excessive intracellular cAMP and learning failure. (c–d) Potential molecular mechanisms underlying Drosophila olfactory learning in the square region as shown in (a). (d) During aversive learning, olfactory stimuli induce depolarization of MBNs, which activates voltage-gated calcium channels and induces calcium influx. Gustatory stimuli, such as quinine, activate DANs and elevate dopamine (DA) release. D1 receptor (dDA1) activates adenylyl cyclase (AC) and elevates cAMP via Gαs, while D2 receptor (D2R) inhibits AC and suppresses cAMP via Gαi/o. In Drosophila, the coincidence detector rutabaga (AC) is activated by the existence of both calcium and Gαs, converging the olfactory and gustatory stimuli. cAMP activates the PKA signaling pathway, elevating the neuronal excitability. D1 and D2 receptors can also form heteromeric receptors and activate the PLC-PKC and CaMKII signaling pathways via Gαq. These pathways inhibit acetylcholine (ACh) release from MBNs to MB output neurons (MBONs), which leads to the avoidance of the learned odor. Abbreviations: AC, adenylyl cyclase; ACh, acetylcholine; ATP, adenosine triphosphate; CaM, calmodulin; CaMKII, Ca2+/calmodulin-dependent protein kinase II; cAMP, cyclic adenosine monophosphate; CS, conditioned stimulus; DA, dopamine; DANs, dopaminergic neurons; DL, dorsolateral; ER, endoplasmic reticulum; Gαi/o, Gi/o protein α subunit; Gαq, Gq protein α subunit; Gαs, Gs protein α subunit; GSNs, gustatory sensory neurons; LP, lower peduncle; MBNs, mushroom body neurons; MBONs, mushroom body output neurons; ML, medial lobe; nAChR, nicotinic acetylcholine receptor; OSNs, olfactory sensory neurons; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; PNs, projection neurons; pPAM, primary protocerebral anterior medial; RyR, ryanodine receptor; US, unconditioned stimulus; VGCC, voltage-gated calcium channel.
Tables
Driver strains screened for dopaminergic neurons in the third-instar larval brain.
Strains are listed with their published names and names used in this work. The numbers of dopaminergic and non-dopaminergic neurons in the third-instar larval brain are described. The identities of dopaminergic neurons from distinct clusters are also listed, especially for those in DL1 and primary protocerebral anterior medial (pPAM). The analogs column lists the labeled neurons in previous publications. Source/gift column shows the original papers in which these strains were described, as well as the laboratories these strains were obtained from. Several, 2–5 neurons; some, 6–10 neurons; lots, >10 neurons.
| Strains | Name in this work | Neurons in third-instar larval brains | Analogs | Stock # | Source/Gift | ||
|---|---|---|---|---|---|---|---|
| DANs | Non-DANs | ||||||
| 1 | TH-Gal4 | – | All DANs except pPAM | Some weak | All DANs except PAM (adult) | – | Dr. J. Hirsh’s Lab |
| 2 | TH-C' | – | 1 DL1, 1 DM1, 3DL2a | Rare | PPL1 +PPM3 (adult) | – | Dr. M. Wu’s lab (Liu et al., 2012) |
| 3 | TH-C1 | – | – | – | PPL1 +PPM3 (adult) | – | |
| 4 | TH-D' | – | 3DL1, 2DL2b | Rare | PPL1 +PPM3 (adult) | – | |
| 5 | TH-D1 | – | – | – | PPL1 +PPM3 (adult) | – | |
| 6 | TH-D4 | – | – | – | PPL1 +PPM3 (adult) | – | |
| 7 | TH-F1 | – | 3DL1 | Rare | PPL1 +PPM3 (adult) | – | |
| 8 | Th-F2 | – | 3 DM1, 2–4 DL1, 2DL2a | – | PPL1 +PPM3 (adult) | – | |
| 9 | Th-F3 | – | 3 DM1b, 1 DL1 | – | PPL1 +PPM3 (adult) | – | |
| 10 | TH-G1 | – | 2–3 DM1, 2–4 DL1, 2 DL2b | – | PPL1 +PPM3 (adult) | – | |
| 11 | R30G08 | – | h1, i1, weak k1 | – | 2 pPAM (L3) | 48101 | BDSC (Rohwedder et al., 2016) |
| 12 | R58E02 | – | h1, i1, j1 | 1 | 3 pPAM (L3) | 41347 | |
| 13 | R64H06 | – | h1, i1, j1, k1 | Lots | 4 pPAM (L3) | 49608 | |
| 14 | MB315C | – | 1–3 pPAM (h1) | Lots | PAM-γ5 (adult) | – | Janelia Farm (Aso et al., 2014a) |
| 15 | MB109B | – | – | – | PAM-β'2a, PAM-γ5 (adult) | – | |
| 16 | MB301B | – | – | – | PAM-β'2m, PAM-β2β'2a (adult) | – | |
| 17 | MB056B | – | – | – | PAM-β'2m, PAM-β'2p (adult) | – | |
| 18 | MB032B | – | – | – | PAM-β'2m, PAM-β'2p, PAM-β2β'2 a, PAM-γ3 (adult) | – | |
| 19 | MB312B | – | – | – | PAM-γ4, PAM-γ4<γ1γ2 (adult) | – | |
| 20 | MB194B | – | – | – | PAM-α1, PAM-β'2a, PAM-β1, PAM-β1ped, PAM-β2 (adult) | – | |
| 21 | MB063B | – | – | – | PAM-β1 (adult) | – | |
| 22 | MB043B | – | h1, i1, j1 | Weak, lots of MBN | PAM-α1, PAM-β'1 ap, PAM-β'1m, PAM-β1 (adult) | – | |
| 23 | MB441B | – | – | – | PAM-γ3 (adult) | – | |
| 24 | MB025B | – | – | – | PAM-β'1ap/m (adult) | – | |
| 25 | MB438B | – | – | – | PPL1-α'2α2, PPL1-α3, PPL1-γ1pedc (adult) | – | |
| 26 | MB296B | DAN-d1 | d1 | Some in VNC | PPL1-γ2α'1 (adult) | – | |
| 27 | MB304B | – | – | – | PPL1-α'3 (adult) | – | |
| 28 | MB058B | – | – | – | PPL1-α'2α'2 (adult) | – | |
| 29 | MB065B | – | c1, f1, e1 | – | PPL1-α'2α2, PPL1-α'3, PPL1-α3, PPL1-γ2α'1 (adult) | – | |
| 30 | GMR_SS01716 | DAN-g1 | g1 | – | g1 (LVL) (L3) | – | Dr. M. Zlatic’s Lab (Saumweber et al., 2018) |
| 31 | GMR_SS01696 | DAN-h1 | h1 +i1 | – | h1 (SHA) (L3) | – | |
| 32 | GMR_SS00864 | DAN-i1 | i1 | 1 | i1 (UT) (L3) | – | |
| 33 | GMR_SS1757 | DAN-k1 | k1 | 1 | k1 (LT) (L3) | – | |
| 34 | R78E04 | – | 1 weak DL1 | Several | d1 (LA) (L3) | 39997 | |
| 35 | R72B05 | – | 1 DL1 | Lots | MBIN-e2 (L3) | 39611 | |
| 36 | R12C11 | – | 2 DM1b | Several | MBON-c2 (L3) | 76324 | |
| 37 | R30F04 | – | weak d1 | Rare | d1 (LA) (L3) | 48614 | |
| 38 | R76C04 | – | c1, d1 | Rare | c1 (LP) (L3) | 48621 | |
| 39 | R37D06 | – | – | – | f1 (IVL) (L3) | 47921 | |
| 40 | R14E06 | – | 2 DL1 | Several | MBIN-e2 (L3) | 48643 | |
| 41 | TH-C-AD;R76F05-DBD | – | 1 weak DL1, 2 strong DM1 | Lots | PPL1 (SMP-PED) (adult) | – | Dr. M. Wu’s Lab (Xie et al., 2018) |
| 42 | TH-F-AD;R61H03-DBD | 2DL2, 3 DM1 | Rare | PPL1 (SMP) (adult) | – | ||
| 43 | R76F02-AD;TH-F-DBD | – | c1, e1 | Lots weak | PPL1 (MB-MP1) (adult) | – | |
| 44 | TH-F-AD;R76F05-DBD | – | 1 weak DL1, 1–2 strong DM1 | Several | PPL1 (SMP-γ) (adult) | – | |
| 45 | R76F02-AD;R60F07-DBD | – | c1, f1, e1 | Rare | PPL1 (PED, MB-MP1) (adult) | – | |
| 46 | R60F07-AD;R76F05-DBD | – | g1, e1 | Strong SOG | PPL1 (SMP-γ) (adult) | – | |
| 47 | DAT-B-AD;R76F05-DBD | – | 2-3pPAM, 1 DL1 | Rare | PPL1 (hSMP-γ) (adult) | – | |
| 48 | R76F02-AD;R55C10-DBD | DAN-c1 | c1 | – | PPL1 (MB-MP1) (adult) | – | |
| 49 | R76F02-AD;R76F01-DBD | – | 1 weak DL1 | Lots | PPL1 (dFB) (adult) | – | |
| 50 | R76F02-AD;R76F05-DBD | – | – | Lots | PPL1 (dFB) (adult) | – | |
| 51 | R76F05-AD;R61H03-DBD | – | 1 DL1, 2 pPAM, 3 DM1 | 1 | PPL1 (MB-MV1) (adult) | – | |
| 52 | WED-1 | – | – | – | WED (adult) | – | Dr. M. Wu’s Lab (Liu et al., 2012) |
| 53 | WED-2 | – | – | – | WED (adult) | – | |
| 54 | TH-FLP/UAS-FRT>>FRT-CD8::GFP;R39G05 | – | – | – | PPL1 (bSMP-γ) (adult) | 50063 | |
| 55 | TH-FLP/UAS-FRT>>FRT-CD8::GFP;R17C11 | – | – | – | PPL1 (V1 and MP1) (adult) | 48763 | |
| 56 | TH-FLP/UAS-FRT>>FRT-CD8::GFP;R39C07 | – | 2 DL1 | – | PPL1 (MV1 and MP1) (adult) | 50039 | |
| 57 | TH-FLP/UAS-FRT>>FRT-CD8::GFP;R67E08 | – | – | – | PPL1 (V1) (adult) | 39445 | |
Additional files
-
Supplementary file 1
Other strains used in this study.
- https://cdn.elifesciences.org/articles/100890/elife-100890-supp1-v1.docx
-
Supplementary file 2
N numbers for brain samples used in Figures.
- https://cdn.elifesciences.org/articles/100890/elife-100890-supp2-v1.docx
-
Supplementary file 3
Summary of R76F02AD;R55C10DBD (DAN-c1) identifying patterns in larval brains.
For the strain R76F02AD; R55C10DBD, 22 third-instar larval brains expressing GFP or SytGFP and DenMark were examined, and all of them clearly identified DAN-c1. Half of them only identified DAN-c1, the rest had 1–5 weakly identified cells without neurites, and barely 1 or 2 strongly identified cells appeared. These non-DAN-c1 neurons were seldom dopaminergic neurons. In the ventral nerve cord (VNC), 8 out of 12 did not have any identified cells, 3 had 2–4 strong identified cells. These data supported that R76F02AD;R55C10DBD exclusively labels DAN-c1 in third-instar larval brains.
- https://cdn.elifesciences.org/articles/100890/elife-100890-supp3-v1.docx
-
Supplementary file 4
N numbers and p-values for learning assays with thermogenetics.
- https://cdn.elifesciences.org/articles/100890/elife-100890-supp4-v1.docx
-
Supplementary file 5
N numbers for D2-like receptor (D2R) knockdown experiments.
- https://cdn.elifesciences.org/articles/100890/elife-100890-supp5-v1.docx
-
Supplementary file 6
N numbers for learning assays with optogenetics.
- https://cdn.elifesciences.org/articles/100890/elife-100890-supp6-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/100890/elife-100890-mdarchecklist1-v1.pdf