Reprograming gene expression in ‘hibernating’ C. elegans involves the IRE-1/XBP-1 pathway
Figures
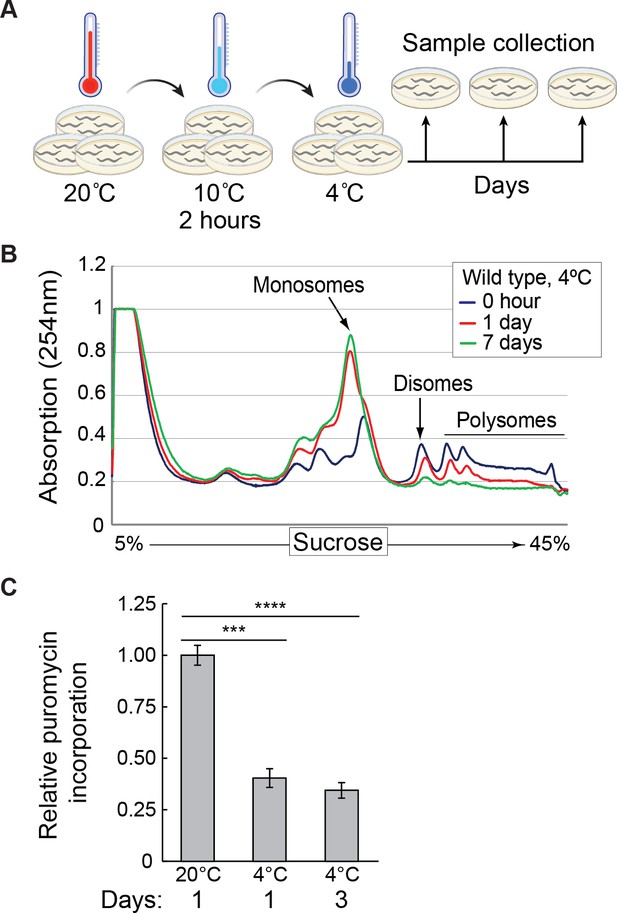
Global protein synthesis is suppressed in hibernating C. elegans.
(A) Schematic of the cooling paradigm used in this study. Young adult nematodes, grown at 20 °C on multiple plates, are first adapted to the cold at 10 °C for 2 hr, and then shifted to 4 °C. At indicated time points, the animals are collected and treated in an experiment-specific manner. Created with BioRender.com. (B) Polysome profiles from wild-type animals were treated as shown in (A) and collected at 4 °C at the indicated times. Marked are the positions of mono-, di-, and polysomes. Note a strong decrease of large polysomes with a concomitant increase of monosomes in cold, which becomes more pronounced with a longer cold exposure. (C) Protein synthesis was evaluated with the SUrface SEnsing of Translation (SUnSET) assay in animals incubated as indicated. The quantification reflects changes in puromycin incorporation (relative to day 1 at 20 °C), normalized to actin as a loading control, and detected by western blotting. Error bars indicate the SEM of three biological replicates. Unpaired two-sided t-test was used for statistical analysis. ***p<0.001, ****p<0.0001.
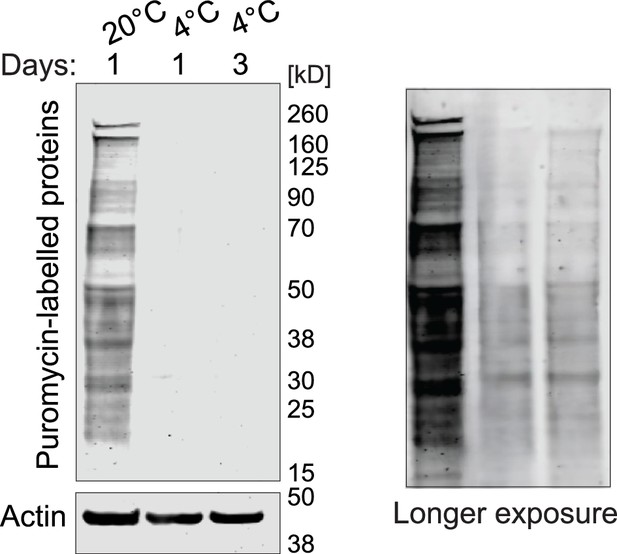
Protein synthesis was evaluated with the SUrface SEnsing of Translation (SUnSET) assay in animals incubated as indicated.
Puromycin incorporation was detected by western blot. Actin (ACT-1) was used as a loading control. A longer exposure of the blot on the right shows that puromycin incorporation, although reduced, continues at 4 °C.
-
Figure 1—figure supplement 1—source data 1
PDF file containing original western blots for Figure 1—figure supplement 1, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/101186/elife-101186-fig1-figsupp1-data1-v1.zip
-
Figure 1—figure supplement 1—source data 2
Original files depicting blots displayed in Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/101186/elife-101186-fig1-figsupp1-data2-v1.zip
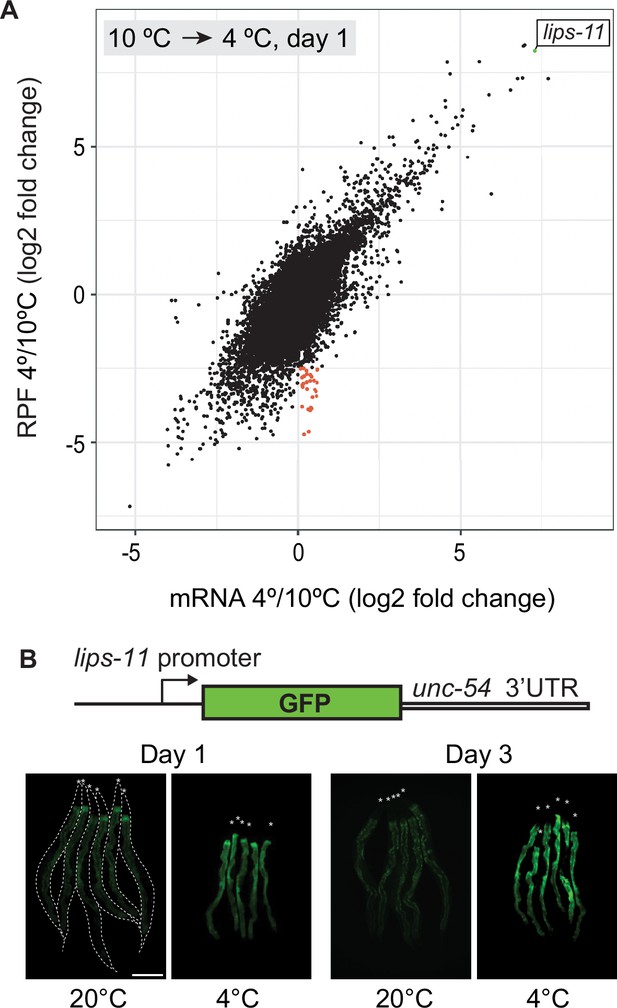
Transcription may be the main determinant of gene expression in the cold.
(A) Changes in mRNA abundance (‘mRNA’ on the x-axis) and translation (ribosome protected fragment, ‘RPF’ on the y-axis) for all transcripts upon shifting wild-type animals from 10°C to 4°C. Each dot represents the log (base 2) fold change of a single transcript. The most upregulated transcript, lips-11, is indicated in green. Overall, note a strong correlation between mRNA levels and translation (Pearson correlation coefficient = 0.7244968). A small subpopulation of transcripts (red) displayed little or no change in mRNA levels but reduced association with ribosomes, suggesting specific translation repression. (B) Top: Diagram representing a reporter construct, wherein GFP is expressed under the control of the lips-11 promoter and unc-54 3’ UTR. Below are representative fluorescent micrographs, taken at the indicated conditions, of several bundled animals carrying the GFP reporter. The animals are outlined in the control panel and the heads are indicated by asterisks. The scale bar = 200 µm.
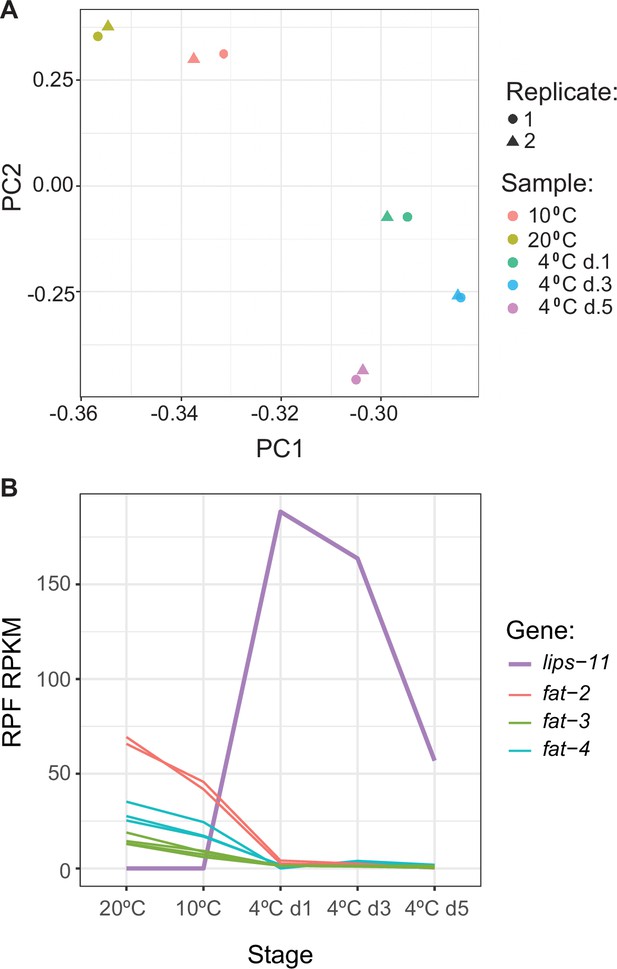
Ribosomal occupacy measured at different temperatures.
(A) PCA plot of ribosome profiling samples and replicates based on mRNA ribosome protected fragment (RPF) normalized according to reads per kilobase exon per million reads (RPKM). Note co-clustering of replicates and temperatures. (B) Changes in translation for selected genes as a function of temperature changes and exposure.
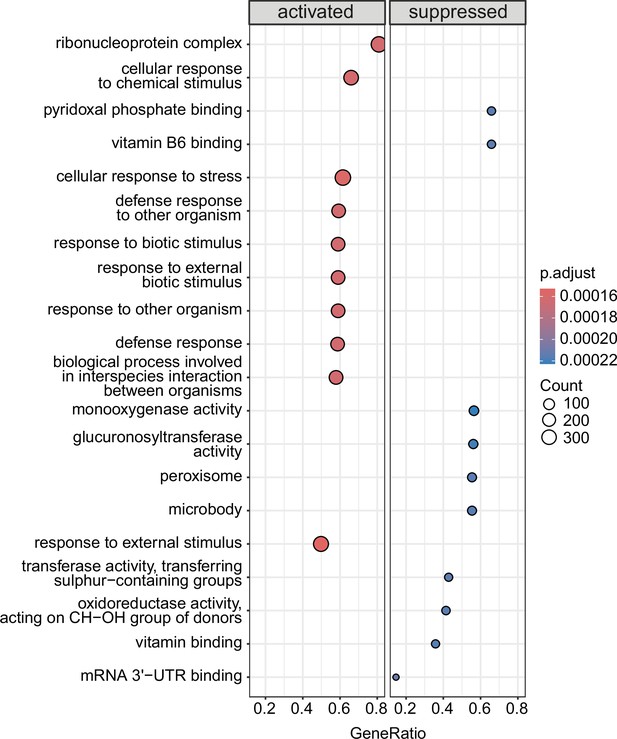
Gene set enrichment analysis depicting the top 10 categories that are activated or suppressed between 10°C and 4°C on day 1 in wild-type animals.
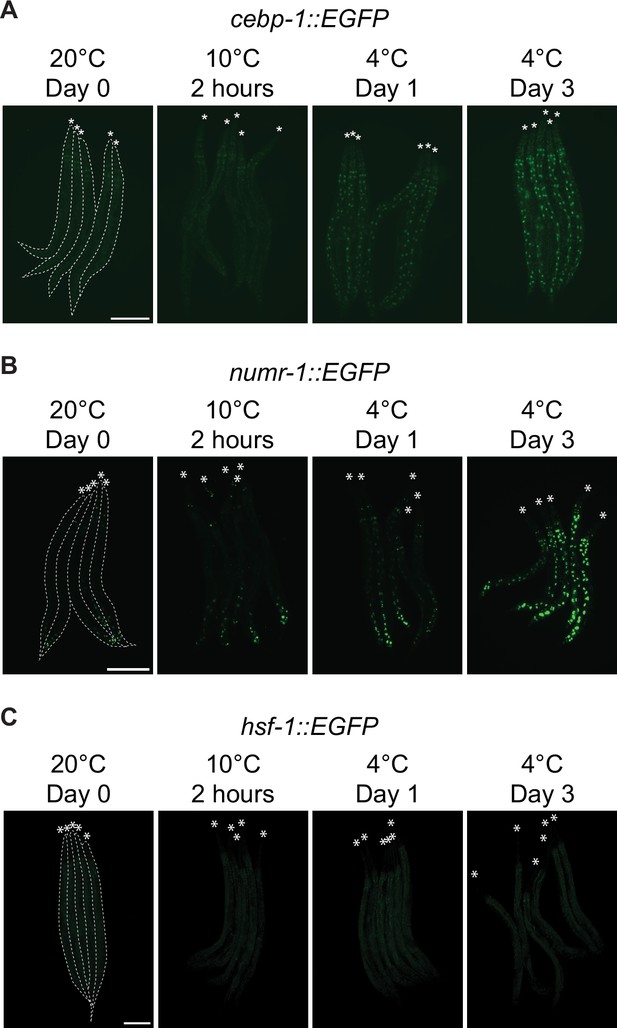
Micrographs of animals expressing EGFP fused to CEBP-1.
(A), NUMR-1 (B), or HSF-1 (C), was taken at the indicated time and temperature. Animals are outlined in the left panels and the heads are indicated with asterisks (A–C). The scale bar = 200 µm.
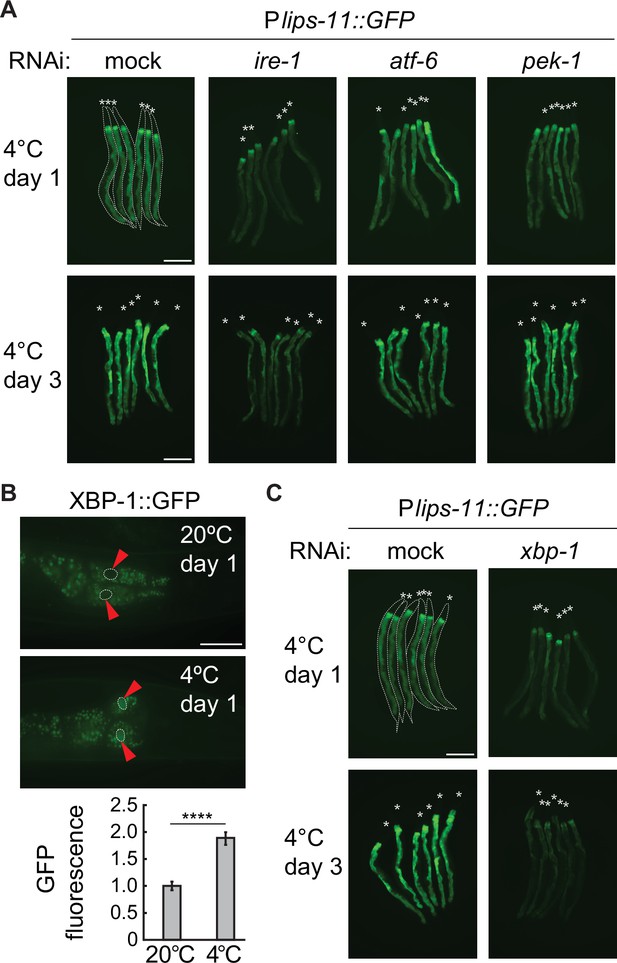
Cold-induced transcription of lips-11 depends on the IRE-1/XBP-1 pathway.
(A) Micrographs of several bundled animals expressing the Plips-11::GFP reporter, taken at the indicated conditions. Note a strong decrease in GFP expression after 1 and 3 days at 4 °C upon RNAi-mediated knockdown of ire-1, but not of atf-6 or pek-1. The animals are outlined in the top-left panel and the heads are indicated by asterisks (A and C). The scale bar = 200 µm. (B) Top: Representative micrographs of adult animals expressing the Pxbp-1::xbp-1::GFP splicing reporter at the indicated temperatures and time points. GFP is expressed in frame only upon removal of the IRE-1-regulated intron in xbp-1 mRNA. Arrowheads indicate the outlined nuclei of the most anterior pair of intestinal cells. The scale bar = 40 µm. Below is the corresponding quantification of the nuclear GFP relative to 20 °C. Between two and five nuclei were analyzed per animal, in at least fifteen animals per condition. Error bars indicate the SEM of three biological replicates. Unpaired two-sided t-test was used for statistical analysis. ****p<0.0001. (C) Micrographs of mock or xbp-1 RNAi-treated Plips-11::GFP reporter animals, kept for 1 or 3 days at 4 °C. The scale bar = 200 µm.
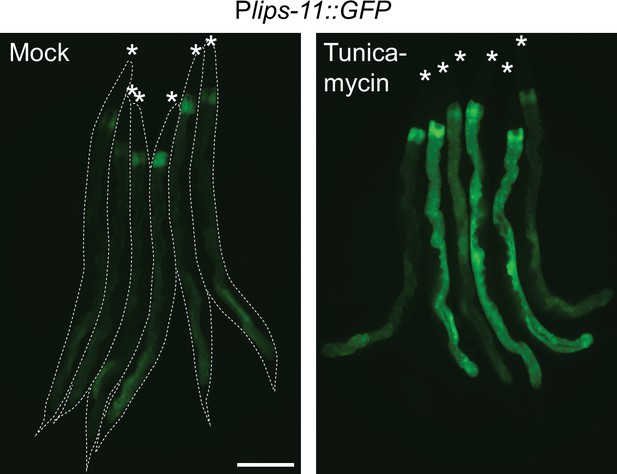
Micrographs of bundled Plips-11::GFP reporter animals after 6 hr of treatment with DMSO (mock) or tunicamycin at 20 °C.
Animals are outlined in the left panel and the heads are indicated with asterisks. The scale bar = 200 µm.
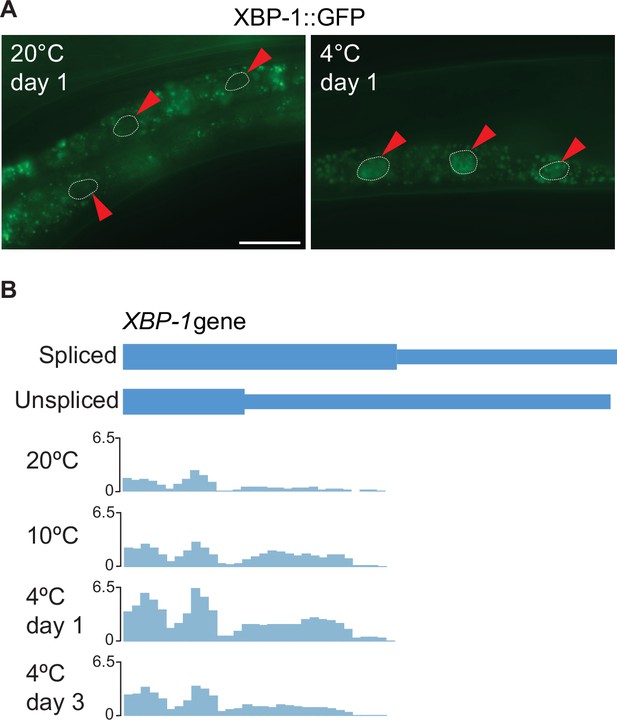
XBP-1 splicing at different temperatures.
(A) Micrographs depicting the middle part of adult animals expressing the Pxbp-1::xbp-1::GFP splicing reporter at the indicated temperatures and time points. GFP is expressed in frame only upon removal of the IRE-1-regulated intron in xbp-1 mRNA. Arrowheads indicate outlined intestinal nuclei. The scale bar = 40 µm. (B) Coverage of ribosome protected fragments (RPF) over the two isoforms of xbp-1 showing the increased translation of the extended (Spliced) proteoform after 2 hr at 10 °C and subsequent days at 4 °C as compared to 20 °C day 0. All tracks are on the same scale, normalized to their respective library sizes and multiplied by a scale factor (10^6).
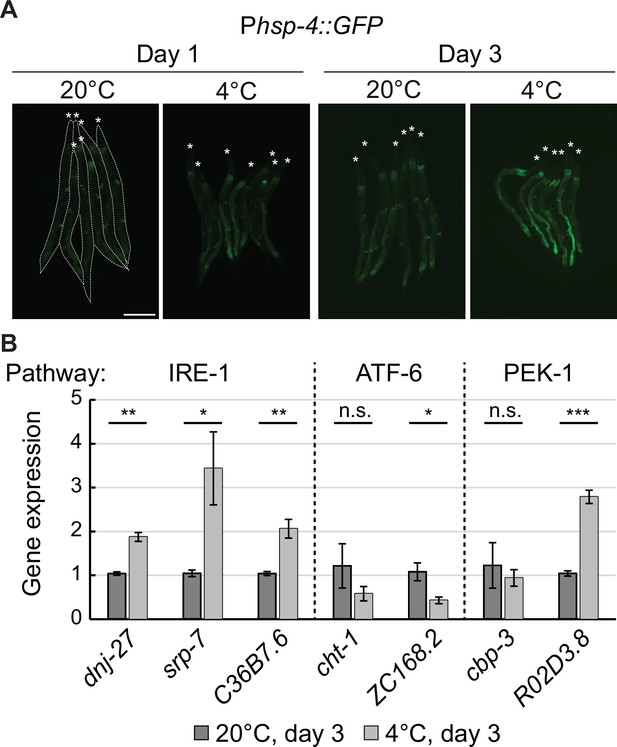
Cold specifically activates UPRER through IRE-1.
(A) Micrographs of several bundled animals carrying the Phsp-4::GFP reporter, taken at the indicated temperature and time. The animals are outlined in the left and the heads are indicated by asterisks. The scale bar = 200 µm. (B) RT-qPCR analysis of changes in mRNA levels for known UPRER target genes after 3 days at 4 °C, relative to 3 days at 20 °C. Dashed lines separate genes that are regulated by different pathways (IRE-1, ATF-6, and PEK-1). Note that the mRNA levels of all IRE-1 responsive genes are significantly upregulated at 4 °C. Error bars indicate the SEM of three biological replicates. Unpaired two-sided t-test was used for statistical analysis. ns: p>0.05, *p<0.05, **p<0.01, ***p<0.001.
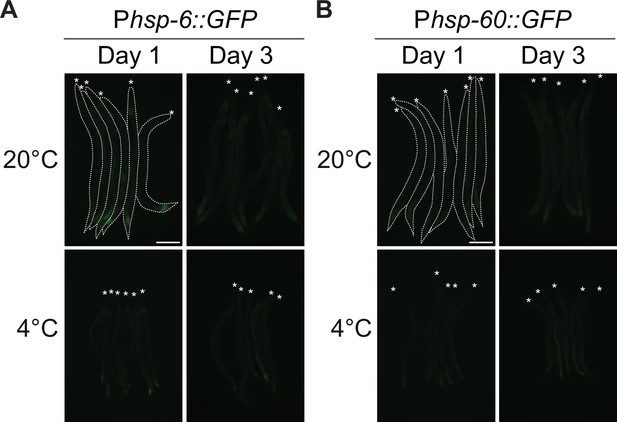
Mitochondrial UPR monitored as indicated.
(A) Micrographs of bundled Phsp-6::GFP UPRmito reporter animals, taken at the indicated time and temperature. Animals are outlined in the top-left panel and the heads are indicated with asterisks. The scale bar = 200 µm. (A and B). (B) Micrographs of bundled Phsp-60::GFP UPRmito reporter animals, taken at the indicated time and temperature.
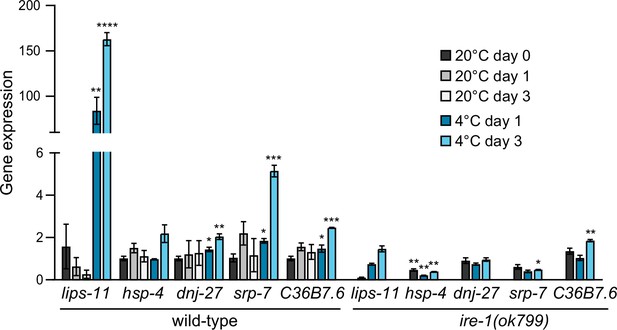
RT-qPCR analysis of changes in mRNA levels for known IRE-1 target genes in wild-type and ire-1(ok799) animals after 1 or 3 d at 20 °C or 4 °C, relative to wild-type animals at 20 °C day 0 (harvested immediately prior to cold adaptation at 10 °C).
Note that the mRNA levels of most IRE-1 responsive genes are significantly upregulated at 4 °C in wild-type and remain unchanged or are significantly downregulated in ire-1(ok799) mutants. Error bars indicate the SEM of three biological replicates. Unpaired two-sided t-test was used for statistical analysis. Unmarked bars: p>0.05 (not significant), *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
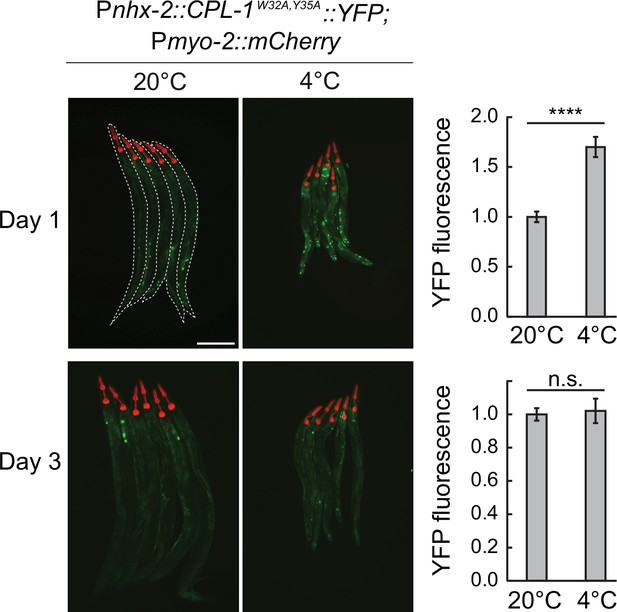
Misfolded protein levels increase transiently during cold exposure.
Left: representative micrographs, taken at the indicated conditions, of bundled animals carrying the CPL-1W32A,Y35A::YFP misfolding reporter. The head of each animal is highlighted by the pharyngeal expression of mCherry, driven by the myo-2 promoter. Animals are outlined in the top-left panel. The scale bar = 200 µm. Right: the corresponding quantification of YFP fluorescence at 4 °C after 1 or 3 days, relative to 20 °C. The YFP fluorescence was analyzed from whole animals, with a minimum of 38 animals per condition. Error bars indicate the SEM of three biological replicates. Unpaired two-sided t-test was used for statistical analysis. ns: p>0.05, ****p<0.0001.
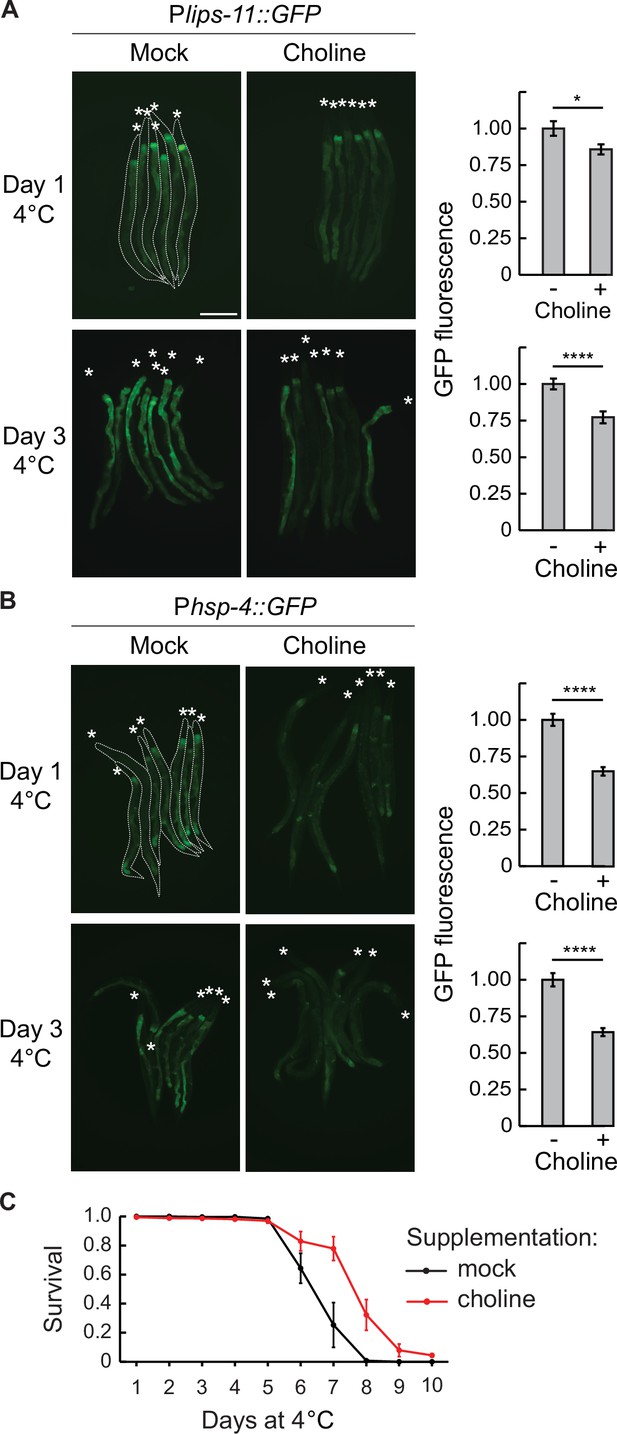
Choline supplementation suppresses UPRER and improves cold survival.
(A) Left: micrographs taken after 1 or 3 days at 4 °C of bundled Plips-11::GFP reporter animals on a mock or 50 mM choline supplemented diet. Animals are outlined in the top-left panel and the heads are indicated with asterisks (A and B). The scale bar = 200 µm. Right: the corresponding quantification of intestinal GFP fluorescence after 1 or 3 days at 4 °C on a choline-supplemented diet, relative to a mock diet. GFP fluorescence was analyzed from a minimum of 38 animals per condition. Error bars indicate the SEM of three biological replicates. Unpaired two-sided t-test was used for statistical analysis. *p<0.05, ****p<0.0001. (B) Left: Micrographs taken after 1 or 3 days at 4 °C of bundled Phsp-4::GFP reporter animals on a mock or 50 mM choline supplemented diet. The scale bar = 200 µm. Right: the corresponding quantification of intestinal GFP fluorescence after 1 or 3 days at 4 °C on a choline supplemented diet, relative to a mock diet. GFP fluorescence was analyzed from a minimum of 28 animals per condition. Error bars indicate the SEM of three biological replicates. Unpaired two-sided t-test was used for statistical analysis. ****p<0.0001. (C) Survival of wild-type animals on a mock or choline-supplemented diet. Error bars indicate the SEM of three biological replicates. A minimum of 200 animals were scored per time point. Wilcoxon signed-rank test was used for statistical analysis; p=0.02.
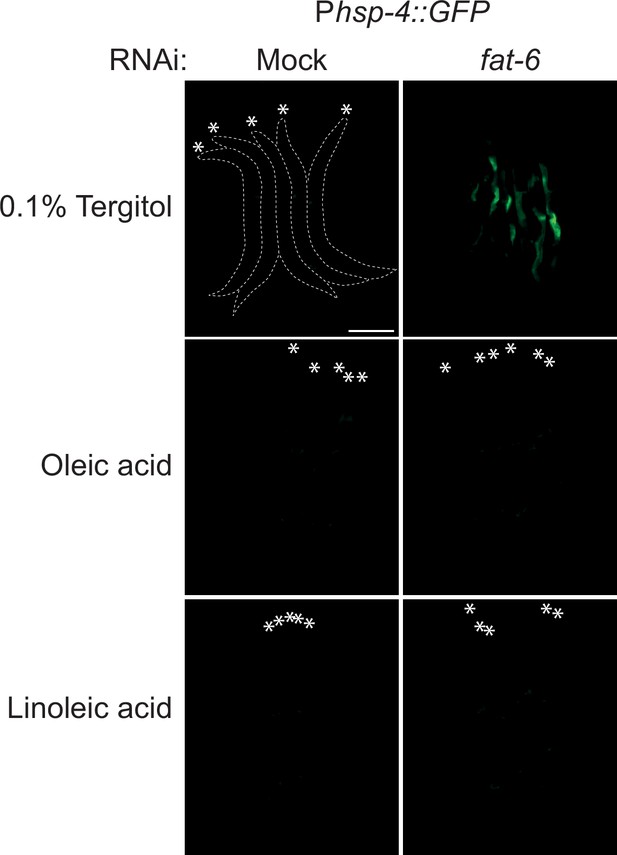
Micrographs of bundled animals expressing the Phsp-4::GFP reporter were subjected to either mock or fat-6 RNAi and supplemented with either 0.1% tergitol (mock), 0.8 mM oleic acid, or 0.8 mM linoleic acid.
Note a strong increase in the GFP reporter upon RNAi-mediated knockdown of fat-6 in animals on the mock diet, which is suppressed by the supplementation with either oleic or linoleic acid. The animals are outlined in the top-left panel and the heads are indicated by asterisks. The scale bar = 200 µm.
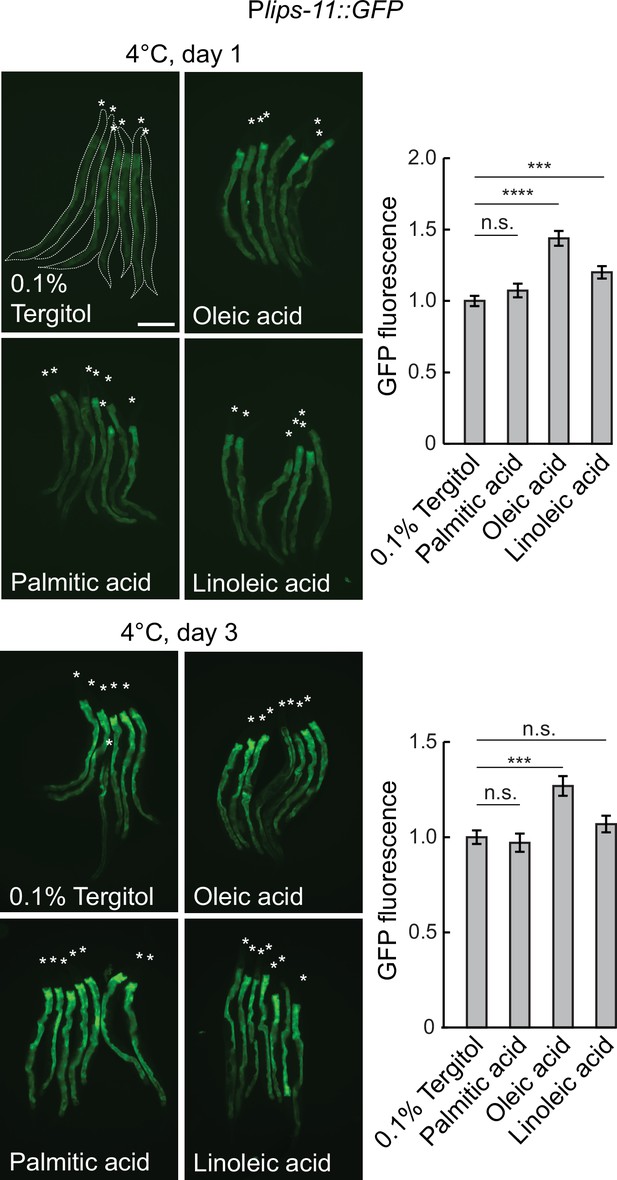
Dietary supplementation of fatty acids.
Left: Micrographs taken after 1 or 3 d at 4 °C of bundled Plips-11::GFP reporter animals on the diet supplemented with 0.1% tergitol (mock), 0.8 mM oleic acid, 0.8 mM palmitic acid, or 0.8 mM linoleic acid. Animals are outlined in the top-left panel and the heads are indicated with asterisks. The scale bar = 200 µm. Right: the corresponding quantification of intestinal GFP fluorescence after 1 or 3 d at 4 °C on different diets, relative to the mock diet. The GFP fluorescence was analyzed from 21 to 31 animals per condition. Error bars indicate the SEM of three biological replicates. Unpaired two-sided t-test was used for statistical analysis. N.s.: p>0.05, ***p<0.001, ****p<0.0001.
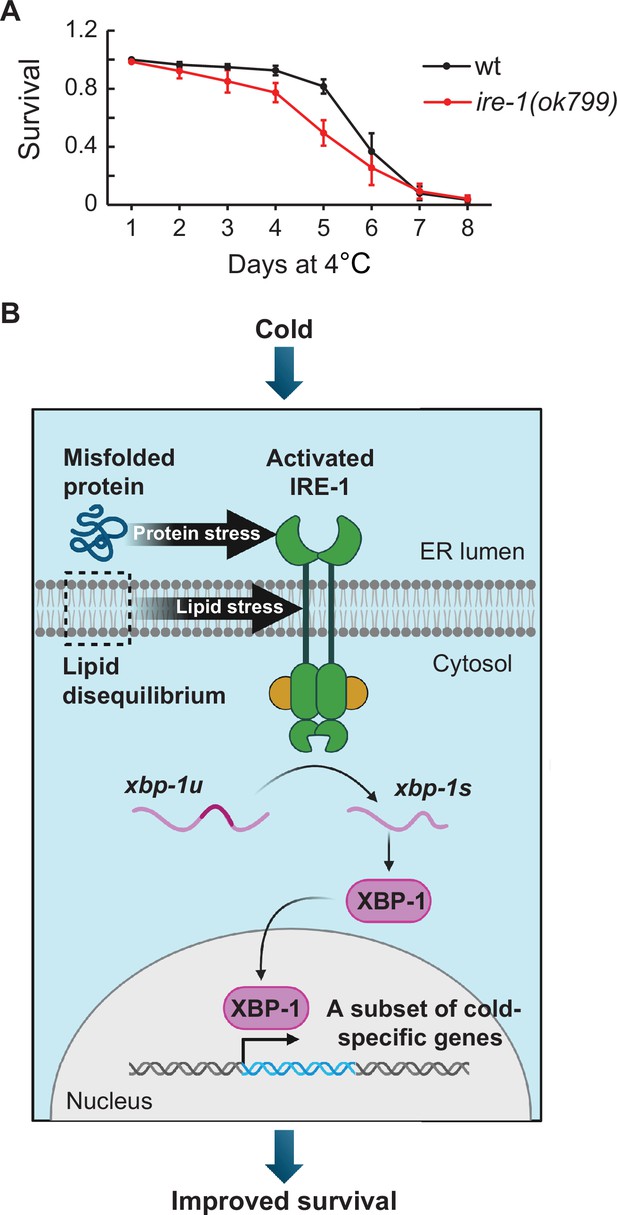
The IRE-1 pathway facilitates C. elegans survival during cold dormancy.
(A) Survival of wild-type and ire-1(ok799) animals at 4 °C. Error bars indicate the SEM from six biological replicates. A minimum of 800 animals were scored per time point. Wilcoxon signed-rank test was used for statistical analysis; p=0.03. (B) A model for the IRE-1 function in hibernating nematodes. Cold exposure leads to increased protein misfolding and lipid disequilibrium in the endoplasmic reticulum (ER). These two stressors trigger the activation of IRE-1, which promotes the downstream processing of xbp-1u to xbp-1s mRNA. The functional XBP-1 transcription factor enters the nucleus to promote transcription of specific genes, including those facilitating cold survival. Created with BioRender.com.
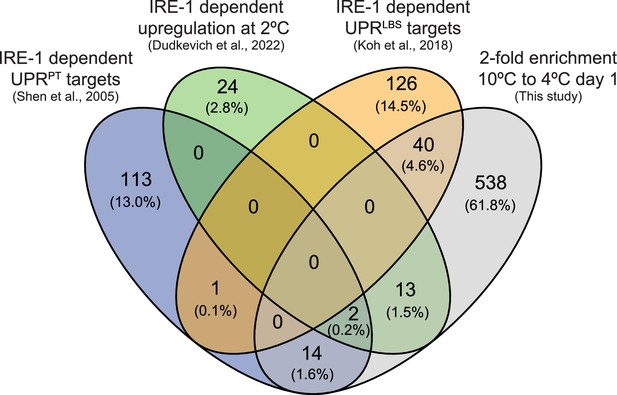
Four-way Venn diagram depicting the overlap between genes with a minimum twofold enrichment (10°C to 4°C day 1) and previously reported IRE-1 responsive genes that are upregulated either during the UPRPT (Shen et al., 2005), upon shifting animals from 15°C to 2°C (Dudkevich et al., 2022), or during the UPRLBS (Koh et al., 2018).
Additional files
-
Supplementary file 1
Total RNA-seq data of wild-type animals kept at the different indicated temperatures.
- https://cdn.elifesciences.org/articles/101186/elife-101186-supp1-v1.csv
-
Supplementary file 2
Ribo-seq data of wild-type animals kept at the different indicated temperatures.
- https://cdn.elifesciences.org/articles/101186/elife-101186-supp2-v1.csv
-
Supplementary file 3
Genes highlighted in Figure 2A in red.
- https://cdn.elifesciences.org/articles/101186/elife-101186-supp3-v1.csv
-
Supplementary file 4
IRE-1 responsive genes from published datasets were used to generate the Venn diagram in Figure 7—figure supplement 1.
The IRE-1 dependent cold genes were selected from Table S2 and Table S3 (Dudkevich et al., 2022): the genes were selected if upregulated in wild-type animals but not ire-1(ok799) mutants at 2 °C.
- https://cdn.elifesciences.org/articles/101186/elife-101186-supp4-v1.csv
-
Supplementary file 5
Shared genes from the Venn diagram in Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/101186/elife-101186-supp5-v1.xlsx
-
Supplementary file 6
The C. elegans strains (A) and oligos (B) were used in this study.
- https://cdn.elifesciences.org/articles/101186/elife-101186-supp6-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/101186/elife-101186-mdarchecklist1-v1.pdf





