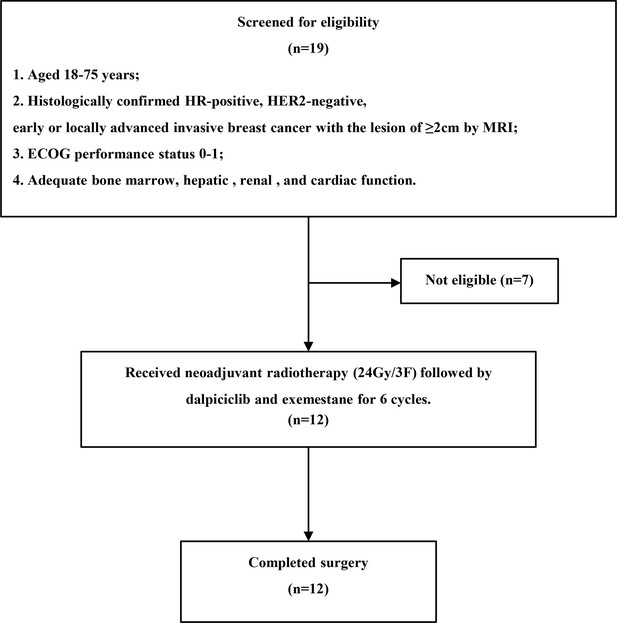
Efficacy and safety of neoadjuvant stereotactic body radiation therapy plus dalpiciclib and exemestane for hormone receptor-positive, HER2-negative breast cancer: A prospective pilot study
Figures
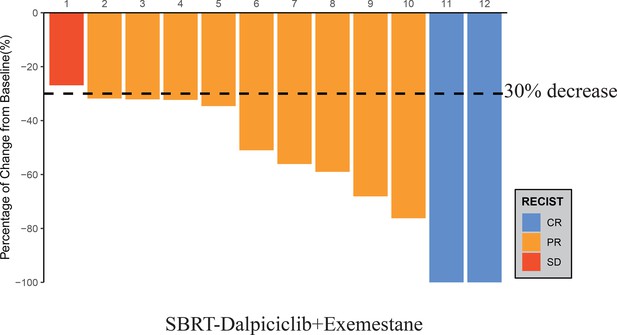
Waterfall plot of best reduction in tumor measurement from baseline.
-
Figure 1—source data 1
Source data for waterfall plot of best reduction in tumor measurement from baseline.
- https://cdn.elifesciences.org/articles/101583/elife-101583-fig1-data1-v1.xlsx
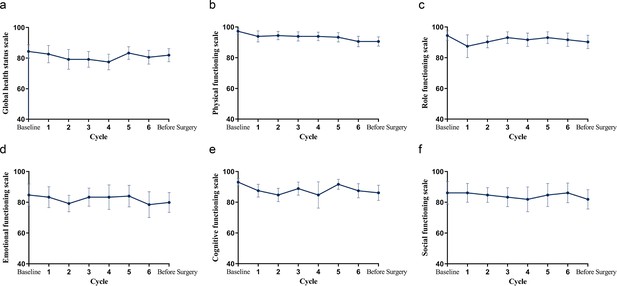
Mean change from baseline in QLQ-C30 over time.
(a) Global health status. (b–f) Physical, role, emotional, cognitive, and social functioning.
-
Figure 2—source data 1
Source data for mean change from baseline in QLQ-C30 over time.
- https://cdn.elifesciences.org/articles/101583/elife-101583-fig2-data1-v1.xlsx
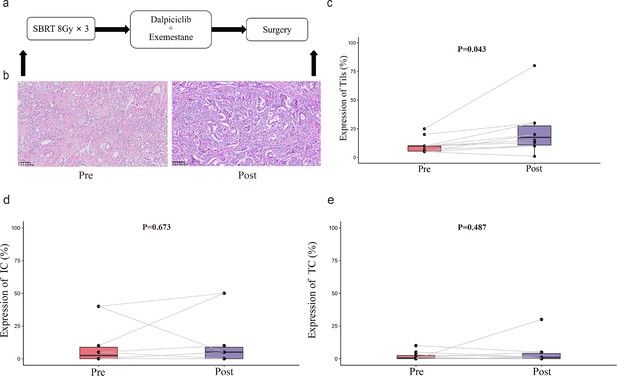
TILs and PD-L1 changes before and after treatment.
(a) Study schema. (b) Representative TILs. Scale bar, 100 μM. (c–e) Percentage of TILs, PD-L1 expression in immune cell and tumor cell in paired specimens (n=10, pre- and post-treatment). Bars, boxes, and whiskers represent median, interquartile range, and range, respectively. Statistical analysis performed using paired t-test, statistical significance was defined as p<0.05.
-
Figure 3—source data 1
Source data for percentage of TILs in paired specimens.
- https://cdn.elifesciences.org/articles/101583/elife-101583-fig3-data1-v1.xlsx
-
Figure 3—source data 2
Source data for PD-L1 expression in immune cell in paired specimens.
- https://cdn.elifesciences.org/articles/101583/elife-101583-fig3-data2-v1.xlsx
-
Figure 3—source data 3
Source data for PD-L1 expression in tumor cell in paired specimens.
- https://cdn.elifesciences.org/articles/101583/elife-101583-fig3-data3-v1.xlsx
Tables
Baseline characteristics of the patients.
| Patients (n=12) | |
|---|---|
| Median age (range), years | 48.5 (36-56) |
| ≤50 | 6 (50.0%) |
| >50 | 6 (50.0%) |
| Menopausal status | |
| Premenopausal | 6 (50.0%) |
| Postmenopausal | 6 (50.0%) |
| Tumor size | |
| T2 | 11 (91.7%) |
| T3 | 1 (8.3%) |
| Lymph node status | |
| N0 | 5 (41.7%) |
| N1 | 2 (16.7%) |
| N2 | 5 (41.7%) |
| Clinical stage | |
| IIA | 4 (33.3%) |
| IIB | 3 (25.0%) |
| IIIA | 5 (41.7%) |
| Tumor grade | |
| II | 11 (91.7%) |
| III | 1 (8.3%) |
| ER expression | |
| >10% | 12 (100.0%) |
| PgR expression | |
| <20% | 3 (25.0%) |
| ≥20% | 9 (75.0%) |
| HER2 expression | |
| 0 | 7 (58.3%) |
| 1+ | 1 (8.3%) |
| 2+, FISH | 4 (33.3%) |
| Median baseline Ki67 (range), % | 35 (15–70) |
| ≤14% | 3 (25.0%) |
| >14% | 9 (75.0%) |
-
Data are n (%) or median (range).
-
ER: estrogen receptor; PgR: progesterone receptor; HER2: human epidermal growth factor receptor 2; FISH: fluorescence in situ hybridization.
Pathological and clinical response (n=12).
| n (%) | |
|---|---|
| Total pathological complete response | 2 (16.7%) |
| Breast pathological complete response | 3 (25.0%) |
| Residual cancer burden score | |
| 0 | 2 (16.7%) |
| I | 0 |
| II | 6 (50.0%) |
| III | 4 (33.3%) |
| Radiological response | |
| Complete response | 2 (16.7%) |
| Partial response | 9 (75.0%) |
| Stable disease | 1 (8.3%) |
| Objective response rate | 11 (91.7%) |
| Preoperative endocrine prognostic index | |
| 0 | 2 (16.7%) |
| 1–3 | 0 |
| ≥4 | 10 (83.3%) |
-
Data are presented as n (%).
Treatment-emergent adverse events (n=12).
| n (%) | |||
|---|---|---|---|
| Grade 1 or 2 | Grade 3 | Grade 4 | |
| Neutropenia | 3 (25.0%) | 8 (66.7%) | 0 |
| Leukopenia | 7 (58.3%) | 3 (25.0%) | 0 |
| Hot flushes | 6 (50.0%) | 0 | 0 |
| Lymphopenia | 6 (50.0%) | 0 | 0 |
| Insomnia | 4 (33.3%) | 0 | 0 |
| Alopecia | 4 (33.3%) | 0 | 0 |
| Anemia | 4 (33.3%) | 0 | 0 |
| Hyperuricemia | 4 (33.3%) | 0 | 0 |
| Blood urea increased | 4 (33.3%) | 0 | 0 |
| Rash | 3 (25.0%) | 0 | 0 |
| Thrombocytopenia | 3 (25.0%) | 0 | 0 |
| Hyperglycemia | 3 (25.0%) | 0 | 0 |
| Creatinine increased | 3 (25.0%) | 0 | 0 |
| Fatigue | 3 (25.0%) | 0 | 0 |
| Arthralgia | 2 (16.7%) | 0 | 0 |
| γ-glutamyl transferase increased | 2 (16.7%) | 0 | 0 |
| Hypocalcemia | 2 (16.7%) | 0 | 0 |
| Abnormal T wave of electrocardiogram | 2 (16.7%) | 0 | 0 |
| Hyponatremia | 2 (16.7%) | 0 | 0 |
| Lactate dehydrogenase increased | 2 (16.7%) | 0 | 0 |
| Diarrhea | 1 (8.3%) | 0 | 0 |
| Headache | 1 (8.3%) | 0 | 0 |
| Mucosal inflammation Constipation | 1 (8.3%) | 0 | 0 |
| Proteinuria | 1 (8.3%) | 0 | 0 |
| Hypertriglyceridemia | 1 (8.3%) | 0 | 0 |
| Alanine aminotransferase increased | 1 (8.3%) | 0 | 0 |
-
Data are presented as n (%).