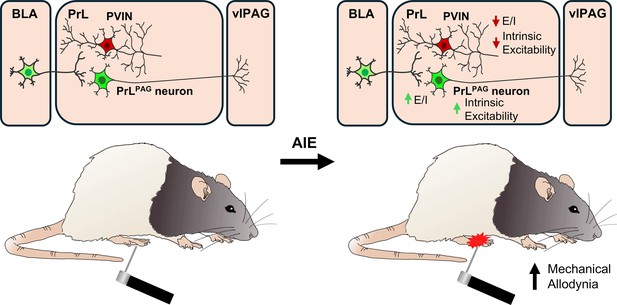
Adolescent alcohol exposure promotes mechanical allodynia and alters synaptic function at inputs from the basolateral amygdala to the prelimbic cortex
Figures
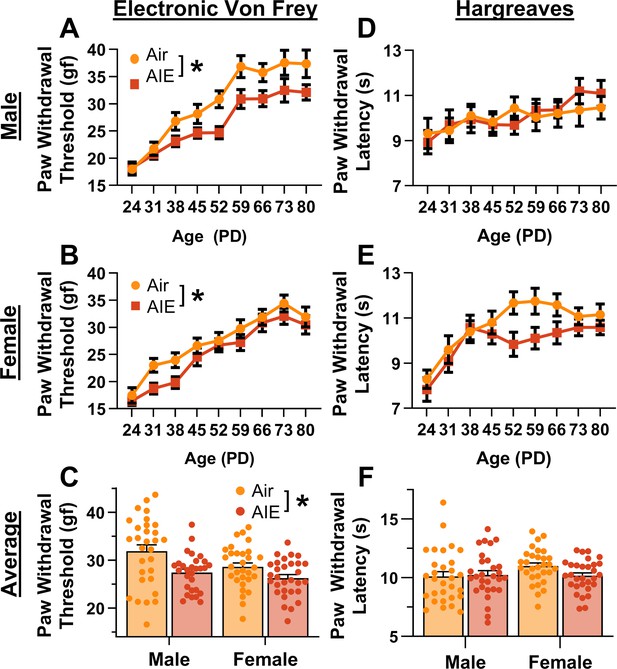
Mechanical and thermal sensitivity of rats during adolescence and early adulthood.
Depiction of the effect of adolescent intermittent ethanol (AIE) exposure on mechanical sensitivity across adolescence and into early adulthood in male (A) and female (B) rats. (C) AIE exposure significantly reduced the average (PD 31–PD 80) electronic Von Frey (eVF) withdrawal threshold, indicating increased mechanical touch sensitivity. Depiction of the effect of AIE exposure on thermal sensitivity during adolescence and early adulthood in male (D) and female (E) rats. (F) AIE exposure did not significantly alter the average (PD 31–PD 80) Hargreaves test withdrawal latency, indicating no change in thermal sensitivity. Data represent the mean ± SEM. Source data for all panels is included in Figure 1—source data 1. Data were analyzed using ANOVA, with exposure (AIR vs. AIE), sex, and age (when applicable) as factors. * indicates a significant difference between the related conditions; p < 0.05; n = 29–30 rats/group.
-
Figure 1—source data 1
Numerical data for mechanical and thermal sensitivity of rats during adolescence.
- https://cdn.elifesciences.org/articles/101667/elife-101667-fig1-data1-v1.xlsx
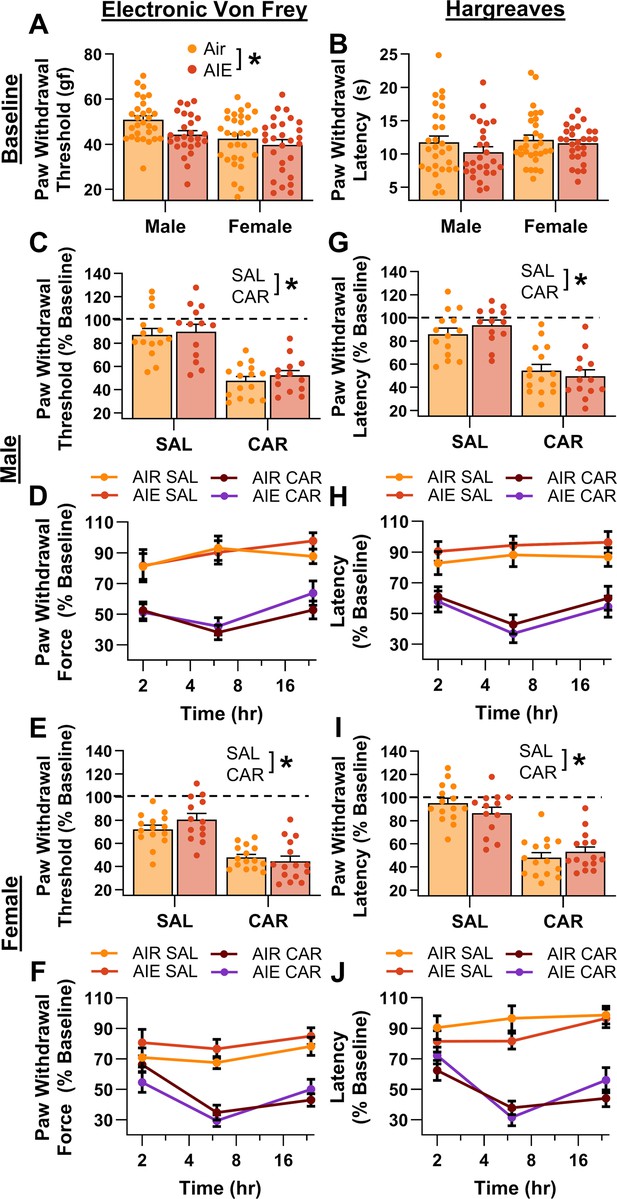
Mechanical and thermal sensitivity of rats in response to a carrageenan paw pain challenge.
(A) Baseline mechanical touch sensitivity was greater in adolescent intermittent ethanol (AIE) exposed rats than in Air control rats. (B) There was no difference in baseline thermal sensitivity between AIE exposed and Air control rats. Male (C, D) and female (E, F) rats injected with carrageenan (CAR) in the right hindpaw displayed mechanical hypersensitivity. This hypersensitivity was not significantly altered by AIE exposure. Average paw withdrawal threshold combining all post-injection timepoints for males (C) and females (E). Paw withdrawal threshold expressed as a percentage of the baseline threshold at 2, 6, and 24 hr post-injection for males (D) and females (F). Similarly, male (G, H) and female (I, J) rats injected with CAR into the right hindpaw displayed thermal hyperalgesia, with no effect of AIE exposure on this hyperalgesia. Average paw withdrawal latency across all post-injection timepoints for male (G) and female (I) rats. Paw withdrawal latency as a percentage of the baseline latency at 2, 6, and 24 hr post-injection for male (H) and female (J) rats. Data represent the mean ± SEM. Source data for all panels is included in Figure 2—source data 1. Data were analyzed using ANOVA, with exposure (AIR vs. AIE), treatment (CAR vs. SAL), and sex as factors. * indicates a significant difference between the related conditions; p < 0.05; n = 13–15 rats/group.
-
Figure 2—source data 1
Numerical data for mechanical and thermal sensitivity of rats in response to a carrageenan paw pain challenge.
- https://cdn.elifesciences.org/articles/101667/elife-101667-fig2-data1-v1.xlsx
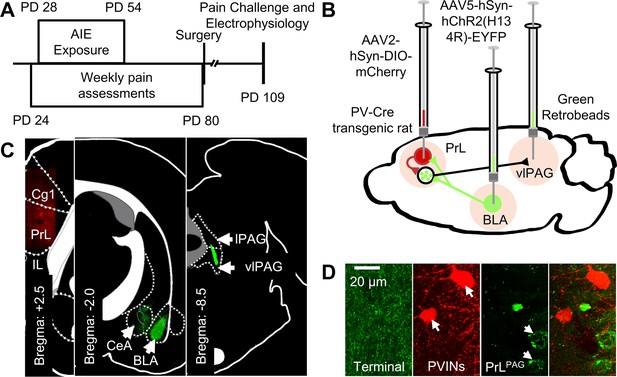
Experimental approach.
(A) Experimental timeline displaying the age of animals at each phase in the study. (B) Diagram showing the viral and retrobead labeling approach used to identify and manipulate specific neuronal populations in the prelimbic (PrL) cortex. (C) Representative images showing injection sites in the PrL (left panel, AAV2-hSyn-DIO-mCherry), basolateral amygdala (BLA, center panel, AAV5-hSyn-hChR2(H134R)-EYFP), and ventrolateral periaqueductal gray (vlPAG, right panel, green retrobeads). (D) Representative images from the PrL cortex (from left to right) of BLA terminals, mCherry-tagged parvalbumin interneurons (PVINs), and green retrobead labeled PrLPAG neurons.
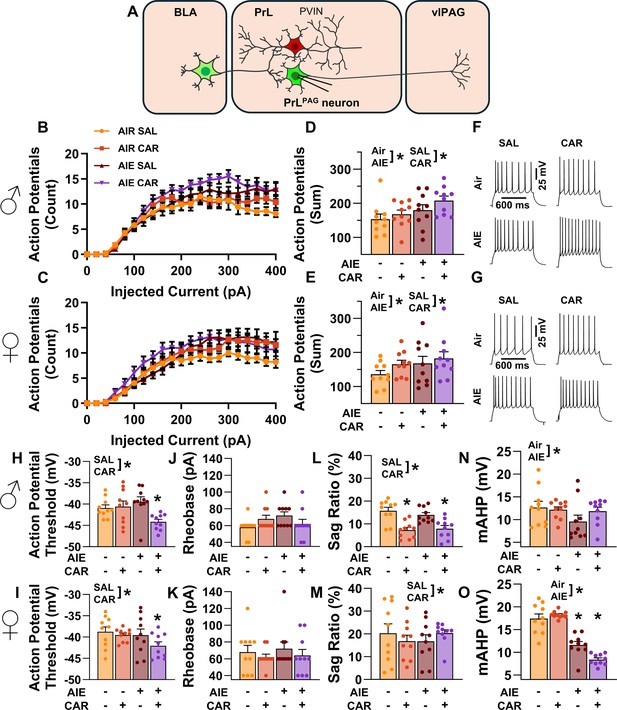
Intrinsic excitability of pyramidal neurons projecting from the prelimbic cortex to the ventrolateral periaqueductal gray (PrLPAG).
(A) Electrophysiological recordings were obtained from PrLPAG neurons in the PrL cortex. Depiction of the relationship between injected current and action potential firing in male (B) and female (C) rats across treatment conditions. The cumulative number of action potentials fired across all current steps was increased by both adolescent intermittent ethanol (AIE) exposure and a carrageenan paw pain challenge (CAR) in male (D) and female (E) rats. Representative traces showing action potential spiking across treatment conditions in male (F) and female (G) rats. The action potential threshold of PrLPAG neurons was reduced in AIE exposed, CAR treated male (H) and female (I) rats. The rheobase of PrLPAG neurons was unaltered by AIE exposure or CAR treatment in male (J) and female (K) rats. Ih-dependent voltage sag was attenuated by carrageenan, with a larger reduction occurring in male rats (L) than in female rats (M). Afterhyperpolarization (mAHP) was attenuated by AIE exposure, with a smaller reduction occurring in male rats (N) than in female rats (O). Data represent the mean ± SEM. Source data for all panels is included in Figure 4—source data 1. Data were analyzed using ANOVA, with exposure (AIR vs. AIE), treatment (CAR vs. SAL), sex, and injected current (when applicable) as factors. * indicates a significant difference between the related conditions; p < 0.05; n = 10 rats/group.
-
Figure 4—source data 1
Numerical data for the intrinsic excitability and selected biophysical properties of PrLPAG neurons.
- https://cdn.elifesciences.org/articles/101667/elife-101667-fig4-data1-v1.xlsx
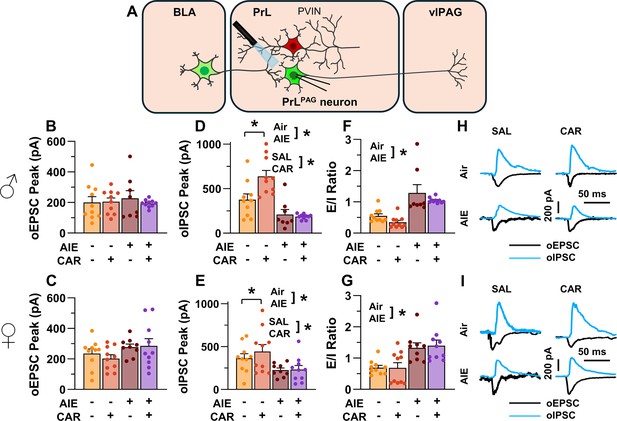
Optically evoked postsynaptic excitatory and inhibitory currents onto pyramidal neurons projecting from the prelimbic cortex to the ventrolateral periaqueductal gray (PrLPAG).
(A) Electrophysiological recordings were obtained from PrLPAG neurons in the PrL cortex. The amplitude of optically evoked excitatory postsynaptic currents (oEPSCs) was not significantly altered by adolescent intermittent ethanol (AIE) exposure or a carrageenan paw pain challenge (CAR) in either male (B) or female (C) rats. In contrast, the amplitude of optically evoked inhibitory postsynaptic currents (oIPSCs) was significantly reduced in both male (D) and female (E) AIE exposed rats. Carrageenan enhanced the amplitude of oIPSCs, but this increase was attenuated in AIE exposed rats. Examination of the oEPSC/oIPSC (excitation/inhibition, E/I) ratios as a measure of excitatory–inhibitory balance at basolateral amygdala (BLA) inputs onto PrLPAG neurons revealed that in AIE exposed animals, the E/I balance was significantly increased in both male (F) and female (G) rats. (H) Representative traces of the oEPSC and oIPSC currents recorded from male rats across all treatment groups. (I) Representative traces of oEPSC and oIPSC currents recorded from female rats across all treatment groups. Data represent the mean ± SEM. Source data for all panels is included in Figure 5—source data 1. Data were analyzed using ANOVA, with exposure (AIR vs. AIE), treatment (CAR vs. SAL), and sex as factors. * indicates a significant difference between the related conditions; p < 0.05; n = 8–10 rats/group.
-
Figure 5—source data 1
Numerical data characterizing optically evoked postsynaptic excitatory and inhibitory currents onto PrLPAG neurons.
- https://cdn.elifesciences.org/articles/101667/elife-101667-fig5-data1-v1.xlsx
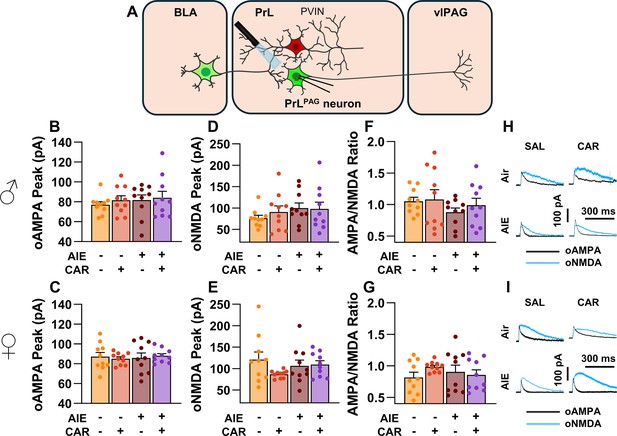
Optically evoked AMPA and NMDA currents at monosynaptic inputs from the basolateral amygdala (BLA) onto pyramidal neurons projecting from the prelimbic cortex to the ventrolateral periaqueductal gray (PrLPAG).
(A) Electrophysiological recordings were obtained from PrLPAG neurons in the PrL cortex. The amplitude of optically evoked AMPA currents was not altered by adolescent intermittent ethanol (AIE) exposure or a carrageenan paw pain challenge (CAR) in either male (B) or female (C) rats. Similarly, the amplitude of optically evoked NMDA currents was unchanged across all treatment conditions in both male (D) and female (E) rats. The AMPA/NMDA ratio was also not significantly altered by AIE or CAR in male (F) or female (G) rats. (H) Representative traces of optically evoked AMPA and NMDA currents recorded from male rats across all treatment groups. (I) Representative traces of optically evoked AMPA and NMDA currents recorded from female rats across all treatment groups. Data represent the mean ± SEM. Source data for all panels is included in Figure 6—source data 1. Data were analyzed using ANOVA, with exposure (AIR vs. AIE), treatment (CAR vs. SAL), and sex as factors. n = 10 rats/group.
-
Figure 6—source data 1
Numerical data characterizing optically evoked AMPA and NMDA currents at monosynaptic inputs from the basolateral amygdala (BLA) onto PrLPAG neurons.
- https://cdn.elifesciences.org/articles/101667/elife-101667-fig6-data1-v1.xlsx
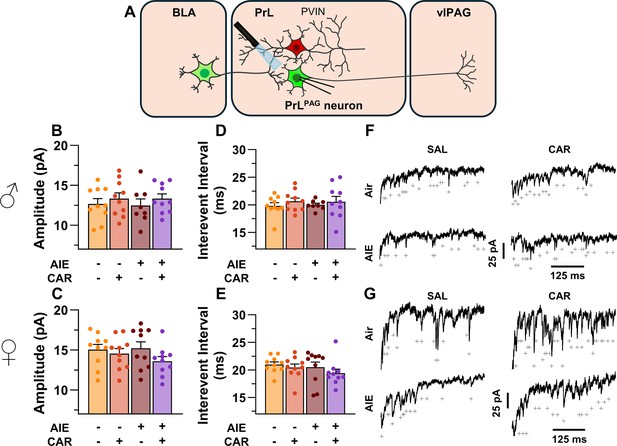
Optically evoked asynchronous excitatory postsynaptic currents (aEPSCs) at monosynaptic inputs from the basolateral amygdala (BLA) onto pyramidal neurons projecting from the prelimbic cortex to the ventrolateral periaqueductal gray (PrLPAG).
(A) Electrophysiological recordings were obtained from PrLPAG neurons in the PrL cortex. When compared across treatment conditions, there were no differences in either the amplitude (B, C) or interevent interval (D, E) of aEPSCs. (F) Representative traces of aEPSCs recorded from male rats across all treatment groups. (G) Representative traces of aEPSCs recorded from female rats across all treatment groups. Data represent the mean ± SEM. Source data for all panels is included in Figure 7—source data 1. On the current traces, a + indicates an asynchronous event. Data were analyzed using ANOVA, with exposure (AIR vs. AIE), treatment (CAR vs. SAL), and sex as factors. n = 8–10 rats/group.
-
Figure 7—source data 1
Numerical data characterizing optically evoked asynchronous excitatory postsynaptic currents (aEPSCs) at monosynaptic inputs from the basolateral amygdala (BLA) onto PrLPAG neurons.
- https://cdn.elifesciences.org/articles/101667/elife-101667-fig7-data1-v1.xlsx
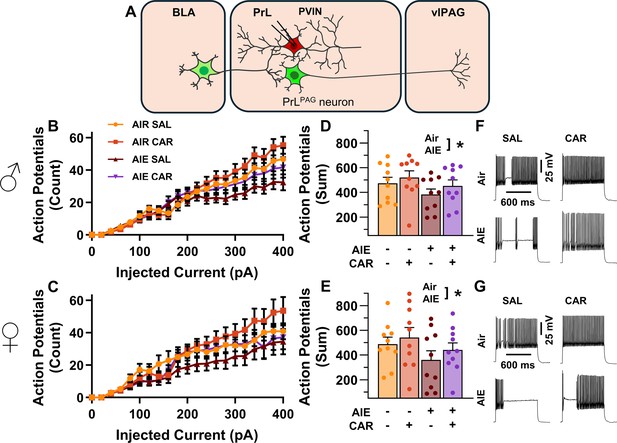
Intrinsic excitability of prelimbic (PrL) parvalbumin interneurons (PVINs).
(A) Electrophysiological recordings were obtained from PVINs in the PrL cortex. (B) Depiction of the relationship between injected current and action potential firing in male rats across treatment conditions. (C) Depiction of the relationship between injected current and action potential firing in female rats across treatment conditions. (D) Adolescent intermittent ethanol (AIE) exposure reduced the cumulative number of action potentials fired across all current steps in male rats. (E) AIE exposure reduced the cumulative number of action potentials fired across all current steps in female rats. (F) Representative traces showing action potential spiking across treatment conditions in male rats. (G) Representative traces showing action potential spiking across treatment conditions in female rats. Data represent the mean ± SEM. Source data for all panels is included in Figure 8—source data 1. Data were analyzed using ANOVA, with exposure (AIR vs. AIE), treatment (CAR vs. SAL), sex, and injected current (when applicable) as factors. * indicates a significant difference between the related conditions; p < 0.05; n = 10 rats/group.
-
Figure 8—source data 1
Numerical data for the intrinsic excitability of prelimbic (PrL) parvalbumin interneurons (PVINs).
- https://cdn.elifesciences.org/articles/101667/elife-101667-fig8-data1-v1.xlsx
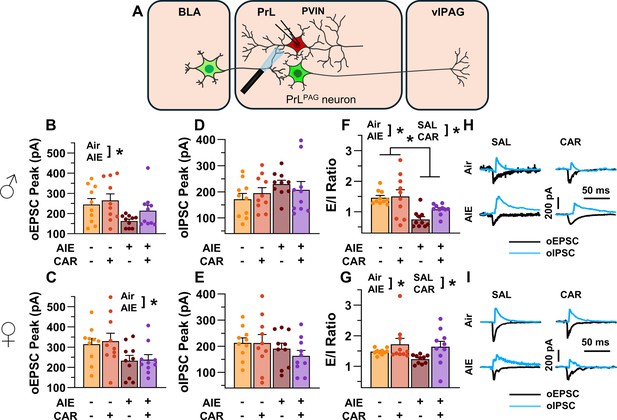
Optically evoked postsynaptic excitatory and inhibitory currents onto prelimbic (PrL) parvalbumin interneurons (PVINs).
(A) Electrophysiological recordings were obtained from PVINs in the PrL cortex. The amplitude of optically evoked excitatory postsynaptic currents (oEPSCs) onto PVINs was found to be significantly reduced by adolescent intermittent ethanol (AIE) exposure in both (B) male and (C) female rats. Quantification of the amplitude of optically evoked inhibitory postsynaptic currents (oIPSCs) revealed that oIPSCs onto PVINs were altered by AIE in a sex-dependent manner, although post hoc analysis did not reveal a significant difference based on any combination of sex and AIE (D, E). Examination of the oEPSC/oIPSC (excitation/inhibition, E/I) ratios as a measure of excitatory–inhibitory balance at basolateral amygdala (BLA) inputs onto PVINs revealed that a carrageenan paw pain challenge (CAR) enhanced the E/I ratio at PVINs in both male (F) and female (G) rats, while AIE reduced the E/I ratio. The effect of AIE on E/I balance was greater in males (F) than in females (G). (H) Representative traces of the oEPSC and oIPSC currents recorded from male rats across all treatment groups. (I) Representative traces of oEPSC and oIPSC currents recorded from female rats across all treatment groups. Data represent the mean ± SEM. Source data for all panels is included in Figure 9—source data 1. Data were analyzed using ANOVA, with exposure (AIR vs. AIE), treatment (CAR vs. SAL), and sex as factors. * indicates a significant difference between the related conditions; p < 0.05; n = 10 rats/group.
-
Figure 9—source data 1
Numerical data characterizing optically evoked postsynaptic excitatory and inhibitory currents onto prelimbic (PrL) parvalbumin interneurons (PVINs).
- https://cdn.elifesciences.org/articles/101667/elife-101667-fig9-data1-v1.xlsx
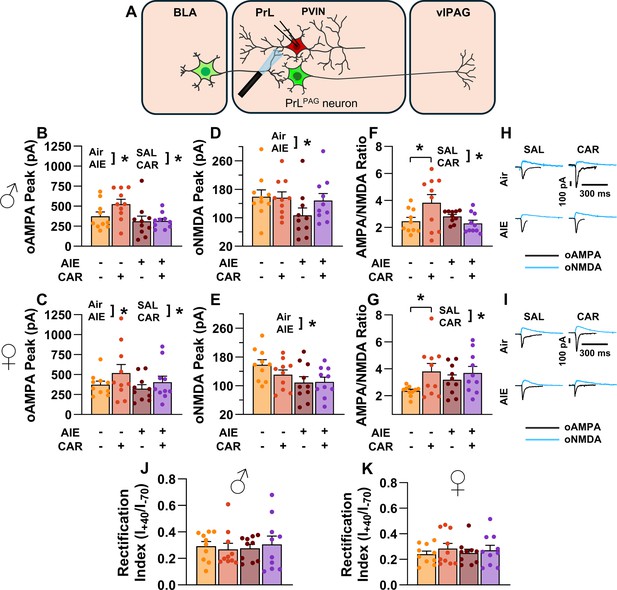
Optically evoked AMPA and NMDA currents at monosynaptic inputs from the basolateral amygdala (BLA) onto prelimbic (PrL) parvalbumin interneurons (PVINs).
(A) Electrophysiological recordings were obtained from PVINs in the PrL cortex. The amplitude of optically evoked AMPA currents was increased by a carrageenan paw pain challenge (CAR) but decreased by adolescent intermittent ethanol (AIE) exposure in both male (B) and female (C) rats. Similarly, AIE reduced the amplitude of optically evoked NMDA currents in both male (D) and female (E) rats. Examination of the AMPA/NMDA ratios revealed that CAR enhanced the AMPA/NMDA ratio at BLA inputs onto PrL PVINs in both male (F) and female (G) rats. However, this increase was attenuated in AIE exposed rats. (H) Representative traces of optically evoked AMPA and NMDA currents recorded from male rats across all treatment groups. (I) Representative traces of optically evoked AMPA and NMDA currents recorded from female rats across all treatment groups. The rectification index was unchanged across treatment conditions in both male (J) and female (K) rats. Data represent the mean ± SEM. Source data for all panels is included in Figure 10—source data 1. Data were analyzed using ANOVA, with exposure (AIR vs. AIE), treatment (CAR vs. SAL), and sex as factors. * indicates a significant difference between the related conditions; p < 0.05; n = 10 rats/group.
-
Figure 10—source data 1
Numerical data characterizing optically evoked AMPA and NMDA currents at monosynaptic inputs from the basolateral amygdala (BLA) onto prelimbic (PrL) parvalbumin interneurons (PVINs).
- https://cdn.elifesciences.org/articles/101667/elife-101667-fig10-data1-v1.xlsx
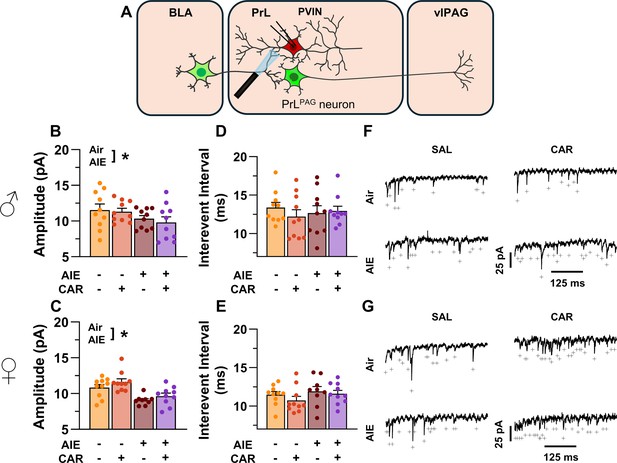
Optically evoked asynchronous excitatory postsynaptic currents (aEPSCs) at monosynaptic inputs from the basolateral amygdala (BLA) onto prelimbic (PrL) parvalbumin interneurons (PVINs).
(A) Electrophysiological recordings were obtained from PVINs in the PrL cortex. Adolescent intermittent ethanol (AIE) exposure was found to decrease the amplitude of aEPSCs from both male (B) and female (C) rats. The interevent interval of aEPSCs, however, was unaltered by either AIE or a carrageenan paw pain challenge (CAR) in male (D) and female (E) rats. (F) Representative traces of aEPSCs recorded from male rats across all treatment groups. (G) Representative traces of aEPSCs recorded from female rats across all treatment groups. Data represent the mean ± SEM. + indicates an asynchronous event. Source data for all panels is included in Figure 11—source data 1. Data were analyzed using ANOVA, with exposure (AIR vs. AIE), treatment (CAR vs. SAL), and sex as factors. * indicates a significant difference between the related conditions; p < 0.05; n = 9–10 rats/group.
-
Figure 11—source data 1
Numerical data characterizing optically evoked asynchronous excitatory postsynaptic currents (aEPSCs) at monosynaptic inputs from the basolateral amygdala (BLA) onto prelimbic (PrL) parvalbumin interneurons (PVINs).
- https://cdn.elifesciences.org/articles/101667/elife-101667-fig11-data1-v1.xlsx
Tables
Biophysical properties of PrLPAG neurons across treatment condition and sex.
| Condition | Sex | Vrest (mV) | Rinput (MΩ) |
|---|---|---|---|
| AIR: SAL | Male | –66.6 ± 0.9 | 75.3 ± 3.5 |
| Female | –65.0 ± 1.7 | 76.3 ± 2.4 | |
| AIR: CAR | Male | –66.2 ± 1.4 | 83.2 ± 5.4 |
| Female | –65.2 ± 1.5 | 83.9 ± 4.1 | |
| AIE: SAL | Male | –65.0 ± 1.4 | 86.9 ± 7.8 |
| Female | –65.6 ± 1.5 | 76.6 ± 2.9 | |
| AIE: CAR | Male | –65.6 ± 1.0 | 88.6 ± 4.3 |
| Female | –66.5 ± 1.2 | 83.4 ± 6.0 | |
Biophysical properties of parvalbumin interneurons (PVINs) across treatment condition and sex.
| Condition | Sex | Vrest (mV) | Rinput (MΩ) | APThresh (mV) | Sag ratio (%) | AHP (mV) |
|---|---|---|---|---|---|---|
| AIR: SAL | Male | –74.3 ± 1.9 | 145.1 ± 5.1 | –42.4 ± 0.7 | 2.6 ± 0.8 | 18.0 ± 0.9 |
| Female | –72.6 ± 1.6 | 155.4 ± 8.8 | –42.3 ± 1.6 | 2.7 ± 0.4 | 15.3 ± 0.2 | |
| AIR: CAR | Male | –72.5 ± 1.9 | 138.1 ± 7.4 | –42.0 ± 1.4 | 1.3 ± 0.5 | 19.4 ± 1.3 |
| Female | –73.7 ± 1.6 | 135.4 ± 8.9 | –41.2 ± 0.8 | 2.3 ± 0.6 | 12.0 ± 1.2 | |
| AIE: SAL | Male | –73.6 ± 1.3 | 155.7 ± 4.9 | –40.4 ± 0.6 | 1.2 ± 0.5 | 16.0 ± 1.8 |
| Female | –74.6 ± 1.8 | 147.0 ± 5.2 | –41.4 ± 1.2 | 3.2 ± 0.8 | 14.1 ± 1.0 | |
| AIE: CAR | Male | –71.6 ± 2.1 | 153.2 ± 8.3 | –44.3 ± 1.6* | 2.6 ± 0.7 | 16.1 ± 1.2 |
| Female | –74.0 ± 1.6 | 141.3 ± 5.9 | –44.0 ± 2.0* | 2.5 ± 0.7 | 14.6 ± 1.8 | |
| * Denotes a significant difference; p < 0.05. | ||||||
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Rattus norvegicus) | Parvalbumin-Cre on a Long-Evans background | Rat Resource and Research Center | RRID:RRRC_00773 | Male and female |
| Recombinant DNA reagent | AAV5-hSyn-hChR2(H134R)-EYFP | Addgene | RRID:Addgene_26973 Cat#:26973-AAV5 | |
| Recombinant DNA reagent | AAV2-hSyn-DIO-mCherry | Addgene | RRID:Addgene_50459 Cat#:50459-AAV2 | |
| Chemical compound, drug | λ-Carrageenan (low viscosity) | Tokyo Chemical Industry | Cat#:C2871 CAS#:9064-57-7 | |
| Chemical compound, drug | Picrotoxin | Ascent Scientific | Cat#:ASC-315 CAS#:124-87-8 | |
| Chemical compound, drug | Kynurenic acid sodium salt | Hello Bio | Cat#:HB0363 CAS#:2439-02-3 | |
| Chemical compound, drug | Tetrodotoxin (citrate) | Cayman Chemical | Cat#:14964 CAS#:18660-81-6 | |
| Chemical compound, drug | 4-Aminopyridine | Sigma-Aldrich | Cat#:A78403 CAS#:504-24-5 | |
| Chemical compound, drug | DL-APV | Cayman Chemical | Cat#:14540 CAS#:76326-31-3 | |
| Software, algorithm | Axograph X | Axograph | RRID:SCR_014284 | |
| Software, algorithm | Stata 15.1 | StataCorp | RRID:SCR_012763 | |
| Other | Green Retrobeads IX | Lumafluor |