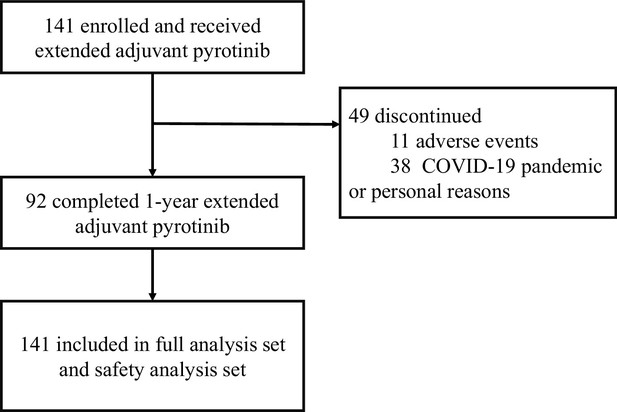
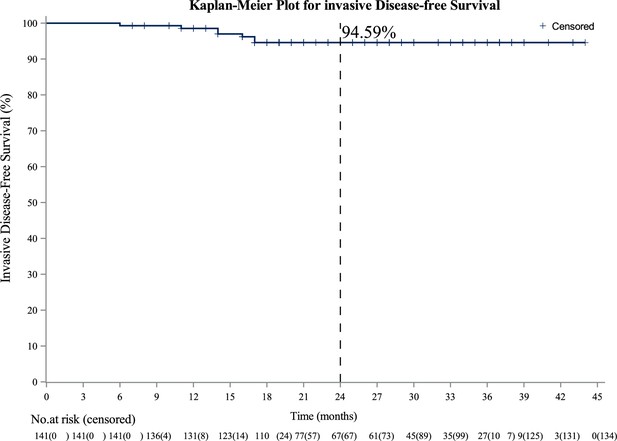
Pyrotinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (PERSIST): A multicenter phase II trial
Figures
Figure 3
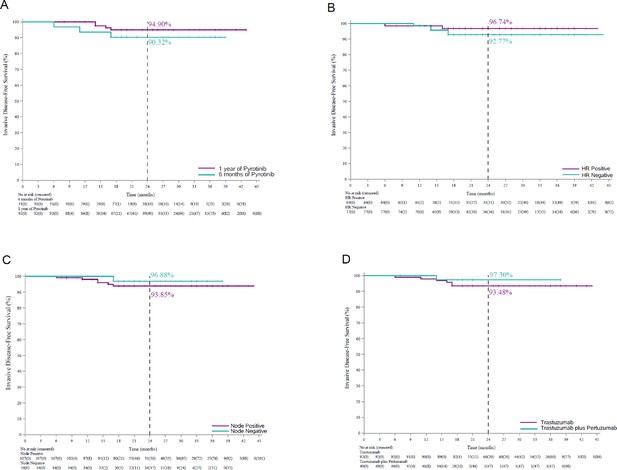
Invasive disease-free survival in different subgroups.
Kaplan–Meier curves for invasive disease-free survival in (A) patients who received 6-month (n = 31) and 1-year pyrotinib treatment (n = 92), (B) patients with hormone receptor-positive breast cancer (n = 64) and hormone receptor-negative breast cancer (n = 77), (C) patients with lymph node-positive breast cancer (n = 107) and patients with lymph node-negative breast cancer (n = 34), and (D) patients who received adjuvant therapy with trastuzumab (n = 92) and trastuzumab plus pertuzumab (n = 49).
Tables
Table 1
Baseline characteristics of patients in the full analysis set.
| Variables | All (n = 141) |
|---|---|
| Age (years), median (range) | 50 (25–72) |
| T stage, n (%) | |
| T1 | 43 (30.5%) |
| T2 | 83 (58.9%) |
| T3 | 11 (7.8%) |
| T4 | 2 (1.4%) |
| Unable to determine | 2 (1.4%) |
| Nodal status, n (%) | |
| Negative | 34 (24.1%) |
| 1–3 positive nodes | 49 (34.8%) |
| >3 positive nodes | 58 (41.1%) |
| Hormone receptor status, n (%) | |
| Negative (ER and PR negative) | 77 (54.6%) |
| Positive (ER positive, PR positive, or both) | 64 (45.4%) |
| Ki-67, n (%) | |
| ≥20% | 119 (84.4%) |
| <20% | 22 (15.6%) |
| Previous neoadjuvant therapy, n (%) | 26 (17.7%) |
| Previous adjuvant therapy, n (%) | |
| Trastuzumab | 92 (65.2%) |
| Trastuzumab plus pertuzumab | 49 (34.8%) |
-
Tumors were assessed as being ER or PR positive with a threshold of 1%.
-
ER, estrogen receptor; PR, progesterone receptor.
Table 2
Treatment-emergent adverse events occurring in at least 10% of the patients.
| Events, n (%) | All (n = 141) | |
|---|---|---|
| Any grade | Grade 3 | |
| Diarrhea | 112 (79.4%) | 43 (30.5%) |
| Fatigue | 52 (36.9%) | 0 |
| Lymphocyte count decreased | 52 (36.9%) | 0 |
| Nausea | 47 (33.3%) | 0 |
| Hand-foot syndrome | 47 (33.3%) | 1 (0.7%) |
| Hyperuricemia | 33 (23.4%) | 0 |
| White blood cell count decreased | 26 (18.4%) | 0 |
| Dizziness | 26 (18.4%) | 0 |
| Anemia | 24 (17.0%) | 0 |
| Vomiting | 22 (15.6%) | 0 |
| Headache | 19 (13.5%) | 0 |
| Creatinine increased | 17 (12.1%) | 0 |
| Neutrophil count decreased | 16 (11.3%) | 0 |
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/101724/elife-101724-mdarchecklist1-v1.docx
-
Source data 1
Processed raw data (with all private patient information removed).
- https://cdn.elifesciences.org/articles/101724/elife-101724-data1-v1.xlsx
Download links
A two-part list of links to download the article, or parts of the article, in various formats.
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Pyrotinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (PERSIST): A multicenter phase II trial
eLife 13:RP101724.
https://doi.org/10.7554/eLife.101724.3