ORMDL3 restrains type I interferon signaling and anti-tumor immunity by promoting RIG-I degradation
Figures
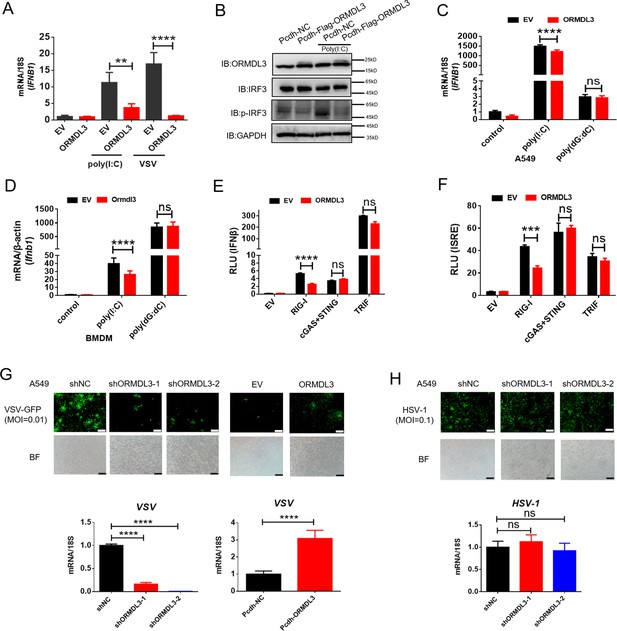
ORMDL3 negatively regulates RIG-I-like receptor (RLR)-induced type I interferon (type I IFN) signaling pathway.
(A) HEK293T cells were transfected with an empty vector (EV) or ORMDL3 plasmid for 12 hr and were then infected with vesicular stomatitis virus (VSV) (MOI = 0.01) or transfected with poly(I:C). The transcription of IFNB1 mRNA was detected using quantitative real-time PCR (qRT-PCR). (B) HEK293T-EV and HEK293T-Flag-ORMDL3 stable cell lines were transfected with or without poly(I:C) and immunoblot analyses of phosphorylated IRF3 (p-IRF3), total IRF3, GAPDH, and ORMDL3 levels were performed. (C) Results of the qRT-PCR assays showing mRNA levels of IFNB1 in A549 cells transfected with EV or ORMDL3 followed by stimulating with poly(I:C) or poly(dG:dC). (D) Results of the qRT-PCR assays showing mRNA levels of Ifnb1 in bone marrow-derived primary macrophages (BMDM) infected with EV or psc-AAV-Ormdl3 virus followed by transfecting with poly(I:C) or poly(dG:dC). (E–F) Results of the luciferase assay showing IFNβ-Luc activity (E) and ISRE-Luc activity (F) in HEK293T cells transfected with EV or ORMDL3 plasmids together with individual EV, RIG-I, cGAS plus STING, or TRIF plasmids for 24 hr. (G) ORMDL3 stable knockdown or overexpression A549 cells, and the control cells were infected with VSV-GFP (MOI = 0.01) for 12 hr. The viral infection was observed using fluorescence microscopy, and the viral amount was detected using qRT-PCR. Scale bars, 200 μm. (H) The control and ORMDL3 stable knockdown A549 cells were infected with herpes simplex virus-1 (HSV-1) (MOI = 0.1) for 24 hr. The viral infection was observed using fluorescence microscopy, and the viral amount was detected using qRT-PCR. Scale bars, 200 μm. Data from three independent experiments are presented as mean ± SD and were analyzed by two-tailed Student’s t test (A, C, D-F, G-H, bottom) ,**p<0.01, ***p<0.001, ****p<0.0001, and ns = no significance.
-
Figure 1—source data 1
Original files for western blots shown in Figure 1, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig1-data1-v1.zip
-
Figure 1—source data 2
Original files for western blots shown in Figure 1.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig1-data2-v1.zip
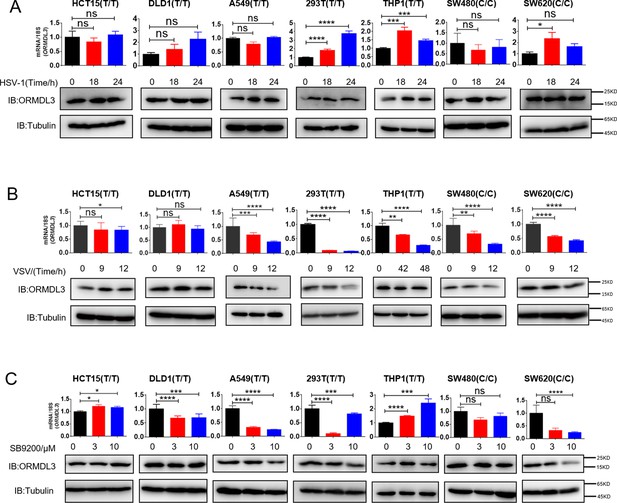
ORMDL3 expression under different treatments.
(A) Results of the WB and quantitative real-time PCR (qRT-PCR) assays showing ORMDL3 protein and RNA levels in indicated cell lines infected with herpes simplex virus-1 (HSV) (MOI = 0.1) for indicated times. (B) Results of the WB and qRT-PCR assays showing ORMDL3 protein and RNA levels in indicated cell lines infected with VSV-1 (MOI = 0.01) for indicated times. (C) Results of the WB and qRT-PCR assays showing ORMDL3 protein and RNA levels in indicated cell lines treated with RIG-I agonist SB9200 for indicated times. Data from three independent experiments are presented as mean ± SD and were analyzed by two-tailed Student’s t test (A-C), *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, and ns = no significance.
-
Figure 1—figure supplement 1—source data 1
Original files for western blots shown in Figure 1—figure supplement 1, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig1-figsupp1-data1-v1.zip
-
Figure 1—figure supplement 1—source data 2
Original files for western blots shown in Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig1-figsupp1-data2-v1.zip
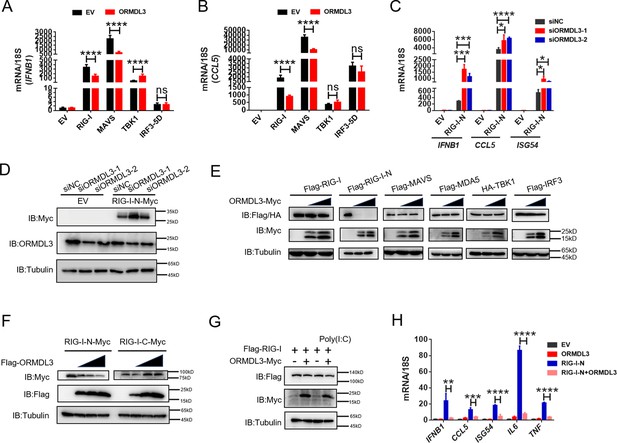
ORMDL3 regulates the protein abundance of RIG-I.
(A, B) Quantitative real-time PCR (qRT-PCR) analyses of the expression of the indicated mRNA levels in HEK293T cells transfected with empty vector (EV) or ORMDL3 plasmids combined with co-transfection of individual plasmids encoding EV, RIG-I, MAVS, TBK1, IRF3-5D (the constitutively activated form of IRF3). (C) Results of the qRT-PCR assays showing mRNA levels of IFNB1, CCL5, and ISG54 in HEK293T cells transfected with control or ORMDL3-specific siRNAs followed by secondary transfection with EV or RIG-I-N plasmids. (D) Results of the WB assays showing protein levels in HEK293T cells transfected with control or ORMDL3-specific siRNAs followed by secondary transfection with EV or RIG-I-N plasmids. (E) Immunoblot analysis of 293T cells transfected with individual plasmid encoding Flag-tagged RIG-I, RIG-I-N, MAVS, MDA5, IRF3, or HA-TBK1, in combination with increasing doses of ORMDL3-Myc plasmids. (F) Immunoblot analysis of 293T cells transfected with plasmid of RIG-I-N-Myc or RIG-I-C-Myc and increasing doses of Flag-ORMDL3 plasmid. (G) HEK293T cells were transfected with Flag-RIG-I and ORMDL3-Myc plasmids, as indicated, with or without poly(I:C) co-transfection. Cell lysates were immunoblotted with anti-Flag and anti-Myc antibodies. (H) Results of the qRT-PCR assays showing IFNB1, CCL5, ISG54, IL6, and TNF mRNA levels in HEK293T cells transfected with EV, ORMDL3, and RIG-I-N plasmids as indicated for 24 hr. Data from three independent experiments are presented as mean ± SD and were analyzed by two-tailed Student’s t test (A-C, H), *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, and ns = no significance.
-
Figure 2—source data 1
Original files for western blots shown in Figure 2, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig2-data1-v1.zip
-
Figure 2—source data 2
Original files for western blots shown in Figure 2.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig2-data2-v1.zip
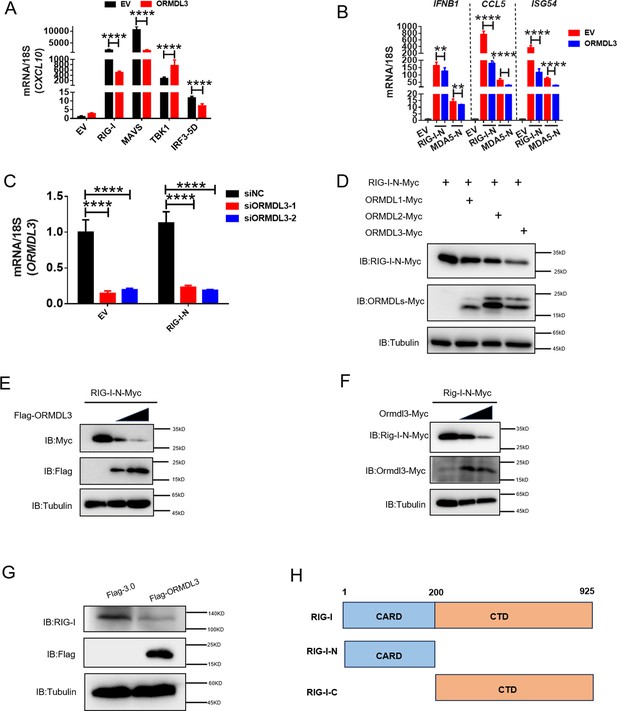
ORMDL3-mediated downregulation of RIG-I is conserved in both human and murine cells.
(A) Quantitative real-time PCR (qRT-PCR) analysis of CXCL10 when ORMDL3 was co-expressed with RIG-I, MAVS,TBK1, or IRF3-5D. (B) Results of the qRT-PCR assays showing IFNB1, CCL5, ISG54 mRNA level in HEK293T cells transfected with empty vector (EV) or ORMDL3 plasmids together with individual RIG-I-N or MDA5-N plasmids for 24 hr. (C) Results of the qRT-PCR assays showing ORMDL3 knockdown efficiency which links to Figure 2D. (D) Immunoblot assay of RIG-I-N expression in HEK293T co-transfected with ORMDLs family members. (E) Immunoblot assay of exogenous RIG-I-N expression in HEK293T cells transfected with human ORMDL3. (F) Immunoblot assay of exogenous Rig-I-N expression in HEK293T cells transfected with murine Ormdl3. (G) Immunoblot assay of endogenous RIG-I expression in HEK293T cells transfected with Flag-ORMDL3. (H) The diagram of RIG-I truncations. Data from three independent experiments are presented as mean ± SD and were analyzed by two-tailed Student’s t test (A-C), **p<0.01, ****p<0.0001.
-
Figure 2—figure supplement 1—source data 1
Original files for western blots shown in Figure 2—figure supplement 1, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig2-figsupp1-data1-v1.zip
-
Figure 2—figure supplement 1—source data 2
Original files for western blots shown in Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig2-figsupp1-data2-v1.zip
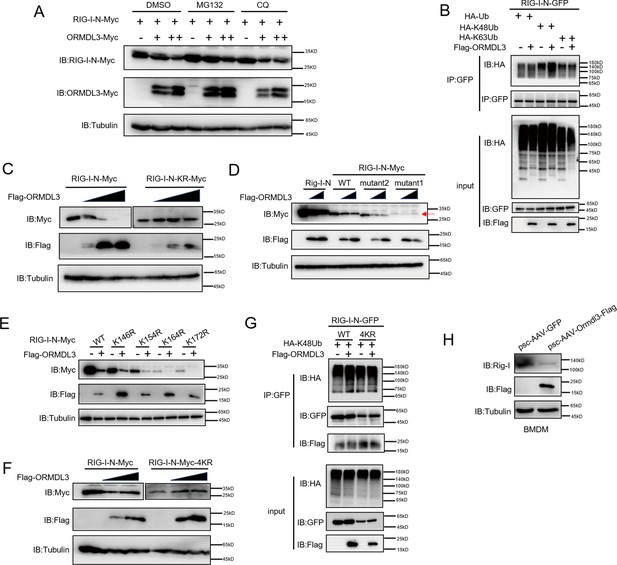
ORMDL3 promotes proteasomal degradation of RIG-I.
(A) HEK293T cells were transfected with plasmids encoding RIG-I-N-Myc together with increasing amounts of Flag-ORMDL3 plasmid treated with MG132 (10 μM) or chloroquine (CQ) (50 μM) for 6 hr and the cell lysates were analyzed by immunoblot. (B) HEK293T cells were transfected with the indicated plasmids, and cell lysates were immunoprecipitated with an anti-GFP antibody followed by immunoblots using anti-GFP and anti-HA antibodies. (C) HEK293T cells were transfected with RIG-I-N-Myc (WT or KR) and increasing doses of plasmid for Flag-ORMDL3. The expression levels of RIG-I-N-Myc were analyzed by immunoblot. (D) HEK293T cells were transfected with Rig-I-N, RIG-I-N-Myc (WT, KR, mutant1, or mutant2) and increasing doses of plasmid for Flag-ORMDL3. The expression levels of RIG-I-N-Myc were analyzed by immunoblot. (E) 293T cells were transfected with RIG-I-N-Myc (WT, K146R, K154R, K164R, or K172R) with or without Flag-ORMDL3. The expression levels of RIG-I-N-Myc and its mutant forms were analyzed by immunoblot. (F) HEK293T cells were transfected with RIG-I-N-Myc (WT or 4KR) and increasing doses of Flag-ORMDL3. The expression levels of RIG-I-N-Myc (WT or 4KR) were analyzed by immunoblot. (G) HEK293T cells were transfected with RIG-I-N-GFP (WT or 4KR) and HA-K48Ub in combination with EV or Flag-ORMDL3, and cell lysates were immunoprecipitated with an anti-GFP antibody followed by immunoblots using anti-GFP, anti-HA, and anti-Flag antibodies. (H) Bone marrow-derived primary macrophages (BMDM) were infected with psc-AAV-GFP or psc-AAV-Ormdl3-Flag virus, followed by immunoblot analysis of Rig-I, Flag, and Tubulin.
-
Figure 3—source data 1
Original files for western blots shown in Figure 3, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig3-data1-v1.zip
-
Figure 3—source data 2
Original files for western blots shown in Figure 3.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig3-data2-v1.zip
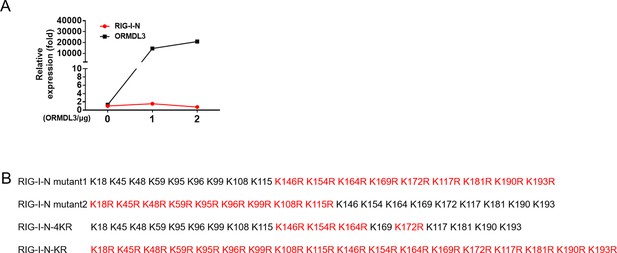
ORMDL3 promotes the degradation of RIG-I.
(A) Quantitative real-time PCR (qRT-PCR) assay of RIG-I-N mRNA levels in HEK293T cells transfected with RIG-I-N and increasing amounts of ORMDL3. (B) Different annotations of KR mutation of RIG-I-N.
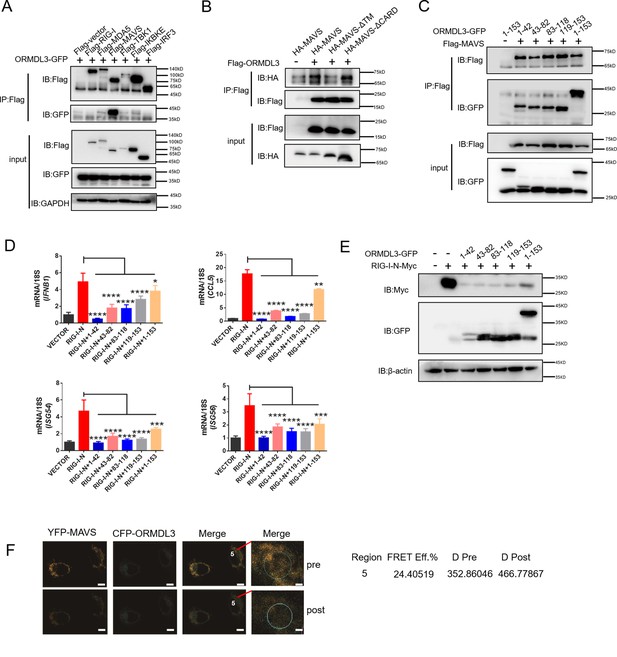
ORMDL3 interacts with signaling adaptor MAVS.
(A) HEK293T cells were transfected with empty vector (EV) or Flag-RIG-I/MAVS/MDA5/TBK1/IRF3/IKBKE with ORMDL3-GFP, and cell lysates were immunoprecipitated with an anti-Flag antibody followed by immunoblots using anti-GFP and anti-Flag antibodies. (B) HEK293T cells were transfected with different MAVS truncations in combination with EV or Flag-ORMDL3, and cell lysates were immunoprecipitated with anti-Flag antibody followed by immunoblots using anti-HA and anti-Flag antibodies. (C) HEK293T cells were transfected with EV or Flag-MAVS in combination with different ORMDL3 truncations, and cell lysates were immunoprecipitated with anti-Flag antibody followed by immunoblots using anti-GFP and anti-Flag antibodies. (D) HEK293T cells were transfected with EV or Flag-MAVS in combination with different ORMDL3 truncations, followed by quantitative real-time PCR (qRT-PCR) analysis of IFNB1, CCL5, ISG54, and ISG56. (E) HEK293T cells were transfected with RIG-I-N-Myc in combination with EV or different ORMDL3 truncations, followed by immunoblots using anti-GFP and anti-Myc antibodies. (F) FRET experiment of YFP-MAVS and CFP-ORMDL3 in HeLa cells. YFP-MAVS is the donor and CFP-ORMDL3 is the acceptor, and FRET efficiency is 24.40519%. Scale bars, 10 μm. Data from three independent experiments are presented as mean ± SD and were analyzed by two-tailed Student’s t test (D), *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
-
Figure 4—source data 1
Original files for western blots shown in Figure 4, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig4-data1-v1.zip
-
Figure 4—source data 2
Original files for western blots shown in Figure 4.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig4-data2-v1.zip
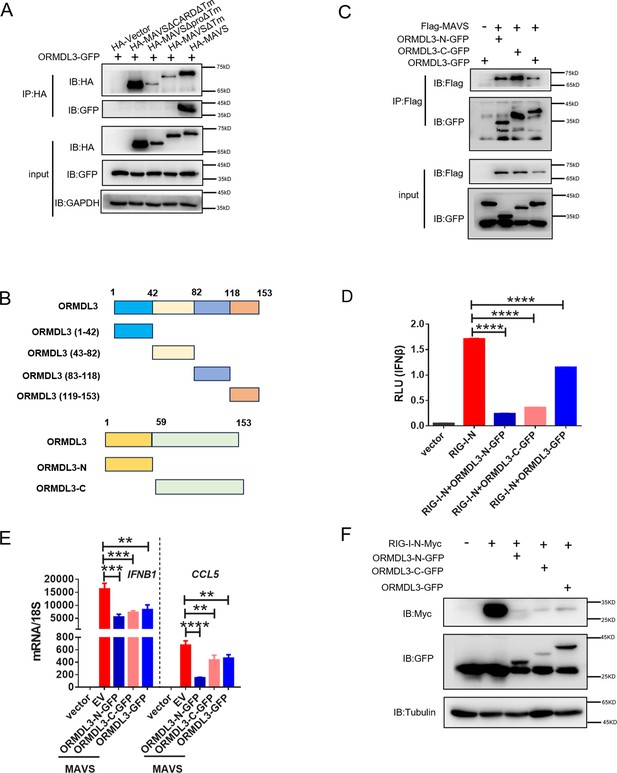
All truncations of ORMDL3 can inhibit type I interferon (type I IFN) production.
(A) HEK293T cells were transfected with the indicated plasmids, and cell lysates were immunoprecipitated with anti-HA antibody followed by immunoblots using anti-GFP and anti-HA antibodies. (B) Scheme of the truncations of ORMDL3. (C) HEK293T cells were transfected with the indicated plasmids, and cell lysates were immunoprecipitated with anti-Flag antibody, followed by immunoblots using anti-GFP and anti-Flag antibodies. (D) Results of the luciferase assays showing IFNβ-Luc activity in HEK293T cells co-transfected with ORMDL3 truncations and RIG-I-N. (E) Results of the quantitative real-time PCR (qRT-PCR) assays showing mRNA levels of IFNB1 and CCL5 in HEK293T cells co-transfected with ORMDL3 truncations and MAVS. (F) HEK293T cells were transfected with RIG-I-N-Myc and different truncation of ORMDL3 followed by immunoblot assay. Data from three independent experiments are presented as mean ± SD and were analyzed by two-tailed Student’s t test (D-E), **p<0.01, ***p<0.001, ****p<0.0001.
-
Figure 4—figure supplement 1—source data 1
Original files for western blots shown in Figure 4—figure supplement 1, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig4-figsupp1-data1-v1.zip
-
Figure 4—figure supplement 1—source data 2
Original files for western blots shown in Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig4-figsupp1-data2-v1.zip
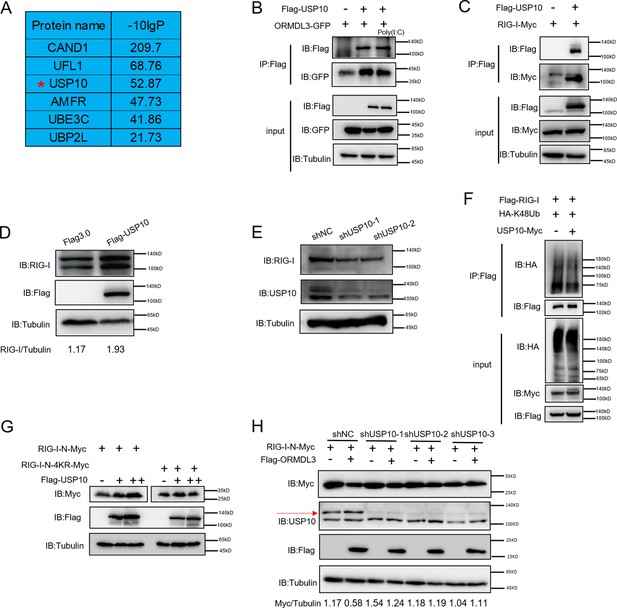
USP10 induces RIG-I stabilization.
(A) Candidate proteins interacted with ORMDL3 screened from mass spectrometry results. (B) HEK293T cells were transfected with ORMDL3-GFP and Flag-USP10 plasmids, as indicated, with or without poly(I:C) co-transfection. Cell lysates were immunoprecipitated with the anti-Flag antibody and immunoblotted with anti-Flag and anti-GFP antibodies. (C) HEK293T cells were transfected with RIG-I-Myc and EV or Flag-USP10 plasmids. Cell lysates were immunoprecipitated with the anti-Flag antibody and immunoblotted with anti-Flag and anti-Myc antibodies. (D) Immunoblot the protein level of RIG-I in USP10 stable overexpression HEK293T cell line. (E) Immunoblot the protein level of RIG-I in USP10 stable knockdown HEK293T cell lines. (F) Immunoprecipitation (IP) and immunoblot analysis of 293T cells transfected with vectors expressing Flag-RIG-I and HA-K48Ub with or without USP10-Myc. (G) HEK293T cells were transfected with RIG-I-N-Myc (WT or 4KR) and increasing doses of expression vector for Flag-USP10. The expression levels of RIG-I-N-Myc were analyzed by immunoblot. (H) USP10 stable knockdown HEK293T cell lines were transfected with RIG-I-N-Myc and Flag-ORMDL3 as indicated. The expression levels of RIG-I-N-Myc were analyzed by immunoblot.
-
Figure 5—source data 1
Original files for western blots shown in Figure 5, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig5-data1-v1.zip
-
Figure 5—source data 2
Original files for western blots shown in Figure 5.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig5-data2-v1.zip
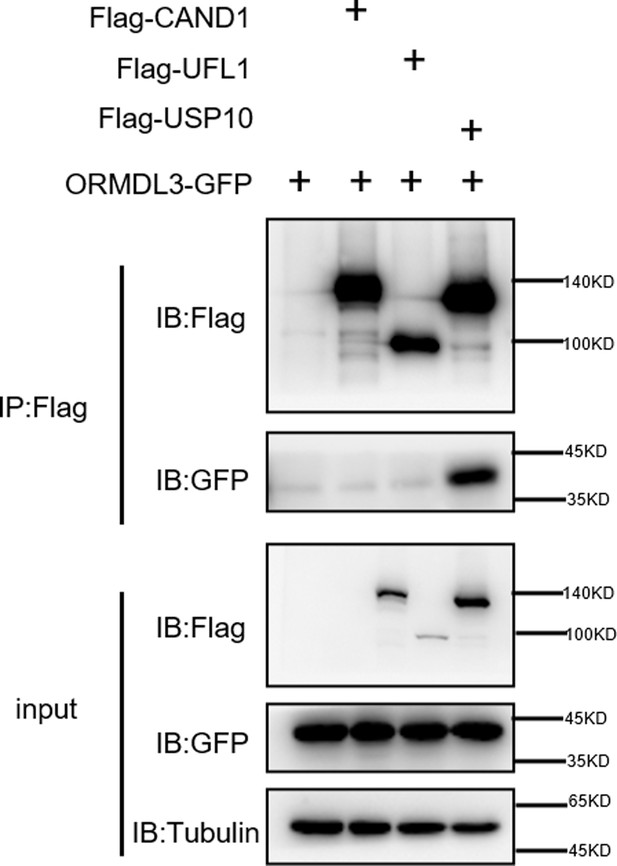
ORMDL3 interacts with USP10 but not CAND1 or UFL1.
HEK293T cells were transfected with the indicated plasmids, and cell lysates were immunoprecipitated with anti-Flag antibody followed by immunoblots using anti-GFP and anti-Flag antibodies.
-
Figure 5—figure supplement 1—source data 1
Original files for western blots shown in Figure 5—figure supplement 1, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig5-figsupp1-data1-v1.zip
-
Figure 5—figure supplement 1—source data 2
Original files for western blots shown in Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig5-figsupp1-data2-v1.zip
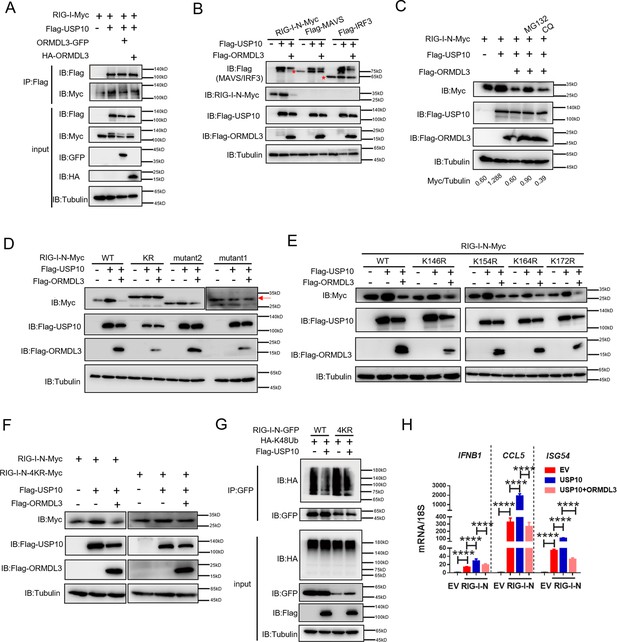
ORMDL3 disturbs USP10-induced RIG-I stabilization.
(A) HEK293T cells were transfected with Flag-USP10 and RIG-I-Myc plasmids, as indicated, with or without ORMDL3 co-transfection. Cell lysates were immunoprecipitated with the anti-Flag antibody and immunoblotted with anti-Flag and anti-Myc antibodies. (B) Immunoblot analysis of HEK293T cells transfected with Flag-USP10 and Flag-MAVS, Flag-IRF3, or RIG-I-N-Myc with or without ORMDL3 co-transfection using indicated antibodies. (C) HEK293T cells were transfected with plasmids encoding RIG-I-N-Myc together with Flag-USP10 with or without Flag-ORMDL3 plasmid followed by MG132 (10 μM) or chloroquine (CQ) (50 μM) treatment for 6 hr. The cell lysates were analyzed by immunoblot. (D) HEK293T cells were transfected with Flag-USP10 and RIG-I-N-Myc (WT, KR, mutant1, or mutant2) plasmids, as indicated, with or without Flag-ORMDL3 co-transfection. Cell lysates were immunoblotted with indicated antibodies. (E) HEK293T cells were transfected with RIG-I-N-Myc (WT, K146R, K154R, K164R, or K172R) and Flag-USP10 with or without Flag-ORMDL3. Cell lysates were immunoblotted with indicated antibodies. (F) HEK293T cells were transfected with Flag-USP10 and RIG-I-N-Myc (WT or 4KR) plasmids, as indicated, with or without Flag-ORMDL3 co-transfection. Cell lysates were immunoblotted with indicated antibodies. (G) Immunoprecipitation (IP) and immunoblot analysis of HEK293T cells transfected with vectors expressing RIG-I-N-GFP/RIG-I-N-4KR-GFP and HA-K48Ub with or without USP10 transfection. (H) HEK293T cells were transfected with Flag-USP10 and RIG-I-N-Myc, with or without ORMDL3 co-transfection followed by quantitative real-time PCR (qRT-PCR) analysis of IFNB1, CCL5, and ISG54. Data from three independent experiments are presented as mean ± SD and were analyzed by two-tailed Student’s t test (H), ****p<0.0001.
-
Figure 6—source data 1
Original files for western blots shown in Figure 6, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig6-data1-v1.zip
-
Figure 6—source data 2
Original files for western blots shown in Figure 6.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig6-data2-v1.zip
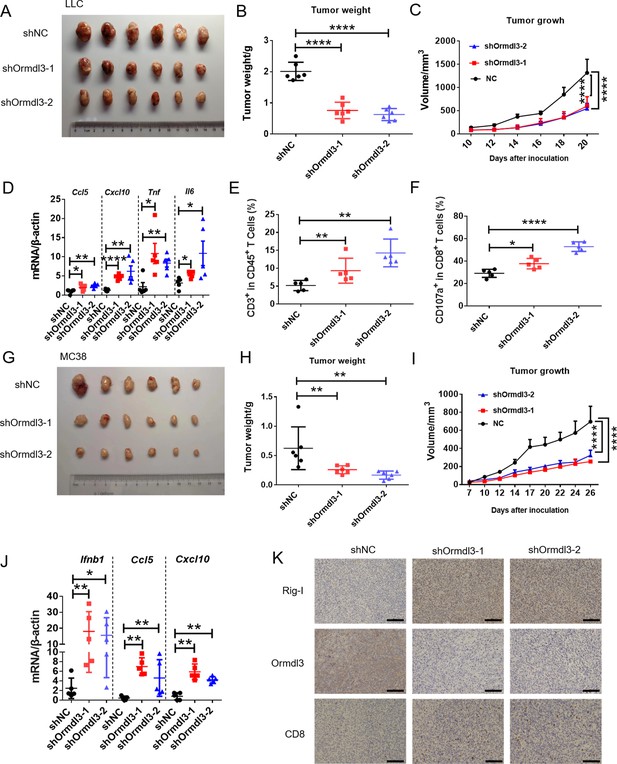
Knockdown of ORMDL3 in LLC/MC38 cells enhances anti-tumor immunity.
(A–C) Representative images (A), tumor weight (B), and tumor growth (C) of LLC tumors on day 21 after inoculation with 1.5×106 LLC cells with or without Ormdl3 stable knockdown into C57BL/6 mice, (n=6). (D) Results of the quantitative real-time PCR (qRT-PCR) assays showing mRNA levels of Ccl5, Cxcl10, Tnf, and Il6 in LLC tumors, (n=5). (E, F) Flow cytometry assay of CD3+ T and CD107a+ CD8+ T cell percentages in indicated population, (n=5). (G–I) Representative images (G), tumor weight (H), and tumor growth (I) on day 27 after tumor inoculation with 5×105 MC38 cells with or without Ormdl3 stable knockdown into C57BL/6 mice, (n=6). (J) Results of the qRT-PCR assays showing mRNA levels of Ifnb1, Ccl5, and Cxcl10 in MC38 tumors, (n=5). (K) Results of the immunohistochemistry (IHC) assay showing expression levels of Ormdl3, Rig-I, and CD8 in MC38 tumors. Scale bars, 50 μm. Data from three independent experiments are presented as mean ± SD and were analyzed by one-way ANOVA (B, D-F, H, and J) or two-way ANOVA (C ,and I), *p<0.05, **p<0.01, ****p<0.0001.
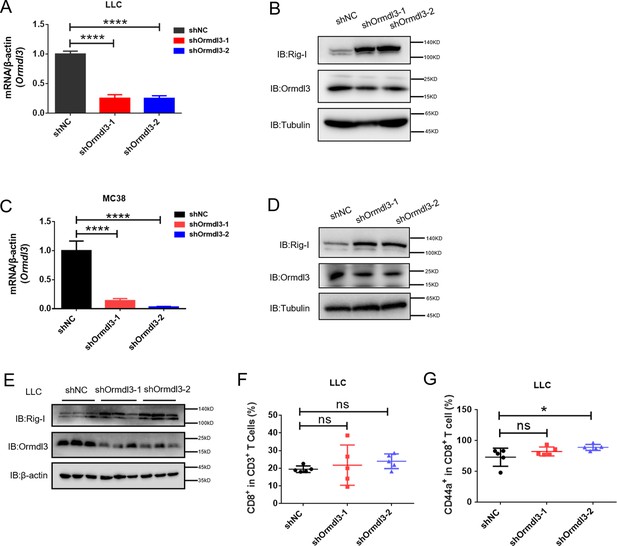
Inhibiting ORMDL3 increases the abundance of RIG-I.
(A) Quantitative real-time PCR (qRT-PCR) showing the shRNA knockdown efficiency of Ormdl3 in LLC lung cancer cells. (B) Immunoblot showing Ormdl3 knockdown and its effects on Rig-I abundance in LLC cells. (C) qRT-PCR showing the shRNA knockdown efficiency of Ormdl3 in MC38 colon cancer cells. (D) Immunoblot showing Ormdl3 knockdown and its effects on Rig-I abundance in MC38 cells. (E) Immunoblot assay of Ormdl3 and Rig-I protein levels in shNC, shOrmdl3-1, and shOrmdl3-2 in LLC tumors. (F, G) Flow cytometry assay of CD8+T and CD44+CD8+ T cell percentages in indicated populations (n=5). Data from three independent experiments are presented as mean ± SD and were analyzed by two-tailed Student’s t test (A, and C) or one way ANOVA (F-G),*p<0.05, ****p<0.0001, and ns = no significance.
-
Figure 7—figure supplement 1—source data 1
Original files for western blots shown in Figure 7—figure supplement 1, indicating relevant bands.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig7-figsupp1-data1-v1.zip
-
Figure 7—figure supplement 1—source data 2
Original files for western blots shown in Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/101973/elife-101973-fig7-figsupp1-data2-v1.zip
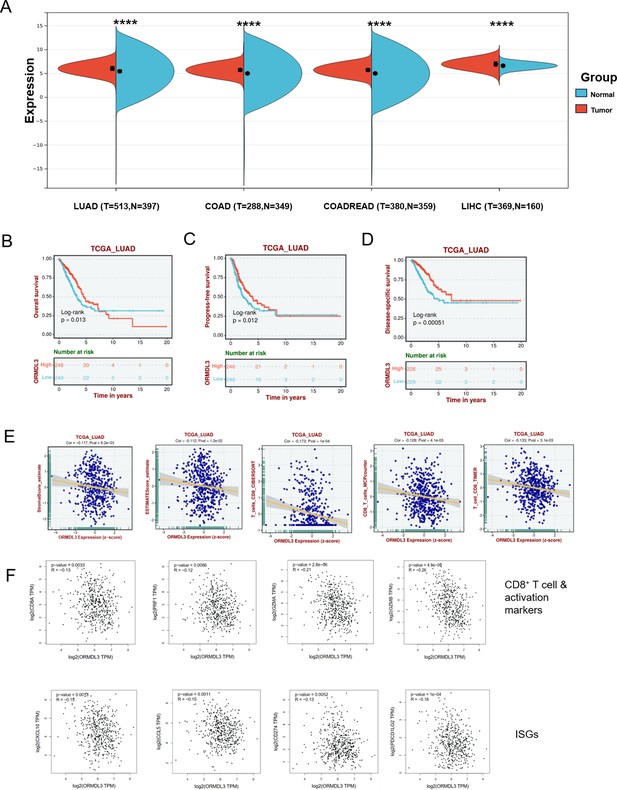
ORMDL3 level is associated with poor survival and reduced immune cell infiltration and interferon-stimulated gene (ISG) expression.
(A) ORMDL3 expression between tumor and adjacent normal tissues in The Cancer Genome Atlas (TCGA) pan-cancer cohorts. (B–D) Association of ORMDL3 level with overall survival (OS), progression-free survival (PFS), and disease-specific survival (DSS) in TCGA-lung adenocarcinoma (LUAD). (E) Correlation among ORMDL3 expression with stromal score and CD8+T cell infiltration in LUAD cohort from TCGA datasets. (F) Correlation among ORMDL3 expression with CD8+T and its activation markers (CD8A, PRF1, GZMA, GZMB) and ISGs (CCL5, CXCL10, CD274, and PDCD1LG2) in TCGA-LUAD from the GEPIA website (Tang et al., 2017). Data were analyzed by Unpaired Wilcoxon Rank Sum and Signed Rank Tests (A), ****p<0.0001.
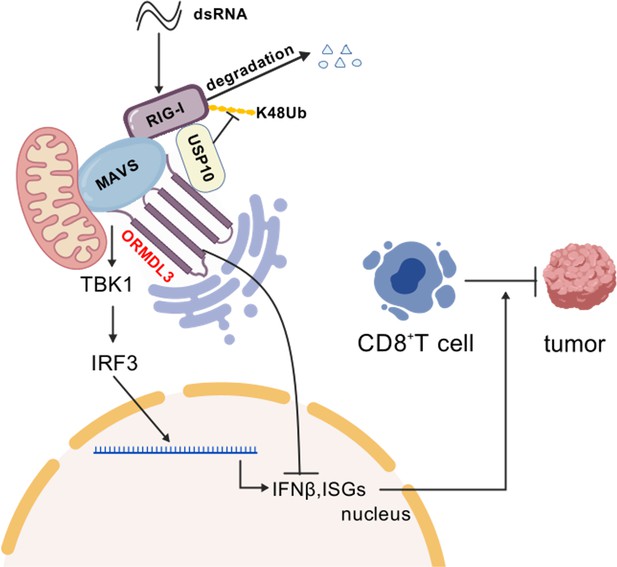
Schematic shows that ORMDL3 promotes the degradation of RIG-I and attenuates type I IFN in cancer.
The image was created with BioGDP.com (Jiang et al., 2025).

PCR amplification of human TLR3 was conducted on cDNA derived from HEK293T and A549 cells (lanes 1 and 2, respectively), and PCR amplification of murine Tlr3 was performed on cDNA from BMDM (lane 3).
Human spleen cDNA (lane 4, TAKARA Human MTCTM Panel I, Cat# 636742) served as a positive control, and 18s rRNA was used as an internal control.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | HEK293T | ATCC | Cat#CRL-11268 | RRID:CVCL_1926 |
| Cell line (Homo sapiens) | A549 | ATCC | Cat#CCL-185 | RRID:CVCL_0023 |
| Cell line (Homo sapiens) | HeLa | ATCC | Cat#CCL-2 | RRID:CVCL_0030 |
| Cell line (Homo sapiens) | DLD1 | ATCC | Cat#CCL-221 | RRID:CVCL_0248 |
| Cell line (Homo sapiens) | HCT15 | ATCC | Cat#CCL-225 | RRID:CVCL_0292 |
| Cell line (Homo sapiens) | SW480 | ATCC | Cat#CCL-228 | RRID:CVCL_0546 |
| Cell line (Homo sapiens) | SW620 | ATCC | Cat#CCL-227 | RRID:CVCL_0547 |
| Cell line (Homo sapiens) | THP1 | This paper | TIB-202 | Cell line maintained in Shuai Chen lab (Sun Yat-sen University Cancer Center) |
| Cell line (Mus musculus) | MC38 | This paper | Gift from Prof. Penghui Zhou (Sun Yat-sen University Cancer Center) | Cell line maintained in Shuai Chen lab (Sun Yat-sen University Cancer Center) |
| Cell line (Mus musculus) | LLC | This paper | Gift from Prof. Penghui Zhou (Sun Yat-sen University Cancer Center) | Cell line maintained in Shuai Chen lab (Sun Yat-sen University Cancer Center) |
| Sequence-based reagent | ORMDL3-shRNA-1 | This paper | PCR primers | CCGGCCCACAGAATGTGATAGTAATCTCGAGATTACTATCACATTCTGTGGGTTTTTG |
| Sequence-based reagent | ORMDL3-shRNA-2 | This paper | PCR primers | CCGGCATGGGCATGTATATCTTCCTCTCGAGAGGAAGATATACATGCCCATGTTTTTG |
| Sequence-based reagent | USP10 shRNA-1 | This paper | PCR primers | CCGGCCTATGTGGAAACTAAGTATTCTCGAGAATACTTAGTTTCCACATAGGTTTTTG |
| Sequence-based reagent | USP10 shRNA-2 | This paper | PCR primers | CCGGCCCATGATAGACAGCTTTGTTCTCGAGAACAAAGCTGTCTATCATGGGTTTTTG |
| Sequence-based reagent | USP10 shRNA-3 | This paper | PCR primers | CCGGCGACAAGCTCTTGGAGATAAACTCGAGTTTATCTCCAAGAGCTTGTCGTTTTTG |
| Sequence-based reagent | Ormdl3-shRNA-1 | This paper | PCR primers | CCGGCCAAGTATGACCAAGTCCATTCTCGAGAATGGACTTGGTCATACTTGGTTTTTG |
| Sequence-based reagent | Ormdl3-shRNA-2 | This paper | PCR primers | CCGGGCCGACTTGGAGTAGCTTGTACTCGAGTACAAGCTACTCCAAGTCGGCTTTTTG |
| Antibody | ORMDL3 (Rabbit, polyclonal) | Abcam | ab211522 | RRID:AB_3102000 WB (1:1000) |
| Antibody | ORMDL3 (Rabbit, polyclonal) | Abcam | ab107639 | RRID:AB_10863267 WB (1:1000) |
| Antibody | RIG-I (Mouse, monoclonal) | Santa Cruz | sc376845 | RRID:AB_2732794 WB (1:1000) |
| Antibody | Flag (Mouse, monoclonal) | Sigma | F1804# | RRID:AB_262044 WB (1:1000) |
| Antibody | GFP (Mouse, monoclonal) | Proteintech | 66002-1-Ig | RRID:AB_11182611 WB (1:1000) |
| Antibody | Myc (Mouse, monoclonal) | Proteintech | 60003-2-Ig | RRID:AB_2734122 WB (1:1000) |
| Antibody | Myc (Rabbit, polyclonal) | Proteintech | 10828-1-AP | RRID:AB_2148585 WB (1:1000) |
| Antibody | HA (Mouse, monoclonal) | Ray antibody | RM1004 | WB (1:1000) |
| Antibody | USP10 (Rabbit, polyclonal) | Abclonal | A13387 | RRID:AB_2760247 WB (1:1000) |
| Antibody | Tubulin (Mouse, polyclonal) | Fdbio | FD0064 | RRID:AB_3076327 WB (1:2000) |
| Antibody | GAPDH (Mouse, monoclonal) | Proteintech | 60004-1-Ig | RRID:AB_2107436 WB (1:2000) |
| Antibody | GAPDH (Rabbit, monoclonal) | ServiceBio | GB15004 | RRID:AB_2943040 WB (1:5000) |
| Antibody | β-Actin (Mouse, monoclonal) | Proteintech | 60008-1-Ig | RRID:AB_2289225 WB (1:2000) |
| Antibody | β-Actin (Rabbit, monoclonal) | ServiceBio | GB15003 | RRID:AB_3083699 WB (1:2000) |
| Antibody | IRF3 (Rabbit, monoclonal) | Cell Signaling Technology | # 4302 | RRID:AB_1904036 WB (1:1000) |
| Antibody | Anti-phospho-IRF-3 (Rabbit, monoclonal) | Cell Signaling Technology | #29047 | RRID:AB_2773013 WB (1:1000) |
| Antibody | CD3ε-APC (anti-Mouse) | BioLegend | 100235 | RRID:AB_2561455 Flow (1:300) |
| Antibody | CD4-Pacific blue (anti-Mouse) | BioLegend | 100428 | RRID:AB_493647 Flow (1:300) |
| Antibody | CD8-PE-cy7 (anti-Mouse) | BioLegend | 100721 | RRID:AB_312760 Flow (1:300) |
| Antibody | CD45-APC-cy7 (anti-Mouse) | BioLegend | 157024 | RRID:AB_2876533 Flow (1:300) |
| Antibody | CD44-FITC (anti-Mouse) | BioLegend | 103006 | RRID:AB_312957 Flow (1:300) |
| Antibody | CD107a-PE (anti-Mouse) | BioLegend | 121611 | RRID:AB_17320511 Flow (1:300) |
| Chemical compound, drug | Poly(I:C) (LMW) | Invivogen | tlrl-picw | |
| Chemical compound, drug | Poly(dG:dC) | Invivogen | tlrl-pgcn | |
| Chemical compound, drug | SB9200 | Bidepharm | CAS:942123-43-5 | |
| Software, algorithm | GraphPad Prism 7 | GraphPad | N/A | |
| Software, algorithm | ImageJ | https://imagej.net/Fiji/Downloads | N/A | |
| Software, algorithm | FlowJo10 | FlowJo | N/A | |
| Commercial assay or kit | Lipo293 | Beyotime | C0521 | |
| Commercial assay or kit | Lipofectamine 2000 | Thermo Fisher Scientific | Cat#11668019 | |
| Commercial assay or kit | Lipofectamine RNAiMAX | Thermo Fisher Scientific | Cat#13778075 | |
| Commercial assay or kit | Dual-Luciferase Reporter Assay System | Promega | Cat#E1960 |
Additional files
-
Supplementary file 1
Primers for qPCR.
Related to Materials and methods.
- https://cdn.elifesciences.org/articles/101973/elife-101973-supp1-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/101973/elife-101973-mdarchecklist1-v1.docx





