Hypothermia protects against ventilator-induced lung injury by limiting IL-1β release and NETs formation
Figures
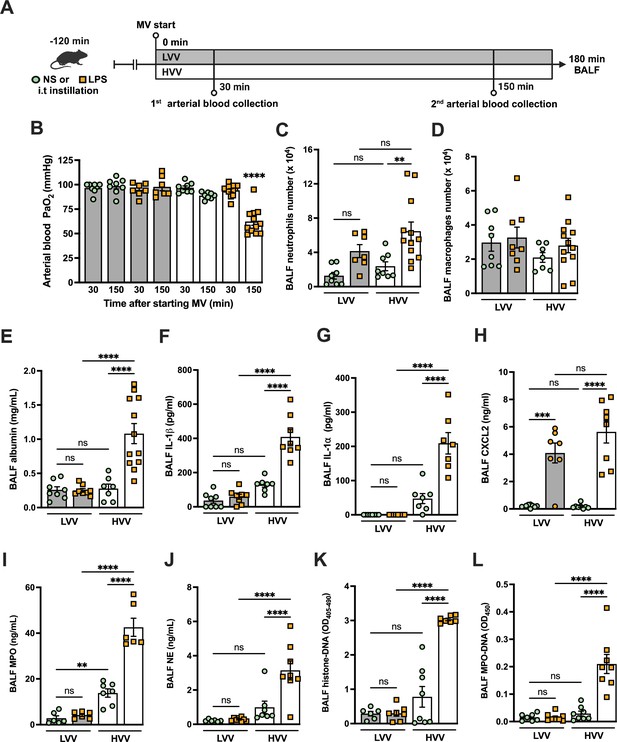
Severe acute lung injury induced by lipopolysaccharide (LPS) plus high-volume mechanical ventilation (MV) is associated with neutrophil extracellular traps (NETs) formation in the alveoli.
LPS or normal saline (NS) was intratracheally instilled into C57BL/6 mice, and after 120 min, the animals were anesthetized and placed on MV for 180 min with the tidal volumes of 30 mL/kg, high-volume ventilation (HVV), or 10 mL/kg low-volume ventilation (LVV) (A). This panel was created using BioRender.com. Arterial blood partial pressure of oxygen (PaO2) was measured at 30 and 150 min after starting MV (B). Absolute counts of neutrophils (C) and macrophages (D) were determined in bronchoalveolar lavage fluid (BALF). The levels of albumin (E), IL-1β (F), IL-1α (G), CXCL2 (H), MPO (I), and NE (J) in BALF collected from euthanized animals after 180 min of MV were determined by ELISA. Cell death in BALF was evaluated by measuring histone-DNA complexes (K). NETs formation was evaluated by the detection of MPO-DNA complex (L). ****, ***, and ** indicate p<0.0001, p<0.001, and p<0.01, respectively, determined by three-way ANOVA (B) and two-way ANOVA (C–L) followed by Tukey’s multiple comparisons test; ns, nonsignificant; the absence of asterisks means nonsignificant between all the groups; **** on B indicate that the group is different from all the other groups; values are the mean ± SEM; n=7–12.
-
Figure 1—source data 1
Raw numerical values for Figure 1 plots.
- https://cdn.elifesciences.org/articles/101990/elife-101990-fig1-data1-v2.xlsx
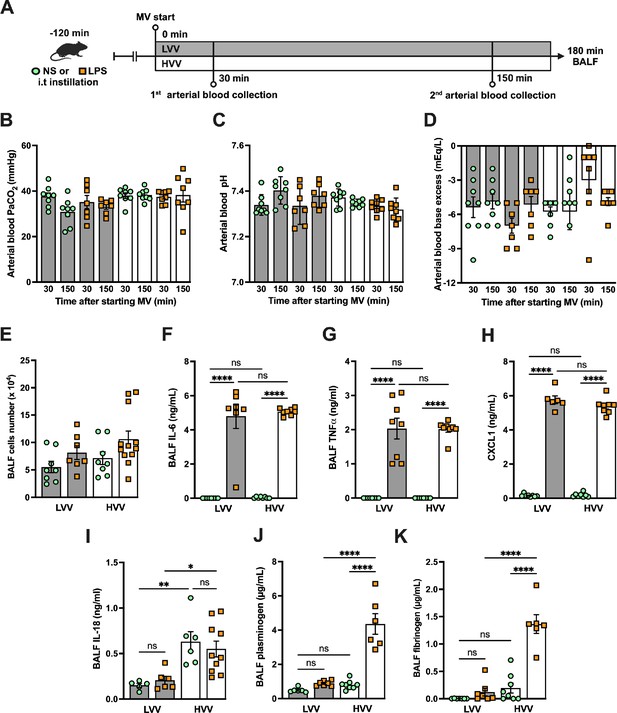
Severe acute lung injury induced by lipopolysaccharide (LPS) plus high-volume mechanical ventilation (MV) occurs without alteration in respiratory acidosis or alkalosis and increased plasminogen and fibrinogen levels in the alveoli.
LPS or normal saline (NS) was i.t. instilled to C57BL/6 mice, and after 120 min, the animals were anesthetized and placed on MV for 180 min with the tidal volumes of 30 mL/kg, high-volume ventilation (HVV), or 10 mL/kg low-volume ventilation (LVV) (A). This panel was created using BioRender.com. Arterial blood partial pressure of carbon dioxide (arterial blood PaCO2) (B), pH (C), and base excess (D) were measured at 30 and 150 min after starting MV. Absolute cell counts (E), IL-6 (F), TNFα (G), CXCL1 (H), IL-18 (I), plasminogen (I), and fibrinogen (K) were quantified in the bronchoalveolar lavage fluid (BALF) collected from euthanized animals after 180 min of MV. ****, **, and * indicate p<0.0001, p<0.01, and p<0.05, respectively, determined by three-way ANOVA (B–D) and two-way ANOVA (E–K) followed by Tukey’s multiple comparisons test; ns, nonsignificant; the absence of asterisks means nonsignificant between all the groups; values are the mean ± SEM; n=7–12.
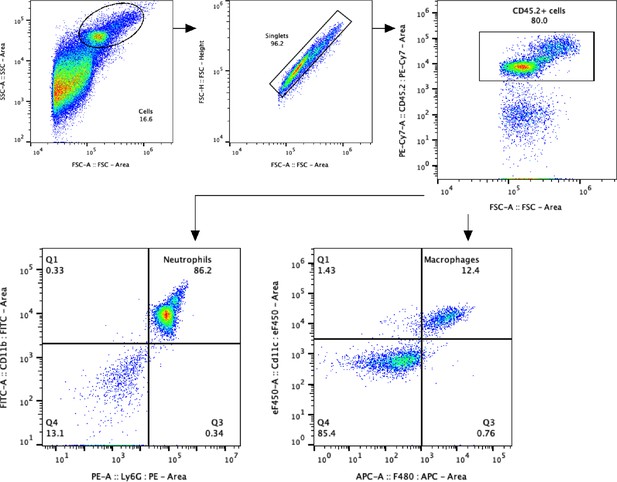
Gate strategy for alveolar neutrophils and macrophages in the two-hit model.
Alveolar neutrophils were identified as CD45.2+ CD11b+ Ly6G+ cells, and alveolar macrophages as CD45.2+ CD11c+ F4/80+ cells in bronchoalveolar lavage fluid (BALF).
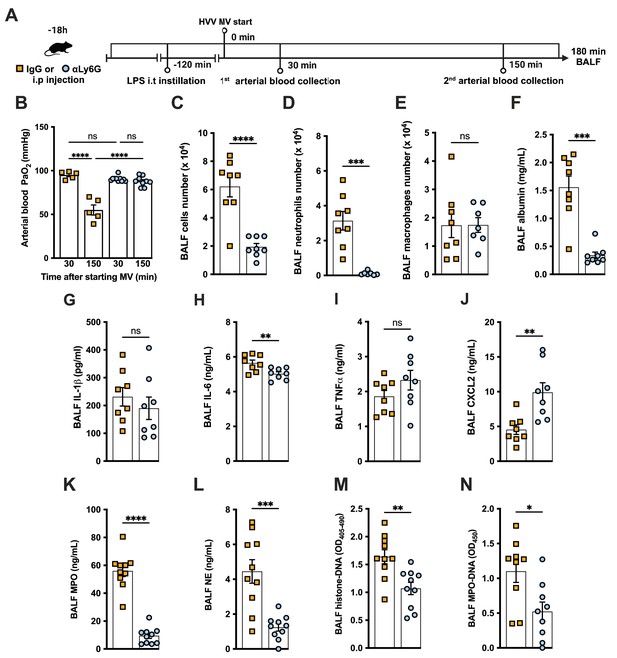
Neutrophils are required for the development of severe acute lung injury in the lipopolysaccharide (LPS)+high-volume ventilation (HVV) model.
Eighteen hours before starting mechanical ventilation (MV), the anti-neutrophil monoclonal antibody (αLy6G [1A8]) or the control IgG was administered i.p. to C57BL/6 mice. Sixteen hours later, LPS was instilled i.t. in the mice, and after 120 min, they were anesthetized and placed on HVV for 180 min (A).This panel was created using BioRender.com. Arterial blood partial pressure of oxygen (PaO2) was measured at 30 and 150 min after starting MV (B). Absolute counts of total cells (C), neutrophils (D), and macrophages (E) in bronchoalveolar lavage fluid (BALF). The concentration of albumin (F), IL-1β (G), IL-6 (H), TNFα (I), CXCL2 (J), MPO (K), and NE (L) was measured in the BALF by ELISA. Cell death and neutrophil extracellular traps (NETs) formation in the BALF were evaluated by histone-DNA (M) and MPO-DNA (N) respectively. ****, ***, **, and * indicate p<0.0001, p<0.001, p<0.01, and p<0.05, respectively, determined by two-way ANOVA followed by Tukey’s multiple comparisons test (B), unpaired two-tailed Student’s t-test (C, E, G–N), or Mann-Whitney test (D, F); ns, nonsignificant; values are the mean ± SEM; n=5–12.
-
Figure 2—source data 1
Raw numerical values for Figure 2 plots.
- https://cdn.elifesciences.org/articles/101990/elife-101990-fig2-data1-v2.xlsx
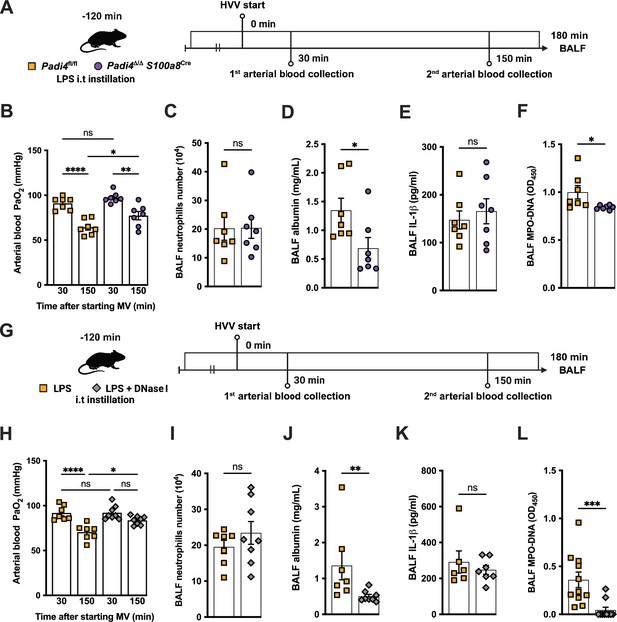
Neutrophil extracellular traps (NETs) contribute to the development of severe hypoxemia in the lipopolysaccharide (LPS)-high-volume ventilation (HVV)-induced acute lung injury (ALI).
LPS was instilled to neutrophil-specific PAD4-deficient (Padi4Δ/ΔS100a8Cre) or the controls (Padi4fl/fl) mice, and after 120 min, the animals were placed on mechanical ventilation (MV) for 180 min (A).This panel was created using BioRender.com. Arterial blood partial pressure of oxygen (PaO2) was measured at 30 and 150 min after starting MV (B). Absolute counts of neutrophils (C) and the levels of albumin (D) and IL-1β (E) were measured in bronchoalveolar lavage fluid (BALF). NETs formations in BALF were evaluated by measuring MPO-DNA (F). LPS and DNase I were instilled i.t. to C57BL/6 mice, and mice were placed on MV (G).This panel waas created using BioRender.com. PaO2 was measured at 30 and 150 min after starting MV (H). Neutrophils (I), albumin (J), IL-1β (K), and MPO-DNA (L) were measured in BALF. ****, ***, **, and * indicate p<0.0001, p<0.001, p<0.01, and p<0.05, respectively, determined by two-way ANOVA followed by Tukey’s multiple comparisons test (B, H), unpaired two-tailed Student’s t-test (C, E, F, I) or Mann-Whitney test (D, J–L); ns, nonsignificant; values are the mean ± SEM; n=7–11.
-
Figure 3—source data 1
Raw numerical values for Figure 3 plots.
- https://cdn.elifesciences.org/articles/101990/elife-101990-fig3-data1-v2.xlsx
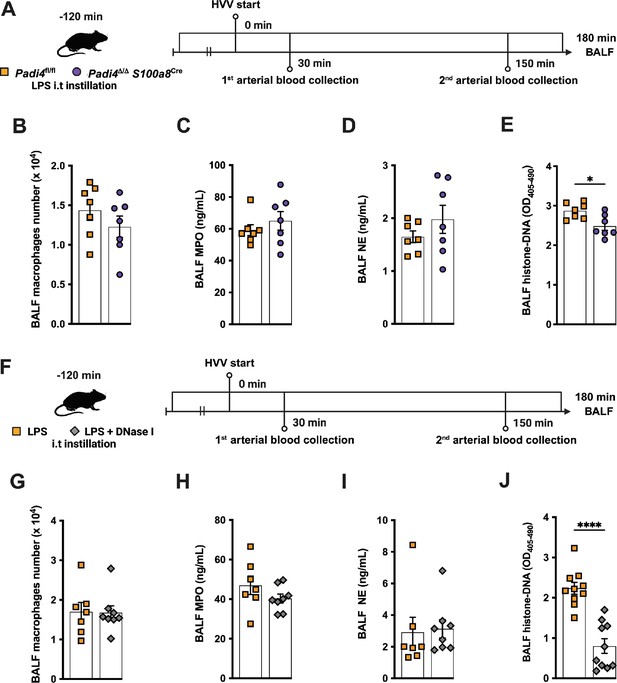
In the lipopolysaccharide (LPS)-high-volume ventilation (HVV) model, neutrophil-specific Padi4 deletion and DNase I treatment reduce the levels of histone-DNA complexes in the alveoli without altering the macrophage number and the levels of myeloperoxidase and neutrophil elastase.
Two different approaches to mitigate neutrophil extracellular trap (NET) effects in the LPS-HVV model are presented. In the first (A), we used neutrophil-specific PAD4-deficient mice (Padi4Δ/Δ S100a8Cre) or the controls (Padi4fl/fl) and the number of macrophages (B), the levels of myeloperoxidase (C) and neutrophil elastase (D), as well as histone-DNA complexes were assessed in the bronchoalveolar lavage fluid (BALF). In the second, simultaneously with the LPS instillation, the animals also received DNase I (F) and the number of macrophages (B), the levels of myeloperoxidase (C) and neutrophil elastase (D), as well as histone-DNA complexes were assessed in the BALF. **** and * indicate p<0.0001 and p<0.05, respectively, determined by unpaired two-tailed Student’s t-test (B, D–H, J) or Mann-Whitney test (C, I); values are the mean ± SEM; n=7–8. Panels A (https://biorender.com/d58i426) and F (https://biorender.com/u98a177) were created using BioRender.com.
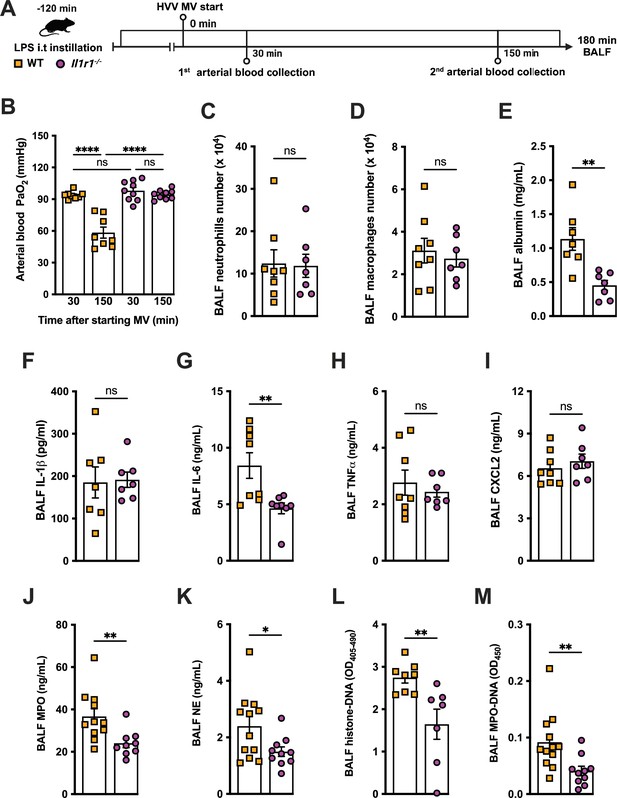
IL-1R1 signaling is required for neutrophil extracellular traps (NETs) formation in lipopolysaccharide (LPS)+high-volume ventilation (HVV)-induced acute lung injury (ALI).
LPS was instilled i.t. into wild-type (WT) and Il1r1-/- mice, and after 120 min, the animals were anesthetized and placed on HVV for 180 min, followed by sacrifice (A). This panel was created using BioRender.com. Arterial blood partial pressure of oxygen was measured at 30 and 150 min after starting mechanical ventilation (MV) (B). Absolute counts of neutrophils (C) and macrophages (D) in bronchoalveolar lavage fluid (BALF) were determined. The levels of albumin (E), IL-1β (F), IL-6 (G), TNFα (H), CXCL2 (I), MPO (J), and NE (K) were measured in the BALF by ELISA. Cell death and NETs formation in the BALF were evaluated by measuring histone-DNA (L) and MPO-DNA (M), respectively. ****, **, and * indicate p<0.0001, p<0.01, and p<0.05, respectively, determined by two-way ANOVA followed by Tukey’s multiple comparisons test (B), unpaired two-tailed Student’s t-test (D–F, H–M), or Mann-Whitney test (C, G); ns, nonsignificant; values are the mean ± SEM; n=7–12.
-
Figure 4—source data 1
Raw numerical values for Figure 4 plots.
- https://cdn.elifesciences.org/articles/101990/elife-101990-fig4-data1-v2.xlsx
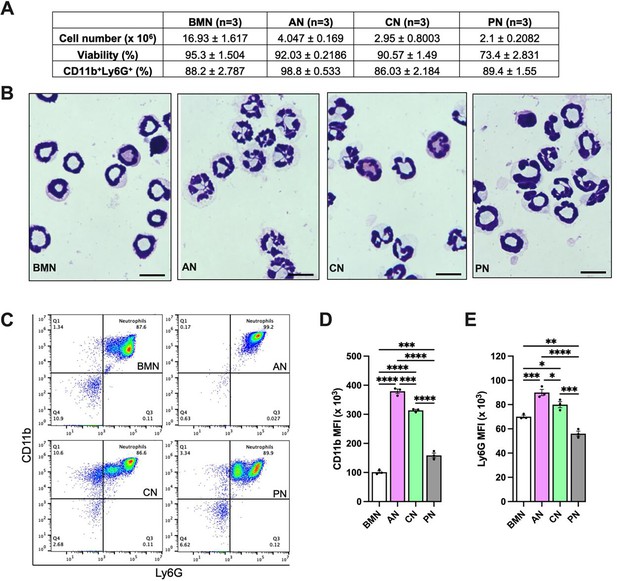
Alveolar neutrophils (AN) present more segmented nuclei and mature CD11b and Ly6G expression.
Bone marrow neutrophils (BMN), AN, circulating neutrophils (CN), and peritoneal neutrophils (PN) were isolated. The total cell number obtained per mouse and the viability are shown in the table (A). Hemacolor-stained cells showing neutrophils nuclei morphology (B). Scale bars, 10 µm. Representative flow cytometry plots of neutrophils, represented as CD11b+Ly6G+ cells in the CD45.2+ gate (C). Mean fluorescence intensity of CD11b (D) and Ly6G (E) on neutrophils. ****, ***, **, and * indicate p<0.0001, p<0.001, p<0.01, and p<0.05, respectively, determined by one-way ANOVA followed by Tukey’s multiple comparisons test; values are the mean ± SEM; n=3.
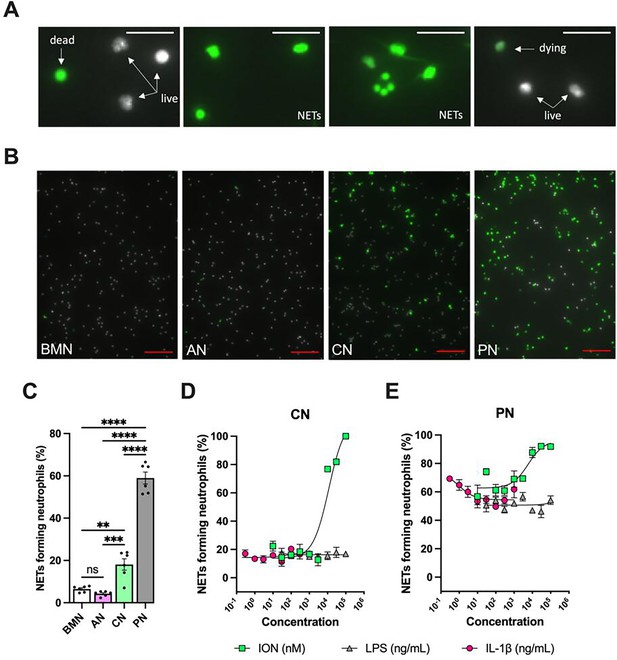
Non-stimulated circulating neutrophils (CN) and peritoneal neutrophils (PN) are more susceptible to forming neutrophil extracellular traps (NETs) compared with bone marrow neutrophils (BMN) and alveolar neutrophils (AN).
NETs are represented by elongated shaped SYTOX Green-positive (green) Hoechst-negative (white) cells. SYTOX Green-negative Hoechst-positive cells are considered live neutrophils, and round-shaped SYTOX Green-positive Hoechst-negative cells are classified as dead neutrophils. Dying neutrophils are shown as SYTOX Green-positive Hoechst-positive cells (A). Hoechst-stained neutrophils were incubated without exogenous stimulations for 5 hr, stained with SYTOX Green, and the images were captured under the microscope. Representative images (A) and NETs forming neutrophils (B) of non-stimulated BMN, AN, CN, and PN. Scale bars: 20 µm in (A) and 100 µm in (B). CN (C) and PN (D) were stimulated with various concentrations of lipopolysaccharide (LPS, 30–100,000 ng/mL), IL-1β (0.3–1000 ng/mL), and ionomycin (ION) (10–100,000 nM), and the curves of NETs forming neutrophils were plotted. ****, ***, and ** indicate p<0.0001, p<0.001, and p<0.01, respectively, determined by one-way ANOVA followed by Tukey’s multiple comparisons test; ns, nonsignificant; values are the mean ± SEM; representative of three independent experiments.
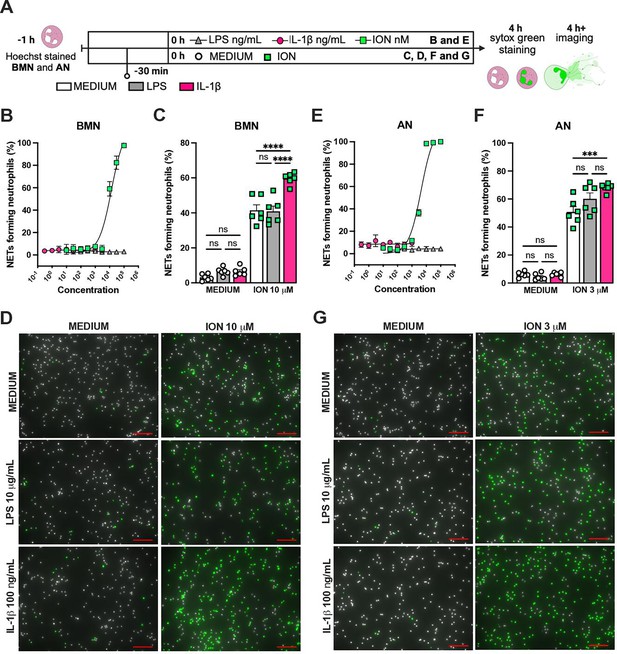
IL-1β enhances ionomycin (ION)-induced neutrophil extracellular traps (NETs) formation in vitro.
Hoechst-stained bone marrow neutrophils (BMN) or alveolar neutrophils (AN) were incubated for 1 hr prior to stimulation at 37°C. At time zero, the different stimuli were added, and the cells were incubated for 4 hr, then stained with SYTOX green, and the images were captured under microscope (A). This panel was created using BioRender.com. BMN (B) and AN (E) were stimulated with several concentrations of lipopolysaccharide (LPS, 30–100,000 ng/mL), IL-1β (0.3–1000 ng/mL), and ION (10–100,000 nM). For combined stimulation, BMN (C and D) and AN (F and G) were first incubated with LPS or IL-1β 30 min prior to ION. The NETs forming neutrophils were analyzed as elongated shaped SYTOX Green-positive cells and expressed as percentage (%). The SYTOX Green- and Hoechst-positive cells are represented by green and white colors, respectively, on the representative images. Scale bars: 100 µm. ****, ***, and ** indicate p<0.0001, p<0.001, and p<0.01, respectively, determined by two-way ANOVA followed by Tukey’s multiple comparisons test; ns, nonsignificant; values are the mean ± SEM; representative of three independent experiments.
-
Figure 5—source data 1
Raw numerical values for Figure 5 plots.
- https://cdn.elifesciences.org/articles/101990/elife-101990-fig5-data1-v2.xlsx
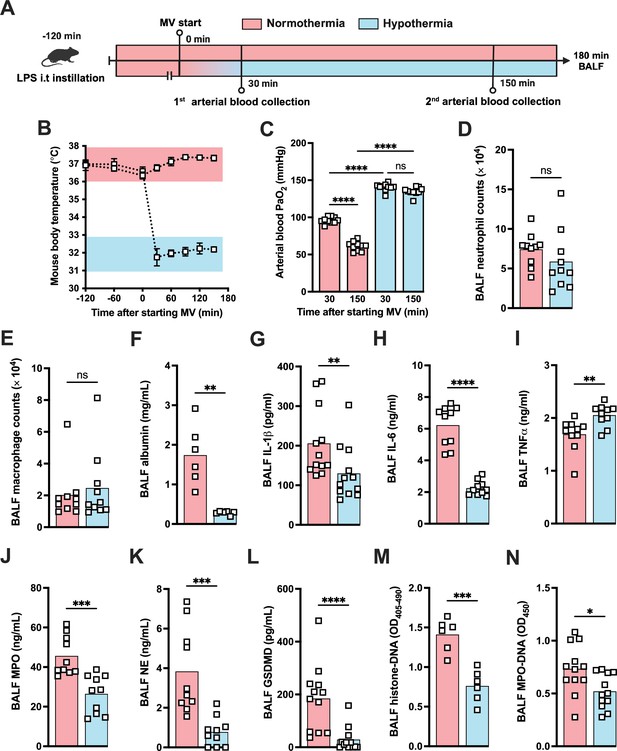
Hypothermia protects against lipopolysaccharide (LPS)+high-volume ventilation (HVV)-induced severe acute lung injury by controlling IL-1β, gasdermin D (GSDMD), and neutrophil extracellular traps (NETs) in the alveoli.
LPS was instilled i.t. to C57BL/6 mice, and after 120 min, the animals were anesthetized and placed on HVV for 180 min under controlled body temperature of 37±1°C or 32±1°C, designated as normothermia and hypothermia, respectively (A). This panel was created using BioRender.com. The body temperature for each group was monitored (B). Arterial blood partial pressure of oxygen was measured at 30 and 150 min after starting mechanical ventilation (MV) (C). Absolute counts of neutrophils (D) and macrophages (E) in the bronchoalveolar lavage fluid (BALF) collected from euthanized animals after 180 min of MV. The levels of albumin (F), IL-1β (G), IL-6 (H), TNFα (I), MPO (J), NE (K), soluble GSDMD (L) in the BALF were determined by ELISA. Cell death and NETs formation in the BALF were evaluated by histone-DNA (M) and MPO-DNA (N), respectively. ****, ***, **, and * indicate p<0.0001, p<0.001, p<0.01, and p<0.05, respectively, determined by two-way ANOVA followed by Tukey’s multiple comparisons test (C), unpaired two-tailed Student’s t-test (D, J, K, M, N), or Mann-Whitney test (E–I, L); ns, nonsignificant; values are the mean ± SEM, n=6–12 mice/group.
-
Figure 6—source data 1
Raw numerical values for Figure 6 plots.
- https://cdn.elifesciences.org/articles/101990/elife-101990-fig6-data1-v2.xlsx
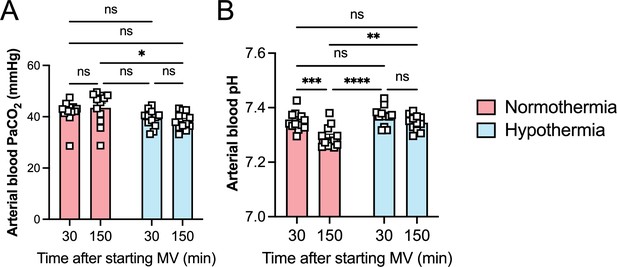
Hypothermia protects against lipopolysaccharide (LPS) plus high-volume mechanical ventilation (MV)-induced severe acute lung injury without respiratory acidosis or alkalosis.
LPS was intratracheally instilled into C57BL/6 mice, and after 120 min, the animals were anesthetized and placed on MV for 180 min with high-volume ventilation (HVV). Arterial blood partial pressure of carbon dioxide (arterial blood PaCO2) (A), pH (B) at 30 and 150 min after HVV. *, **, ***, and **** were determined by two-way ANOVA followed by Tukey’s multiple comparisons test.
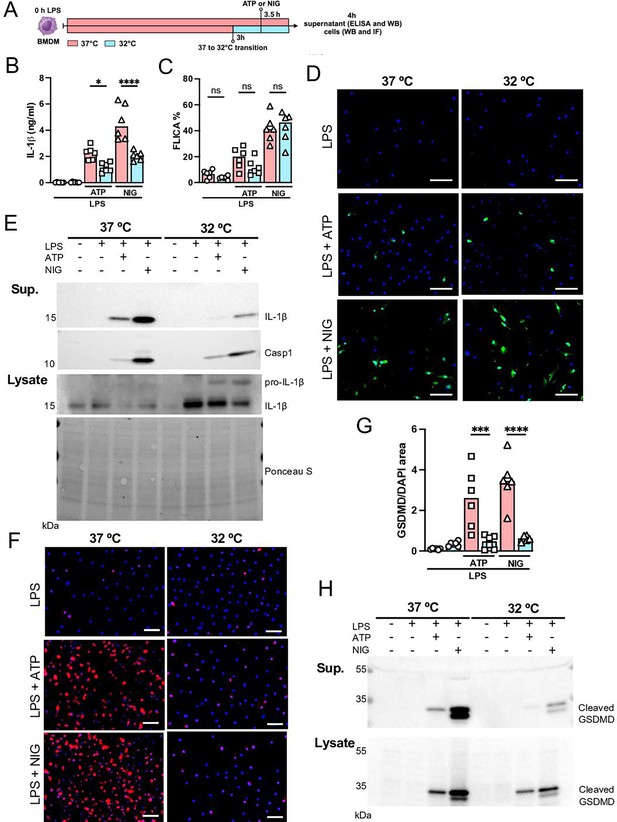
Hypothermia inhibits macrophage IL-1β release by modulating NLRP3 inflammasome-induced gasdermin D (GSDMD) cleavage.
Bone marrow-derived macrophages (BMDMs) were primed with lipopolysaccharide (LPS) for 3 hr at 37°C, then incubated at 37°C or 32°C for 30 min prior to adenosine triphosphate (ATP) or nigericin (NIG) treatments for another 30 min (A). This panel was created using BioRender.com. In the supernatant, IL-1β concentration was determined by ELISA (B). The supernatant (C) and cell lysate (D) were used for western blotting (WB) analysis, and the resulting membranes were stained for IL-1β, caspase-1, and GSDMD (C). WB analysis was also made in the cell lysate, where we investigated the expression of IL-1β and GSDMD. The protein distribution in the cell lysate samples was certified by Ponceau S staining. BMDMs were stained with anti-GSDMD (red) and DAPI (blue), as shown in the representative images. The GSDMD area was analyzed and normalized by DAPI area (E). To evaluate caspase-1 activity, the cells were stained with FAM-YVAD-FMK FLICA and analyzed under the microscope (F). Scale bars: 50 µm. ****, ***, and * indicate p<0.0001, p<0.001, and p<0.05, respectively, determined by two-way ANOVA followed by Tukey’s multiple comparisons test; ns, nonsignificant; values are the mean ± SEM; representative of three independent experiments.
-
Figure 7—source data 1
Annotated western blot images corresponding to Figure 7E and H.
- https://cdn.elifesciences.org/articles/101990/elife-101990-fig7-data1-v2.pdf
-
Figure 7—source data 2
Original, uncropped western blot images used for Figure 7E and H.
- https://cdn.elifesciences.org/articles/101990/elife-101990-fig7-data2-v2.zip
-
Figure 7—source data 3
Raw numerical values for Figure 7 plots.
- https://cdn.elifesciences.org/articles/101990/elife-101990-fig7-data3-v2.xlsx
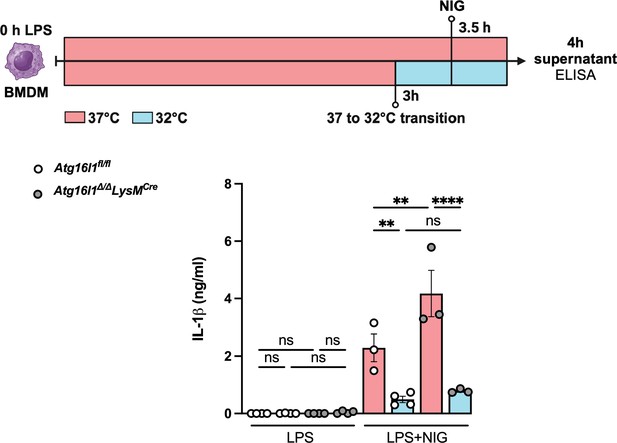
Hypothermia-induced NLRP3 inflammasome inhibition is independent of autophagy.
Bone marrow-derived macrophages (BMDMs) isolated from Atg16l1fl/fl or Atg16l1Δ/Δ Lyz2Cre were primed with lipopolysaccharide (LPS) for 3 hr at 37°C, incubated at 37°C or 32°C for 30 min prior to nigericin (NIG) treatment for another 30 min. The IL-1β concentration in the culture supernatants was determined by ELISA. ** indicates p<0.01 determined by three-way ANOVA followed by Tukey’s multiple comparisons test; ns, nonsignificant; values are the mean ± SEM; representative of three independent experiments. This figure was created using BioRender.com.
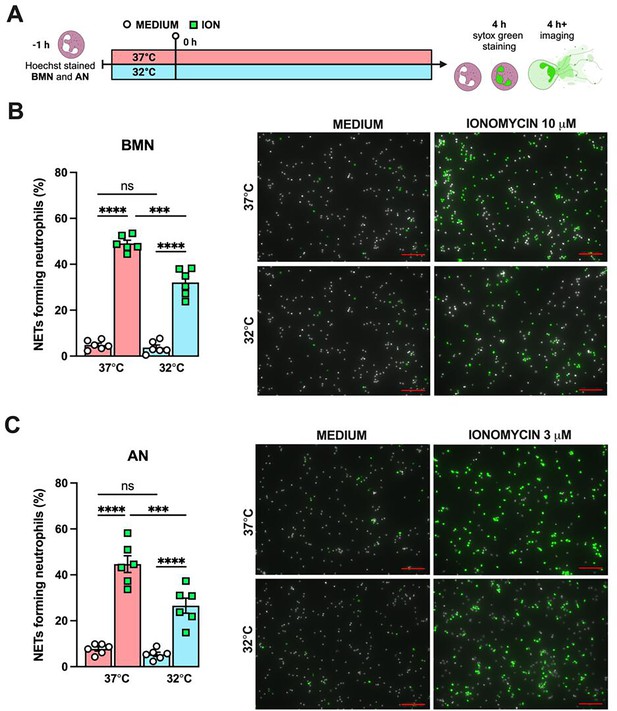
Hypothermia inhibits neutrophil extracellular traps (NETs) formation in vitro.
Bone marrow neutrophils (BMN) and alveolar neutrophils (AN) were incubated for 1 hr prior to stimulation at 37°C or 32°C. Neutrophils were stimulated with ionomycin (ION) for 4 hr at their respective temperatures in the presence of Hoechst, then stained with SYTOX Green, and the images were captured (A). This panel was created using BioRender.com. BMN were stimulated with 10 µM of ION (B) while AN were stimulated with 3 µM of ION (D). Scale bars: 100 µm. NETs formation was then evaluated. **** and *** indicate p<0.0001 and p<0.001, respectively, determined by two-way ANOVA followed by Tukey’s multiple comparisons test; ns, nonsignificant; values are the mean ± SEM, representative of three independent experiments.





