Neuroprotective role of Hippo signaling by microtubule stability control in Caenorhabditis elegans
Figures
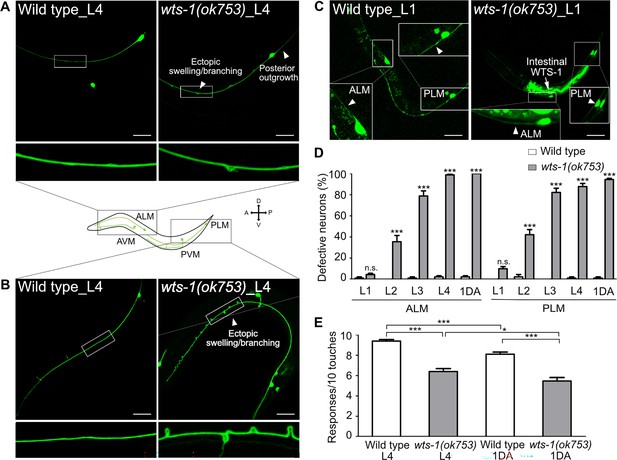
wts-1 mutant shows the premature structural and functional decline of touch receptor neurons (TRNs).
(A, B) Representative images of TRNs (A: ALM; B: PLM) in the wild-type and wts-1(ok753) mutant at the L4 stage. In the mutant, morphologically disrupted ALM and PLM were observed to exhibit abnormalities including ectopic swelling/branching on the processes and posterior outgrowth (arrowheads). (C) Newly developed ALM and PLM in the wild-type and the wts-1(ok753) mutant at the early L1 stage. The intact neuronal process is indicated using arrowheads. Intestinal expression of the WTS-1 rescue construct (Popt-2::WTS-1::GFP) is indicated using an arrow. (A–C) TRNs were visualized by expressing GFP under the control of the mec-7 promoter (muIs35[Pmec-7::GFP]) and anterior is to the left unless otherwise noted. Scale bar = 20 μm. (D) Penetration of defective TRNs of the wild-type and the wts-1(ok753) at different developmental stages. Neurons displaying any morphological abnormalities such as swelling, branching, somatic outgrowth, and extended distal process were scored as defective neurons. In one experiment, 30 cells were observed for each strain and each stage and the experiments were repeated three times. Statistical significance was determined by a two-way ANOVA followed by Bonferroni’s post-test. (E) Quantified touch responses of the wild-type and the wts-1(ok753) mutant at L4 and first day of adulthood (1DA). N = 30. Statistical significance was determined by an unpaired t-test. ***p<0.001, **p<0.01, *p<0.05, n.s., not significant, compared to WT or control, unless otherwise marked on the graph. All data are presented as means ± SEM, unless otherwise noted.
-
Figure 1—source data 1
Raw data for panels D and E.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig1-data1-v1.xlsx
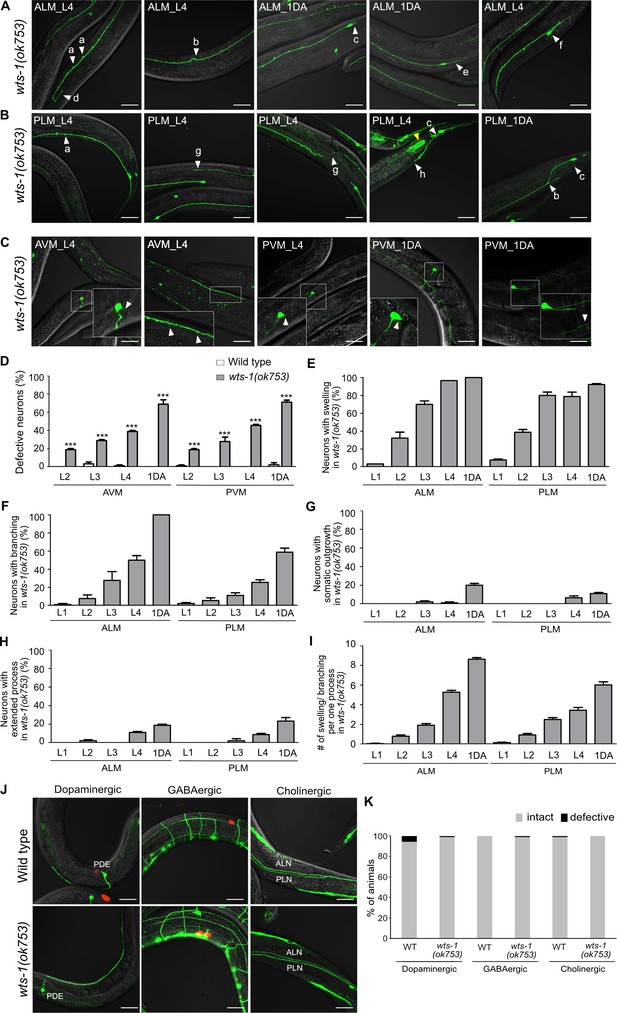
Loss of wts-1 leads to touch neuronal-specific structural decline.
(A, B) Various morphological deformations observed in wts-1 mutant (A) ALM and (B) PLM. In the mutants, both ALM and PLM show ectopic swelling (a), branching (b) and somatic outgrowth (c). ALM also displays an extended distal process (d), posterior outgrowth (e), or abnormalities in the cell body (f). PLM exhibits extended (g) or shortened (h) process. Touch receptor neurons (TRNs) were analyzed at the L4 or first day of adulthood (1DA) stage. Intestinal expression of the WTS-1 rescue construct is indicated using a yellow arrowhead. (C) Structural decline of AVM and PVM in the wts-1 mutants. Somatic outgrowth or ectopic branching/swelling in the process is occasionally noted in both AVM and PVM. (D) Quantified structural defects of AVM and PVM in wild-type and wts-1 mutants at different stages. Statistical significance was determined using a two-way ANOVA followed by Bonferroni’s post-test. (E–H) Quantified structural defects of ALM and PLM observed in wts-1 mutants at different stages. (E) Ectopic swelling or (F) branching on the neuronal process occurs the most frequently, followed by (G) somatic outgrowth and (H) extended distal process. Structural deformations of each category tend to increase as the worms grow. (I) The average number of ectopic lesions on a neuronal process of ALM or PLM of wts-1 at different stages. The total number of ectopic swelling or branching was scored. (D–I) At each time point, 90 ALM or PLM were analyzed (N = 30, repeated three times). (J) Representative images of dopaminergic, GABAergic, and cholinergic neurons in wts-1 mutants at the L4 stage. Each neuron was visualized using a fluorescent marker under the control of a specific promoter; dat-1 promoter, unc-47 promoter, cho-1 promoter, respectively. (K) Quantified structural defects of dopaminergic, GABAergic, and cholinergic neurons in wild-type and wts-1 mutants. N = 90. ***p<0.001, **p<0.01, *p<0.05, n.s., not significant, compared to WT or control, unless otherwise marked on graph. All data are presented as means ± SEM, unless otherwise noted. Scale bar = 20 μm.
-
Figure 1—figure supplement 1—source data 1
Raw data for panels D–I and K.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig1-figsupp1-data1-v1.xlsx
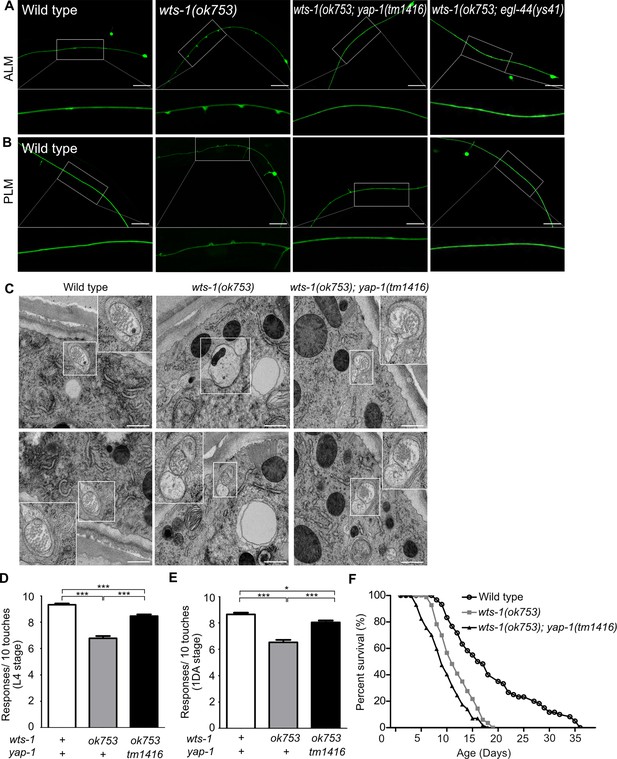
yap-1 or egl-44 suppresses premature neuronal decline in wts-1 mutants.
(A, B) Representative images of (A) ALM and (B) PLM in wild-type, wts-1(ok753), wts-1(ok753); yap-1(tm1416), and wts-1(ok753); egl-44(ys41) at the L4 stage. Loss of yap-1 or egl-44 completely restores structural integrity of the wts-1 mutants. Scale bar = 20 μm. (C) Electron microscope images of wild-type, wts-1(ok753) and wts-1(ok753); yap-1(tm1416) at 1DA. Touch receptor neurons (TRNs) are indicated using boxes. Scale bar = 500 nm. (D, E) Touch responses of wild-type, wts-1(ok753) and wts-1(ok753); yap-1(tm1416) at (D) L4 stage and (E) 1DA stage. For each strain and each stage, 90 animals were tested. Statistical significance was determined using a one-way ANOVA, followed by Tukey’s multiple comparison test. (F) Survival curve of wild-type, wts-1(ok753) and wts-1(ok753); yap-1(tm1416). Worms were maintained at 20°C, and all lines used for the analysis have muIs35.
-
Figure 2—source data 1
Raw data for panels D–F.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig2-data1-v1.xlsx
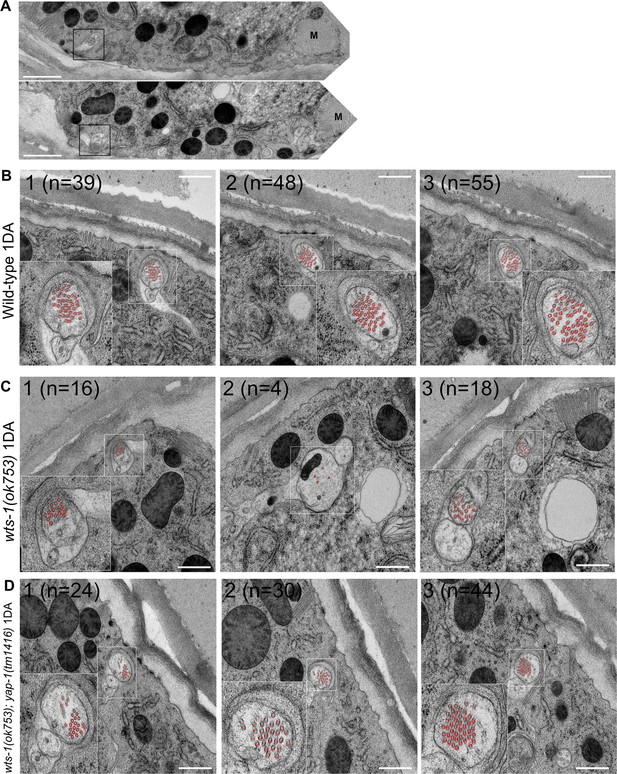
yap-1 suppresses structural deformities of wts-1 mutant neurons.
(A) Electron microscope images show that touch receptor neurons (TRNs) (ALM) normally dissociate from the muscles (M) and innervate the epidermis in the wts-1 mutants. TRNs are indicated using boxes. Scale bar = 1 μm. (B–D) Microtubules of TRNs in (B) control wild-type, (C) wts-1(ok753), and (D) wts-1(ok753); yap-1(tm1416). Decreased number of microtubules in wts-1 mutant ALM is restored in wts-1(ok753); yap-1(tm1416). Microtubules are indicated using red numbers, and the total number of microtubules present in each section is indicated in the upper-left area of each image. Imaging was performed at 1DA. Sections were cut from the head and serial sections for 5~10 μm of one individual are presented. Scale bar = 500 nm.
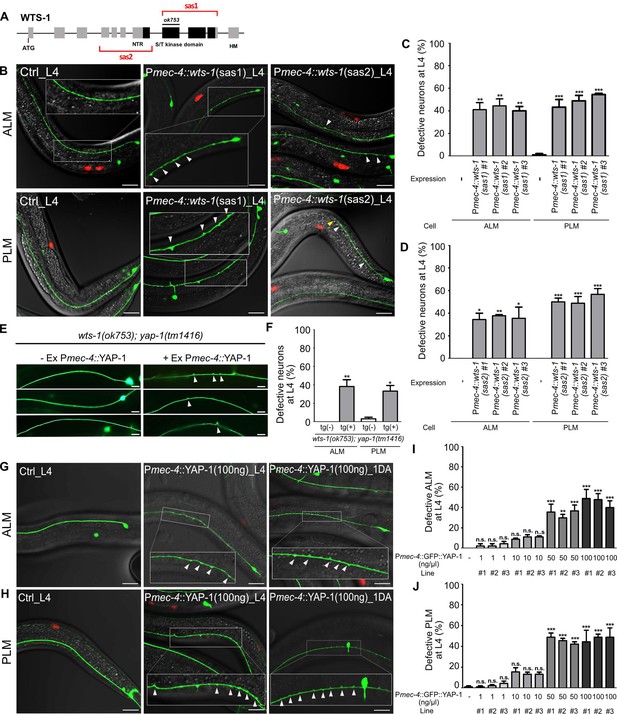
wts-1-yap-1 act in a cell-autonomous manner to maintain touch neuronal integrity.
(A) The targeting regions for touch neuronal-specific RNAi of wts-1. Sense and antisense (sas) genomic fragments were cloned under the touch receptor neurons (TRNs)-specific promoter, Pmec-4. (B) Representative images and (C, D) quantified neuronal abnormalities of TRNs in the controls and wts-1-knockdowned animals. Both TRNs-specific-sas1- and sas2-based knockdown of wts-1 efficiently induce neuronal abnormalities as seen in wts-1 mutant. (E, F) Touch neuronal rescue of YAP-1 is sufficient to re-induce neuronal disintegrity in wts-1(ok753); yap-1(tm1416) mutant. (E) Representative images of TRNs of wts-1(ok753); yap-1(tm1416) mutant with the rescue construct or its sibling without transgenes. (F) Quantified results. 60 ALM and PLM of transgenic worms and their siblings were observed. (G, H) Touch neuronal overexpression of YAP-1 is sufficient to induce neuronal abnormalities in wild-type animals. Final concentration of Pmec-4::GFP::YAP-1 in the injection mixture is 100 ng/μl. (I, J) Quantified neuronal defects of (I) ALM and (J) PLM induced by overexpression of Pmec-4::GFP::YAP-1 at each concentration in the injection mixtures. (C, D, I, J) 90 ALM and PLM were observed in each of the three independent lines. Neuronal morphology was examined at L4 stage. Statistical significance was determined by a one-way ANOVA followed by Dunnett’s multiple comparison test. An unpaired t-test was used for (F). Red: expression of injection marker, Punc-122::RFP; white arrowhead: neuronal swelling; yellow arrowhead: shortened process. Scale bar = 20 μm.
-
Figure 3—source data 1
Raw data for panels C, D, F, I and J.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig3-data1-v1.xlsx
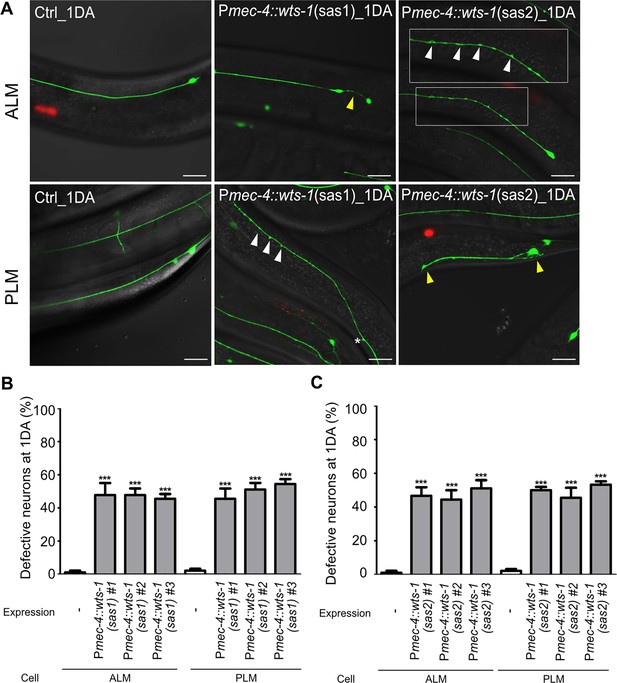
Cell-autonomous function of wts-1.
(A) Representative images of ALM (upper panels) and PLM (lower panels) of control animals and wts-1 touch receptor neurons (TRNs)-specific-knockdown animals at 1DA. Both sas1 and sas2 constructs effectively induce structural defects in TRNs. Ectopic neuronal swelling is labeled with white arrowheads and other structural defects, including abnormal cell body, shortened process of PLM are labeled with yellow arrowheads. (B, C) Quantified neuronal defects induced by (B) sas1- or (C) sas2-based knockdown of wts-1. Neurons were observed at 1DA. N = 30/1 experiment, repeated three times. Statistical significance was determined by a one-way ANOVA, followed by Dunnett’s multiple comparison test. Scale bar = 20 μm.
-
Figure 3—figure supplement 1—source data 1
Raw data for panels B and C.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig3-figsupp1-data1-v1.xlsx
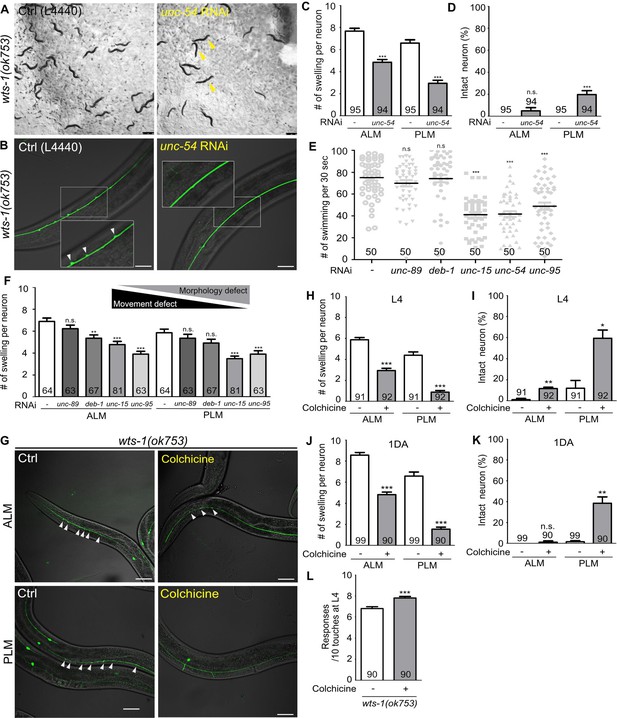
Abnormal microtubules are responsible for neuronal disintegrity observed in the wts-1 mutants.
(A–D) Reduced movement resulting from unc-54 knockdown diminishes ectopic swelling or branching in the wts-1(ok753) mutant. (A) unc-54 knockdown leads to uncoordinated movement of worms (yellow arrowhead). (B, C) unc-54 knockdown reduces the number of neuronal lesions on the ALM and PLM. (D) unc-54 knockdown slightly, but significantly, increases the percentages of intact PLM and not ALM. Neurons without any structural defects, including swelling or branching, were considered intact. (E) Quantified motor deficits in animals knocking down various muscle machinery genes. RNAi against unc-15, unc-54, or unc-95 impairs swimming behavior, whereas unc-89 and deb-1 make no differences compared to control. (F) Quantified morphological abnormalities of touch receptor neurons (TRNs) in muscle machinery-knockdown animals. Knockdown of unc-89 or deb-1 does not alleviate structural decline of both ALM and PLM. (G–K) Treatment with colchicine reduces ectopic neuronal swelling and increases the percentages of intact neurons in the wts-1(ok753) mutants. F1 progenies grown on the drug-contained plates were scored at (H, I) L4 stage or (J, K) 1DA. (L) Colchicine treatment improves touch responses of the wts-1 mutant. The behavior test was done at L4 stage. Statistical significance was determined using an unpaired t-test (C, D, H, L) or a one-way ANOVA, followed by Dunnett’s multiple comparison test (E, F). The total number of cells or animals analyzed is indicated in each column. Asterisks indicate differences from L4440-fed or drug-untreated-control neurons. Ectopic neuronal swelling and branching are labeled with white arrowheads. Scale bar = 20 μm.
-
Figure 4—source data 1
Raw data for panels C–F and H–L.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig4-data1-v1.xlsx
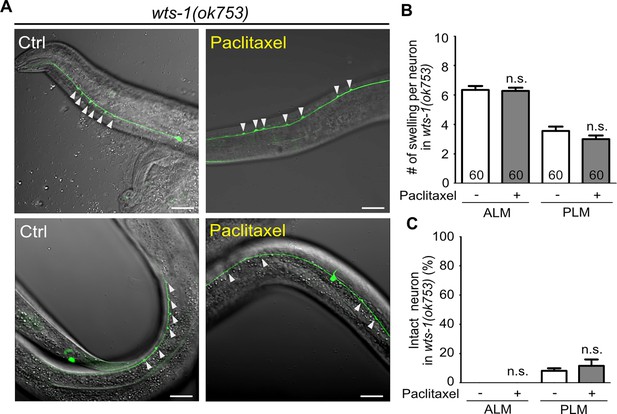
Paclitaxel treatment fails to mitigate wts-1 neuronal defects.
(A) Representative images of ALM (upper panels) and PLM (lower panels) of wts-1 mutants treated with paclitaxel and untreated control worms. Unlike colchicine treatment, paclitaxel treatment fails to lessen the touch neuronal deformation of the mutants. (B) Quantified results of ectopic neuronal lesion scoring. In total, 60 cells were analyzed for each neuron. (C) Quantified results of the percentage of intact ALM and PLM in the wts-1(ok753); bus-17(br12) under the condition of paclitaxel-treated and untreated worms. Statistical significance was determined using an unpaired t-test. Ectopic neuronal swelling is labeled with arrowheads. Scale bar = 20 μm.
-
Figure 4—figure supplement 1—source data 1
Raw data for panels B and C.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig4-figsupp1-data1-v1.xlsx
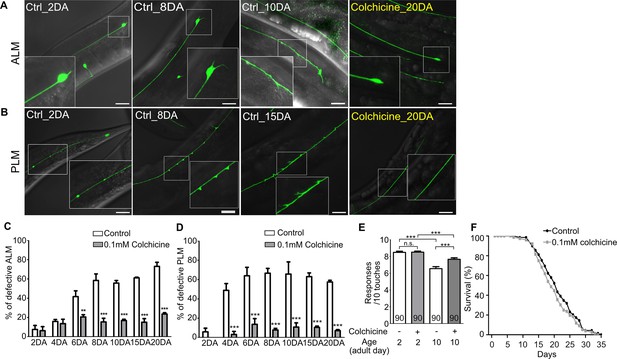
Hyper-stabilized microtubules might be responsible for age-associated morphological deformation of touch receptor neurons.
(A, B) Representative images of (A) ALM and (B) PLM of colchicine-treated or untreated wild-type worms. The age of worms is indicated in the image. (C, D) Quantified defects of (C) ALM or (D) PLM in colchicine-treated or untreated worms. Age-synchronized worms were transferred to drug-containing plates on the 1DA and phenotypes were scored on every 2nd, 4th, 6th, 8th, 10th, 15th, and 20th day of adulthood. At every time point and in each group, 20 neurons were scored per one experiment and the experiments were repeated three times. (E) Colchicine treatment alleviates impaired touch responses of aged animals. Touch responses were scored at 2DA and 10DA. Statistical significance was determined by a two-way ANOVA, followed by Bonferroni’s post-test (C, D) or a one-way ANOVA with Bonferroni’s multiple comparison test (E). (F) Survival curves of colchicine-treated and untreated control animals. Scale bar = 20 μm.
-
Figure 5—source data 1
Raw data for panels C–F.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig5-data1-v1.xlsx
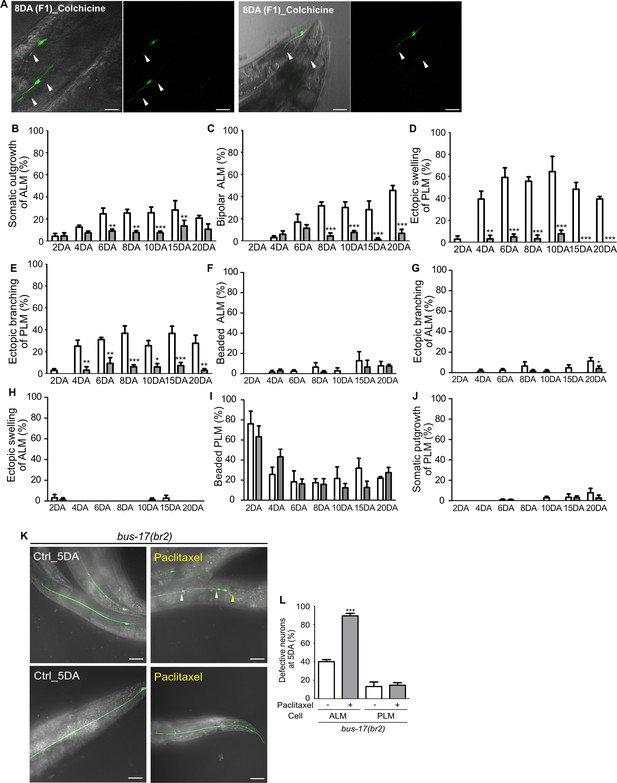
Colchicine treatment affects touch receptor neurons (TRNs) morphology.
(A) Representative images of TRNs of 8-day-old adult wild-type worms fed with colchicine from parental generations. Arrowheads indicate highly degenerated TRNs of F1 progenies. Almost all processes of ALM or PLM degraded, and the cell bodies showed irregular shape. (B–J) Quantified results of the effect of colchicine on age-related structural deformations of touch neurons in each category. Colchicine (0.1 mM) was used on worms from the 1DA and phenotypes were scored at every 2nd, 4th, 6th, 8th, 10th, 15th, and 20th day of adulthood (N = 20/1 experiment, repeated three times). Among the structural deformations, some features that are particularly associated with age, such as (B) somatic outgrowth and (C) posterior outgrowth of ALM or (D) swelling and (E) branching of PLM, have been significantly reduced by colchicine treatment. Colchicine treatment has only limited effects on (G) branching of ALM or no effect on (H–J) the other deformations. Statistical differences were determined using a two-way ANOVA, followed by Bonferroni’s post-test. Asterisks indicate differences from untreated control ALM or PLM. (K, L) Paclitaxel treatment significantly increases neuronal defects of ALM. (K) Representative images of TRNs of control animals and paclitaxel-treated animals at 5DA. (L) Quantified neuronal defects in controls and drug-treated animals (N = 30/1 experiment, repeated three times). Statistical significance was determined using an unpaired t-test. Scale bar = 20 μm.
-
Figure 5—figure supplement 1—source data 1
Raw data for panels B–J and L.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig5-figsupp1-data1-v1.xlsx
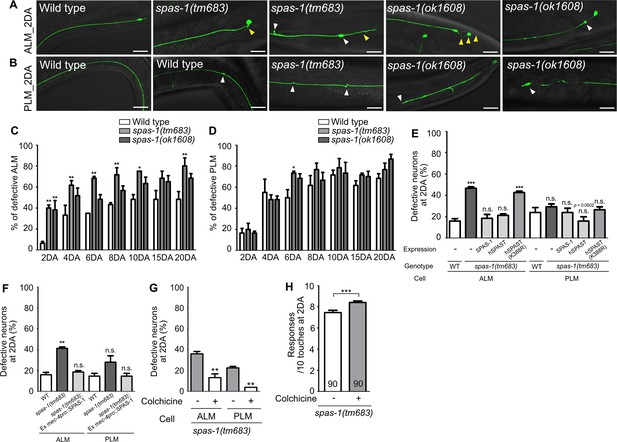
Loss of spas-1, a microtubule-severing enzyme, results in premature structural decline.
(A, B) Representative images of defective touch receptor neurons (TRNs) of spas-1 mutant on the 2DA. Both deletion mutants, tm683 and ok1608, display age-associated morphological alterations of ALM or PLM including ectopic swelling and branching on the neuronal process (white arrowhead), somatic outgrowth and irregular shape of the cell body (yellow arrowhead) precociously. (C, D) Quantified results of structural defects of ALM or PLM in spas-1 mutants. At every time point, 20 neurons were scored and the experiments were repeated three times. (E) Results of the rescue experiment of premature neuronal degeneration of spas-1(tm683) with SPAS-1, human SPAST(wt), and human SPAST(K388R). In all cases, the C. elegans spas-1 promoter was used to induce the C-terminally mCherry tagged transgene. (F) Touch neuronal-specific expression of SPAS-1 was sufficient to rescue neuronal defects of ALM. (E, F) Neuronal morphology were scored at 2DA. For each rescue construct, three independent transgenic lines were observed that yielded similar results and the results of one line are presented. (G, H) Quantified (G) structural defects of TRNs or (H) touch responses of colchicine-treated and untreated spas-1(tm683) mutant. Analyses were done at 2DA (N = 30/1 experiment, repeated three times). Statistical significance was determined using a two-way ANOVA, followed by Bonferroni’s multiple comparison test (C, D), a one-way ANOVA with Dunnett’s multiple comparison test (E, F) or with Turkey’s multiple comparison test (G). Unpaired t-test was used in (H). Scale bar = f20 μm.
-
Figure 6—source data 1
Raw data for panels C–H.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig6-data1-v1.xlsx
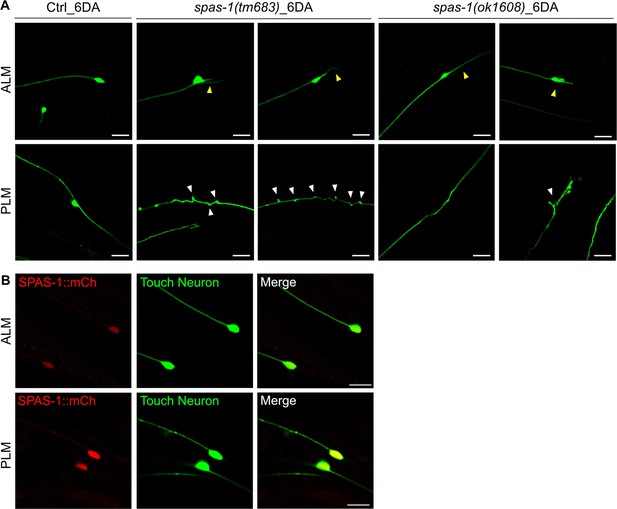
spas-1 also acts cell-autonomously to maintain neuronal morphology of touch receptor neurons (TRNs).
(A) Both spas-1 deletion mutants display structural decline of TRNs precociously. Neurons were imaged at 6DA. Neuronal abnormalities are labeled with arrowheads. (B) SPAS-1 expression in TRNs (red: Pspas-1:: spas-1:: mCherry; green: Pmec-7::GFP). Scale bar = 20 μm.
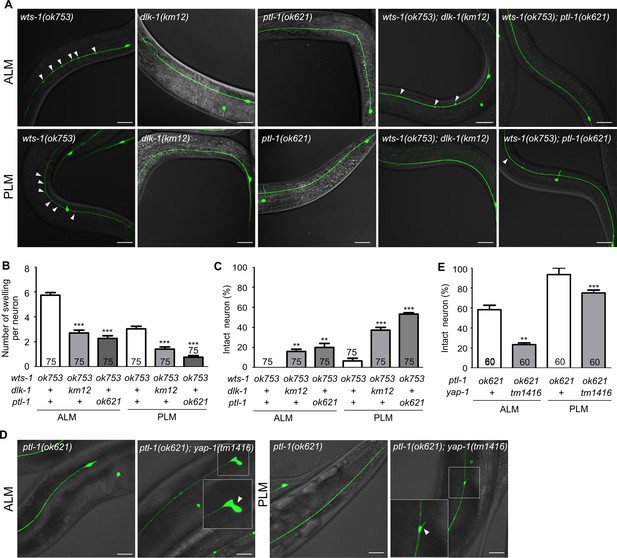
wts-1-yap-1 affect neuronal integrity possibly by modulating microtubule stability.
(A–C) Loss of dlk-1 or ptl-1 significantly mitigates the structural deformation of wts-1-mutant neurons. (A) Representative images of ALM (upper panels) and PLM (lower panels) of wts-1(ok753), dlk-1(km12), ptl-1(ok621), wts-1(ok753); dlk-1(km12), and wts-1(ok753); ptl-1(ok621) at L4 stage. Ectopic lesions are labeled with arrowhead. (B) Average number of ectopic lesions per neuronal process of each strain. (C) Percentage of intact neurons of each mutant. Loss of dlk-1 or ptl-1 protects touch receptor neurons (TRNs) of the wts-1 from premature deformation. (D, E) Loss of yap-1 worsens the neuronal deformation as seen in the ptl-1 mutant. (D) Unlike ptl-1(ok621) single mutant, ptl-1(ok621); yap-1(tm1416) double mutant exhibits severe deformation in ALM and PLM, such as the irregular shape of cell body and ectopic branching of the neuronal process. (E) Percentage of undamaged touch neurons in ptl-1(ok621) and ptl-1(ok621); yap-1(tm1416). (A–E) Neurons were analyzed at the L4 stage. The number of scored neurons is indicated in each column. Statistical significance was determined using a one-way ANOVA, followed by Dunnett’s multiple comparison test. Asterisks indicate differences from the wts-1 or the ptl-1 single mutant. Scale bar = 20 μm.
-
Figure 7—source data 1
Raw data for panels B, C, and E.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig7-data1-v1.xlsx
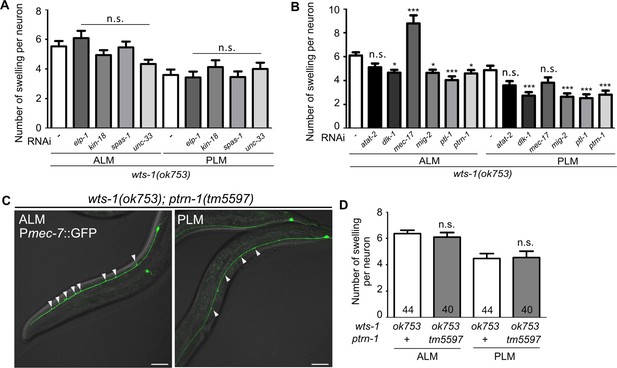
Microtubule-destabilizing genes, not stabilizing genes, mitigate morphological deformations of wts-1 mutant neurons.
(A, B) Knockdown of genes required for microtubule stabilization, except for mec-17, lessens the morphological abnormalities of wts-1 mutant neurons. In contrast, the knockdown of genes acting as microtubule destabilizers does not affect the wts-1 neuron. (C) ALM and PLM of the wts-1(ok753); ptrn-1(tm5597). Unlike dlk-1 or ptl-1, the wts-1; ptrn-1 double mutant fails to recapitulate the knockdown effect of ptrn-1. Arrowheads labels neuronal lesions. (D) Average number of the total ectopic lesions per neuronal process of the wts-1; ptrn-1 mutant. (A–D) ALM or PLM at the L4 stage was scored. Statistical significances were determined by a one-way ANOVA, followed using Dunnett’s multiple comparison test. Asterisks indicate differences from L4440-fed ALM or PLM (A, B) or the wts-1 single mutant neurons (D). Scale bar = 20 μm.
-
Figure 7—figure supplement 1—source data 1
Raw data for panels A, B and D.
- https://cdn.elifesciences.org/articles/102001/elife-102001-fig7-figsupp1-data1-v1.xlsx
Additional files
-
Supplementary file 1
Summary of C. elegans strains used in this study.
- https://cdn.elifesciences.org/articles/102001/elife-102001-supp1-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/102001/elife-102001-mdarchecklist1-v1.docx






