β-Glucan reprograms alveolar macrophages via neutrophil/IFNγ axis in a murine model of lung injury
Figures
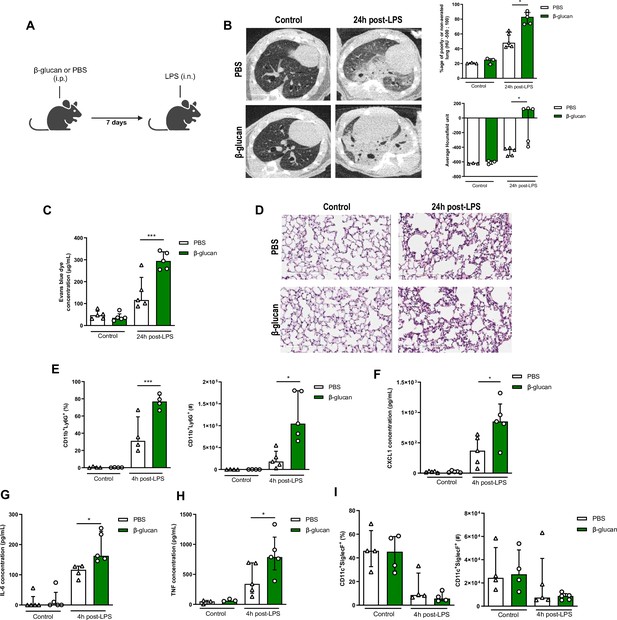
β-Glucan-mediated trained immunity increases lipopolysaccharide (LPS)-induced acute lung injury (ALI).
(A) Schematic of the β-glucan-induced training 7 days before LPS-induced ALI model. Experiments were performed in sex- and age-matched 10- to 12-week-old control (i.p. PBS, white bars) and trained (i.p. β-glucan, green bars) WT mice. (B) Lung micro-CT scan, percentage of poorly or non-aerated lung and average lung Hounsfield unit. (C) Alveolar capillary membrane permeability assessed by lung Evans blue dye concentration. (D) Lung histology after staining with haematoxylin and eosin (Scale bar = 200 μm). (E) Quantification of bronchoalveolar lavage (BAL) neutrophils frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c−, Siglec-F−, CD11b+, Ly6G+). (F–H) BAL chemokine and pro-inflammatory cytokines concentrations (left to right) (CXCL1: chemokine C-X-C motif ligand 1, IL-6: interleukin-6, and TNF: tumour necrosis factor). (I) Quantification of BAL alveolar macrophages frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c+, Siglec-F+). Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. *p < 0.05, ***p < 0.001. Schematics created using BioRender.com.
-
Figure 1—source data 1
Individual measurements, cytokine concentrations, cell frequencies and cell numbers.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig1-data1-v1.xlsx

Weight loss following lipopolysaccharide (LPS) instillation.
(A) Schematic of the β-glucan-induced training 7 days before LPS-induced acute lung injury (ALI) model for weight loss monitoring. (B) Weight loss curve post-LPS instillation (n = 10). Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. *p < 0.05. Schematic created using BioRender.com.
-
Figure 1—figure supplement 1—source data 1
Animal weights overtime post-LPS.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig1-figsupp1-data1-v1.xlsx

Alveolar macrophage (AM) characterization in β-glucan-treated mice.
(A) CD11b expression on CD11c+SiglecF+ cells in the bronchoalveolar lavage (BAL) (n = 4). (B) Expression of AM-associated marker (left to right: MHCII, F4/80, CD169, CD64, CD80) on CD11c+SiglecF+ cells in the BAL (n = 5). Data were analysed using unpaired t-test and one-way ANOVA followed by Dunn’s multiple comparisons test. *p < 0.05.
-
Figure 1—figure supplement 2—source data 1
Cell frequencies.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig1-figsupp2-data1-v1.xlsx

Long-term effects of β-glucan-mediated trained immunity on lipopolysaccharide (LPS)-induced acute lung injury (ALI).
(A) Schematic of the β-glucan-induced training 28 days before LPS-induced ALI model. Experiments were performed in sex- and age-matched 10- to 12-week-old control (i.p. PBS, white bars) and trained (i.p. β-glucan, green bars) WT mice. (B) Alveolar capillary membrane permeability assessed by lung Evans blue dye concentration. (C) Lung histology after staining with haematoxylin and eosin (Scale bar = 200 μm). (D) Bronchoalveolar lavage (BAL) chemokine C-X-C motif ligand 1 (CXCL1) concentration (left) and pro-inflammatory cytokines (IL-6: interleukin-6 (middle) and TNF: tumour necrosis factor (right)). (E) Quantification of BAL neutrophils frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c−, Siglec-F−, CD11b+, Ly6G+). (F) Quantification of BAL alveolar macrophages frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c+, Siglec-F+). Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001. Schematic created using BioRender.com.
-
Figure 1—figure supplement 3—source data 1
Individual measurements, cytokine concentrations, cell frequencies and cell numbers.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig1-figsupp3-data1-v1.xlsx

Long-term effects of β-glucan-mediated trained immunity on alveolar macrophages (AMs).
(A) Schematic of control (i.p. PBS, white bars) or 28 days β-glucan-trained (i.p. β-glucan, green bars) AMs collected from adult WT mice ex vivo stimulation with lipopolysaccharide (LPS). (B) Chemokine C-X-C motif ligand 1 (CXCL1) and tumour necrosis factor (TNF) concentrations after ex vivo LPS stimulation. Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. **p < 0.01 Schematic created using BioRender.com.
-
Figure 1—figure supplement 4—source data 1
Cytokine concentrations.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig1-figsupp4-data1-v1.xlsx

β-Glucan-mediated trained immunity increases poly(I:C)-induced acute lung injury (ALI).
(A) Schematic of the β-glucan-induced training 7 days before poly(I:C)-induced ALI model. Experiments were performed in sex- and age-matched 10- to 12-week-old control (i.p. PBS, white bars) and trained (i.p. β-glucan, green bars) WT mice. (B) Alveolar capillary membrane permeability assessed by lung Evans blue dye concentration. (C) Lung histology after staining with haematoxylin and eosin (Scale bar = 200 μm). (D) Bronchoalveolar lavage (BAL) chemokine C-X-C motif ligand 1 (CXCL1) concentration (left) and pro-inflammatory cytokines (IL-6: interleukin-6 (middle) and TNF: tumour necrosis factor (right)). (E) Quantification of BAL neutrophils frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c−, Siglec-F−, CD11b+, Ly6G+). (F) Quantification of BAL alveolar macrophages frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c+, Siglec-F+). Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001. Schematic created using BioRender.com.
-
Figure 1—figure supplement 5—source data 1
Individual measurements, cytokine concentrations, cell frequencies and cell numbers.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig1-figsupp5-data1-v1.xlsx
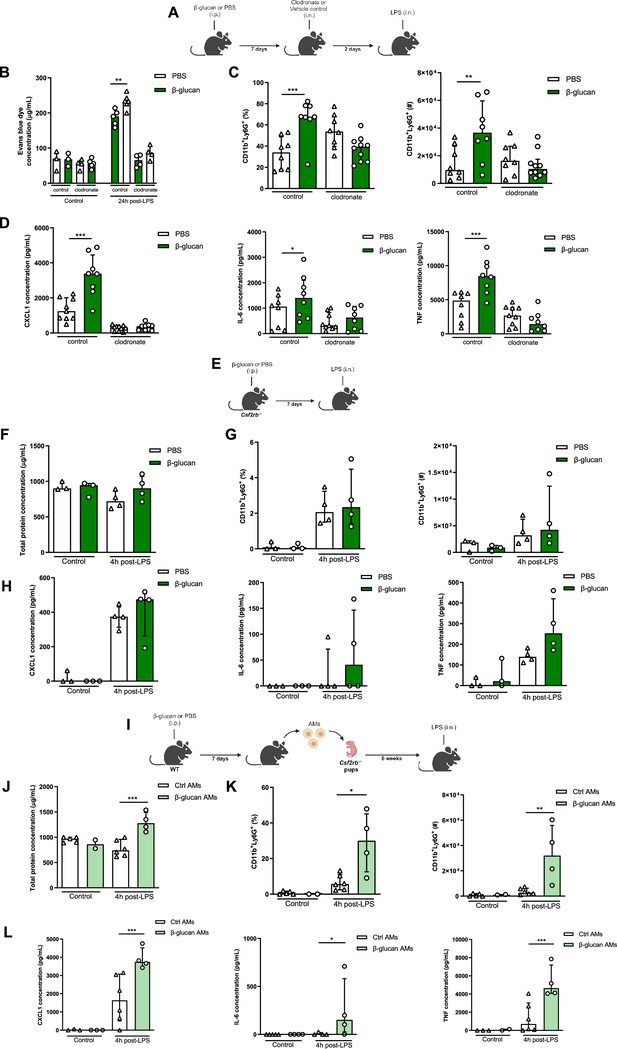
Systemic administration of β-glucan enhances acute lung injury (ALI) via alveolar macrophages (AMs).
(A) Schematic of the clodronate-mediated AMs depletion experiments, performed in sex- and age-matched 10- to 12-week-old control (i.p. PBS, white bars) and trained (i.p. β-glucan, green bars) WT mice. (B) Alveolar capillary membrane permeability assessed by lung Evans blue dye concentration. (C) Quantification of bronchoalveolar lavage (BAL) neutrophils frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c−, Siglec-F−, CD11b+, Ly6G+). (D) BAL chemokine C-X-C motif ligand 1 (CXCL1) and pro-inflammatory cytokines (IL-6: interleukin-6 and TNF: tumour necrosis factor) concentrations. (E) Schematic of the β-glucan-induced training and lipopolysaccharide (LPS)-induced ALI model in sex- and age-matched 6-week-old control (i.p. PBS, white bars) and trained (i.p. β-glucan, green bars) Csf2rb-/- mice. (F) BAL total protein concentration. (G) Quantification of BAL neutrophils frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c−, Siglec-F−, CD11b+, Ly6G+). (H) BAL CXCL1, IL-6, and TNF concentrations. (I) Schematic of the adoptive transfer of control (i.p. PBS, white bars) or β-glucan-trained (i.p. β-glucan, green bars) AMs collected from adult WT mice to 2 days old Csf2rb−/− mice. LPS-induced ALI was performed 6 weeks after adoptive transfer. (J) BAL total protein concentration. (K) Quantification of BAL neutrophils frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c, Siglec-F−, CD11b+, Ly6G+). (L) BAL CXCL1, IL-6, and TNF concentrations. Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001. Schematics created using BioRender.com.
-
Figure 2—source data 1
Individual measurements, cytokine concentrations, cell frequencies and cell numbers.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig2-data1-v1.xlsx
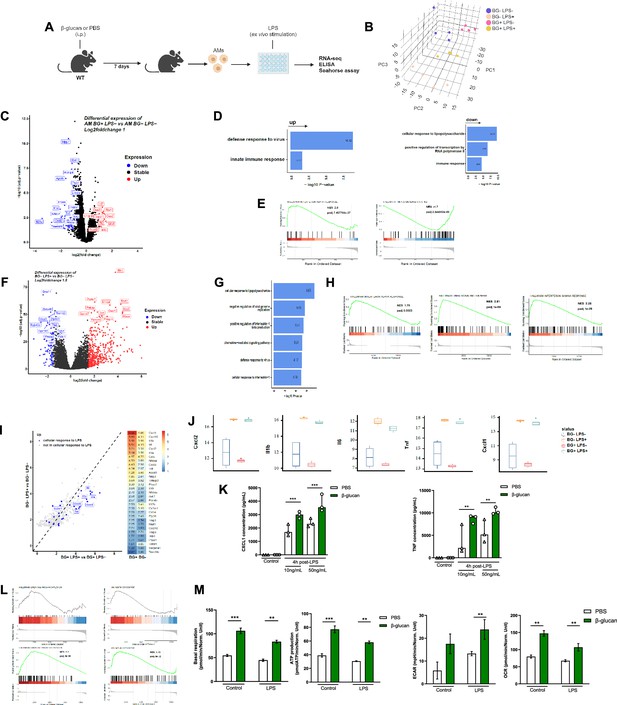
β-Glucan reprograms alveolar macrophages (AMs).
(A) Schematic of control (i.p. PBS) or β-glucan-trained (i.p. β-glucan) AMs collected from adult WT mice ex vivo stimulation with lipopolysaccharide (LPS) (LPS−: unstimulated, LPS+: stimulated in RNA-seq analysis). (B) Discovery plot. (C) AM differential expression of genes in response to β-glucan training. (D) Gene ontology in response to β-glucan training. (E) Gene set enrichment analysis (GSEA) in response to β-glucan training. (F) AM differential expression of genes in response to LPS stimulation. (G) Gene ontology in response to LPS stimulation. (H) GSEA in response to LPS stimulation. (I) AM gene expression in response to LPS in β-glucan-trained AMs. (J) Examples of genes expression in response to LPS in control versus β-glucan-trained AMs. (K) Chemokine C-X-C motif ligand 1 (CXCL1) and tumour necrosis factor (TNF) concentrations after ex vivo LPS stimulation. (L) GSEA of oxidative phosphorylation (left) and glycolysis (right) pathways according to β-glucan-training in unstimulated (LPS−) and LPS stimulated (LPS+) AMs. (M) Evaluation of AM metabolism: basal respiration (upper left), ATP production (upper right), extracellular acidification rate (ECAR, lower left), and oxygen consumption rate (OCR, lower right). Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. **p < 0.01, ***p < 0.001. Schematics created using BioRender.com.
-
Figure 3—source data 1
Cytokine concentrations and Seahorse measurements.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig3-data1-v1.xlsx

Expression of virus response genes in β-glucan-trained alveolar macrophages (AMs).
Schematic of control and β-glucan-trained AMs collected from adult WT mice ex vivo stimulation with lipopolysaccharide (LPS). Differential expression of viral defense genes in response to LPS in β-glucan-trained AMs. Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. Schematic created using BioRender.com.
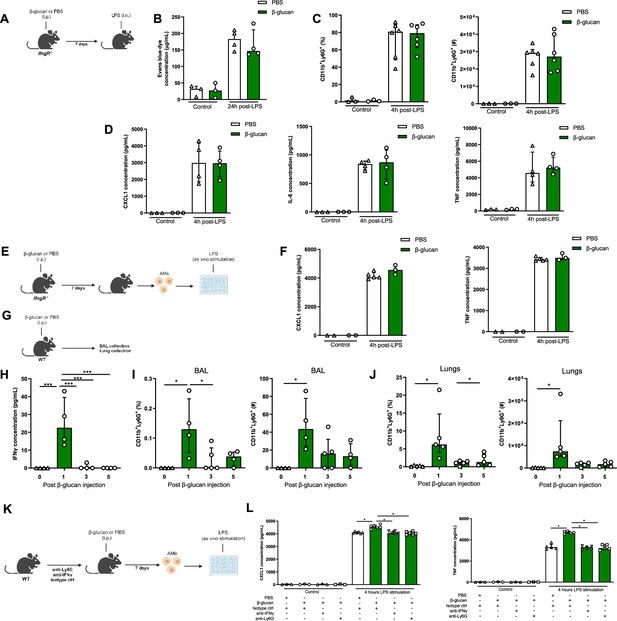
IFNγ and neutrophils are required in β-glucan-mediated alveolar macrophage (AM) reprogramming.
(A) Schematic of the β-glucan-induced training and lipopolysaccharide (LPS)-induced acute lung injury (ALI) model. Experiments were performed in sex- and age-matched 10- to 12-week-old control (i.p. PBS, white bars) and trained (i.p. β-glucan, green bars) IfngR−/− mice. (B) Alveolar capillary membrane permeability assessed by lung Evans blue dye concentration. (C) Quantification of bronchoalveolar lavage (BAL) neutrophils proportion (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c−, Siglec-F−, CD11b+, Ly6G+). (D) BAL chemokine C-X-C motif ligand 1 (CXCL1) concentration (left) and pro-inflammatory cytokines (IL-6: interleukin-6 (middle) and TNF: tumour necrosis factor (right)) concentrations. (E) Schematic of control (i.p. PBS, white bars) or β-glucan-trained (i.p. β-glucan, green bars) AMs collected from adult IfngR−/− mice ex vivo stimulation with LPS. (F) Chemokine C-X-C motif ligand 1 (CXCL1) and tumour necrosis factor (TNF) concentrations after ex vivo LPS stimulation. (G) Schematic of the analysis of the effect of i.p. β-glucan injection on interferon-γ (IFNγ) production and neutrophils expansion before (white bars) and days 1, 3, 5, and 7 post-injection (green bars). (H) BAL IFNγ concentrations. (I) Quantification of BAL neutrophils proportion (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c−, Siglec-F−, CD11b+, Ly6G+). (J) Quantification of lung neutrophils proportion (left) and absolute count (right). (K) Schematic of control (i.p. PBS, white bars) or β-glucan-trained (i.p. β-glucan, green bars) AMs ex vivo stimulation with 50 ng/ml LPS. AMs were collected from control (i.p. injection of isotypes), neutrophils depleted (i.p. injection of anti-Ly6G antibodies), or IFNγ antibody-depleted (i.p. injection of anti-IFNγ antibodies) adult WT mice. (L) CXCL1 and TNF concentrations after ex vivo LPS stimulation. Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. *p < 0.05, ***p < 0.001. Schematics created using BioRender.com.
-
Figure 4—source data 1
Individual measurements, cytokine concentrations, cell frequencies and cell numbers.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig4-data1-v1.xlsx

β-Glucan-aggravated acute lung injury (ALI) is independent of Dectin-1.
(A) Schematic of the β-glucan-induced training 7 days before lipopolysaccharide (LPS)-induced ALI model. Experiments were performed in sex- and age-matched 10- to 12-week-old control (i.p. PBS, white bars) and trained (i.p. β-glucan, green bars) Dectin1−/− mice. (B) Bronchoalveolar lavage (BAL) chemokine C-X-C motif ligand 1 (CXCL1) concentration (left) and pro-inflammatory cytokines (IL-6: interleukin-6 (middle) and TNF: tumour necrosis factor (right)). (C) Quantification of BAL neutrophils frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c−, Siglec-F−, CD11b+, Ly6G+). (D) Quantification of BAL alveolar macrophages frequency (left) and absolute count (right) (gated on single live cells, CD45.2+, CD11c+, Siglec-F+). Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. *p < 0.05, **p < 0.01. Schematic created using BioRender.com.
-
Figure 4—figure supplement 1—source data 1
Cytokine concentrations, cell frequencies and cell numbers.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig4-figsupp1-data1-v1.xlsx

β-Glucan-mediated alveolar macrophage (AM) reprogramming is independent of Dectin-1.
(A) Schematic of control (i.p. PBS, white bars) β-glucan-trained (i.p. β-glucan, green bars) AMs collected from adult Dectin1−/− mice ex vivo stimulation with lipopolysaccharide (LPS). (B) Chemokine C-X-C motif ligand 1 (CXCL1) and tumour necrosis factor (TNF) concentrations after ex vivo LPS stimulation. Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. ***p < 0.001. Schematic created using BioRender.com.
-
Figure 4—figure supplement 2—source data 1
Cytokine concentrations.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig4-figsupp2-data1-v1.xlsx

β-Glucan-mediated alveolar macrophage (AM) reprogramming is independent of type I interferon signalling.
(A) Schematic of control (i.p. PBS, white bars) β-glucan-trained (i.p. β-glucan, green bars) AMs collected from adult Ifnar−/− mice ex vivo stimulation with lipopolysaccharide (LPS). (B) Chemokine C-X-C motif ligand 1 (CXCL1) and tumour necrosis factor (TNF) concentrations after ex vivo LPS stimulation. Data were analysed using one-way ANOVA followed by Dunn’s multiple comparisons test. **p < 0.01, ***p < 0.001. Schematic created using BioRender.com.
-
Figure 4—figure supplement 3—source data 1
Cytokine concentrations.
- https://cdn.elifesciences.org/articles/102068/elife-102068-fig4-figsupp3-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | C57BL/6 | Jackson Laboratories | Strain #: 000664; RRID:IMSR_JAX:000664 | |
| Strain, strain background (M. musculus) | Csf2rb−/− | Jackson Laboratories | Strain #: 005940; RRID:IMSR_JAX:005940 | |
| Strain, strain background (M. musculus) | Clec7a (Dectin1)−/− | Jackson Laboratories | Strain #: 012337; RRID:IMSR_JAX:012337 | |
| Strain, strain background (M. musculus) | Ifnar−/− | Jackson Laboratories | Strain #: 028288; RRID:IMSR_JAX:028288 | |
| Strain, strain background (Mus musculus) | IfngR−/− | Jackson Laboratories | Strain #: 003288; RRID:IMSR_JAX:003288 | |
| Commercial assay or kit | Mouse CXCL1 DuoSet ELISA | R&D Systems | Cat #: DY453 | |
| Commercial assay or kit | Mouse TNF-alpha DuoSet ELISA | R&D Systems | Cat #: DY410 | |
| Commercial assay or kit | Mouse IL-6 DuoSet ELISA | R&D Systems | Cat #: DY406 | |
| Commercial assay or kit | Mouse IFNγ DuoSet ELISA | R&D Systems | Cat #: DY485 | |
| Commercial assay or kit | Pierce BCA assay | Thermo Fisher | Cat #: 23225 | |
| Commercial assay or kit | Seahorse XF Cell Mito Stress Test Kit | Agilent | Cat #: 103015-100 | |
| Antibody | Fixable Viability Dye eFluor 506 | Invitrogen | Cat #: 65-0866-14 | FACS (1:1000) |
| Antibody | Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block) | BD Bioscience | Cat #: 553141; RRID:AB_394656 | FACS (1:200) |
| Antibody | PE-Cy7-conjugated anti-CD11c (Mouse monoclonal) | BD Bioscience | Cat #: 561022; RRID:AB_647251 | FACS (1:200) |
| Antibody | BV786-conjugated anti-Siglec-F (Mouse monoclonal) | BD Bioscience | Cat #: 740956; RRID:AB_2740581 | FACS (1:200) |
| Antibody | BUV395-conjugated anti-CD11b (Mouse monoclonal) | BD Bioscience | Cat #: 565976; RRID:AB_2738276 | FACS (1:200) |
| Antibody | APC-Cy7-conjugated anti-Ly6G (Mouse monoclonal) | BD Bioscience | Cat #: 560600; RRID:AB_1727561 | FACS (1:200) |
| Antibody | BUV737-conjugated anti-CD45.2 (Mouse monoclonal) | BD Bioscience | Cat #: 612778; RRID:AB_2870107 | FACS (1:200) |
| Antibody | PE-conjugated anti-IFNγ (Mouse monoclonal) | BD Bioscience | Cat #: 554412; RRID:AB_395376 | FACS (1:200) |
| Antibody | FITC-conjugated anti-CD45.2 (Mouse monoclonal) | BD Bioscience | Cat #: 553772; RRID:AB_395041 | In vivo (2 µg per mouse) |
| Antibody | BUV395-conjugated anti-CD45.2 (Mouse monoclonal) | BD Bioscience | Cat #: 564616; RRID:AB_2738867 | FACS (1:200) |
| Antibody | Depleting anti-IFNγ (rat IgG1k) (Mouse monoclonal) | Biolegend | Cat #: 505801; RRID:AB_315395 | In vivo (200 µg per mouse) |
| Antibody | Depleting anti-Ly6G (rat IgG2a,k) (Mouse monoclonal) | Biolegend | Cat #: 127601; RRID:AB_1089179 | In vivo (70 µl per mouse) |
| Chemical compound | Clodronate liposomes | Liposoma BV | Cat #: C-005 | In vivo (200 µg per mouse) |
| Other | Escherichia coli O55:B55 LPS | Sigma-Aldrich | Cat #: L2637 | In vivo (50 µg per mouse) |
| Other | β-1,3-Glucan purified from Saccharomyces cerevisiae | Sigma-Aldrich | Cat #: G5011 | In vivo (1 mg per mouse) |
| Other | Poly(I:C) HMW | Invivogen | Cat #: tlrl-pic | In vivo (50 µg per mouse) |