Decapping activators Edc3 and Scd6 act redundantly with Dhh1 in post-transcriptional repression of starvation-induced pathways
Figures
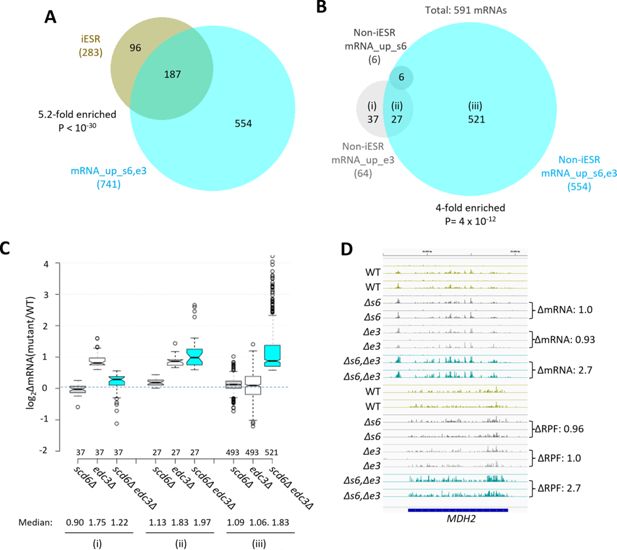
Most mRNAs up-regulated in the scd6∆edc3∆mutant are not iESR transcripts and exhibit Scd6/Edc3 functional redundancy in repression of transcript abundance.
(A) Venn diagram of overlap between the 741 mRNAs up-regulated in the scd6Δedc3Δ mutant vs. wild-type (WT) (mRNA_up_s6,e3) and the 283 induced ESR (iESR) mRNAs, indicating fold-enrichment and p value of overlap determined by the hypergeometric distribution. (B) Venn diagram of overlaps involving all 591 non-iESR mRNAs up-regulated in abundance by scd6Δ (6 Non-iESR mRNA_up_s6 transcripts), edc3Δ (64 mRNA_up_e3 transcripts), or scd6Δedc3Δ mutations (554 Non-iESR mRNA_up_s6,e3 transcripts). (C) Notched box-plot analyses of log2 changes in mRNA abundance (log2∆mRNA) determined by DESeq2 analysis between the indicated mutants vs. WT for mRNAs belonging to the specified sectors of the Venn diagram in (B). The numbers of mRNAs in each group for which data were obtained are indicated immediately above the x-axis; unlogged median values are indicated for each column at the bottom. (D) Gene browser image for MDH2 showing the mRNA (top 16 tracks) and ribosome-protected fragment (RPF) (bottom 16 tracks) reads measured by parallel RNA-Seq and ribosome profiling analyses for two biological replicates of WT and the indicated mutants with fold-changes in mRNA or RPFs between mutant and WT indicated to the right of each track. In panels A-D, results for the scd6∆, edc3∆, and scd6∆edc3∆ mutations, abbreviated as ∆s6, ∆e3, and ∆s6,∆e3, are shown in gray, white or light gray, and cyan, respectively.
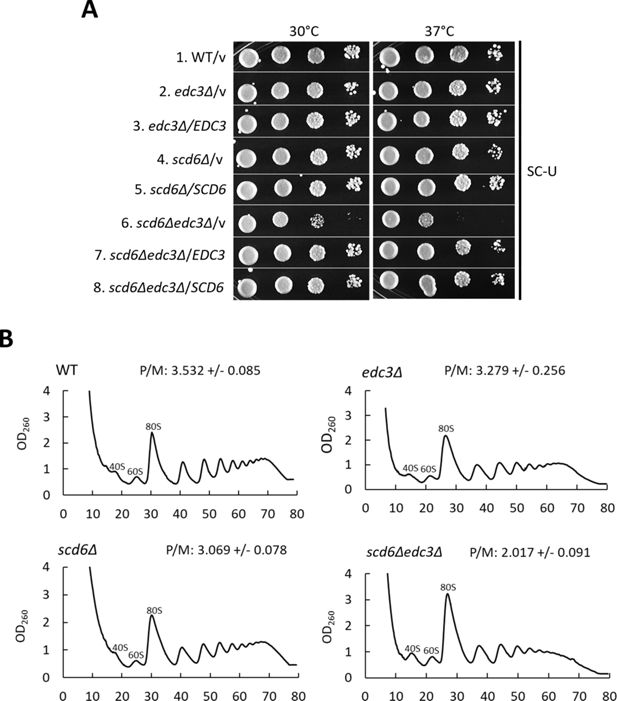
Combining scd6Δ and edc3∆ mutations confers synthetic reductions in cell growth and polysome assembly.
(A) Serial dilutions of wild-type (WT) strain HFY114, edc3∆ strain FZY862, scd6∆ strain SYY2352, and scd6∆edc3∆ strain FZY858 transformed with empty URA3 CEN vector YCplac33 or derivatives of this vector containing EDC3 (pLfz614-7) or SCD6 (pLfz615-5) were spotted on synthetic complete plates minus uracil (SC-Ura) and incubated at the indicated temperatures. (B) Polysome profiles of the strains in (A) but lacking plasmids cultured in YPD medium at 30 C to log-phase growth and treated with cycloheximide prior to harvesting to block run-off of elongating ribosomes during cell lysis. Cell extracts were resolved by sedimentation through 10–50% sucrose gradients, and gradients were scanned at 260 nm to yield the indicated tracings. Average polysome/monosome ratios (P/M) from two biological replicates are shown, with means +/- SEMs.
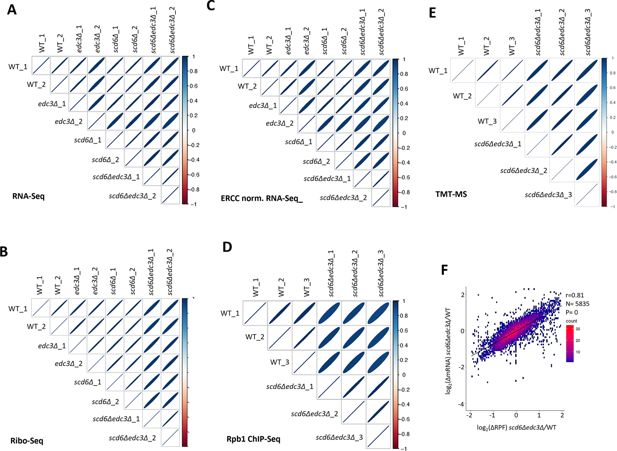
Reproducibility among biological replicates of RNA-Seq, Ribo-Seq, External RNA Controls Consortium (ERCC)-normalized RNA-Seq, Rpb1 ChIP-seq, and TMT mass spectrometry (TMT-MS) data.
(A) Correlation matrix showing Spearman correlation coefficients calculated for pair-wise comparisons of numbers of RPKM-normalized RNA-Seq reads for all expressed genes among all eight RNA-Seq libraries generated for two biological replicates (_1,_2) of the indicated genotypes. The correlation coefficients between replicates are ≥0.98 and for all comparisons >0.95 with p-values of ≈0. For this and all similar plots below, the eccentricity of the ellipses are scaled parametrically to the correlation value between the two samples. (B) Same as (A) but for Ribo-Seq data, with correlation coefficients between replicates >0.995 and for all comparisons >0.96 with p-values of ≈0. (C) Same as (A) but for ERCC-normalized RNA-Seq data, with correlation coefficients between replicates >0.97 and for all comparisons >0.94 with p-values of ≈0. (D) Same as (A) but for Rpb1 ChIP-Seq data comparing three normalized occupancies averaged across the coding sequences (CDS) of all 5770 expressed genes, with correlation coefficients between replicates >0.98 and for all comparisons >0.91 with p-values of ≈0. (E) Same as (A) but for TMT-MS data, comparing log2 cyclic Loess-normalized exclusive MS1 intensities for all expressed proteins with correlation coefficients between replicates >0.96 and for all comparisons >0.95 with p-values of ≈0. (F) Correlation between changes in abundance of RNA vs. ribosome-protected fragments (RPFs) for 5835 expressed transcripts in the scd6Δedc3Δ double mutant compared to WT, calculated from RPKM-normalized RNA-Seq and Ribo-Seq reads obtained after combining data from biological replicates, with the Pearson correlation coefficient (r) and p-value of the correlation indicated.
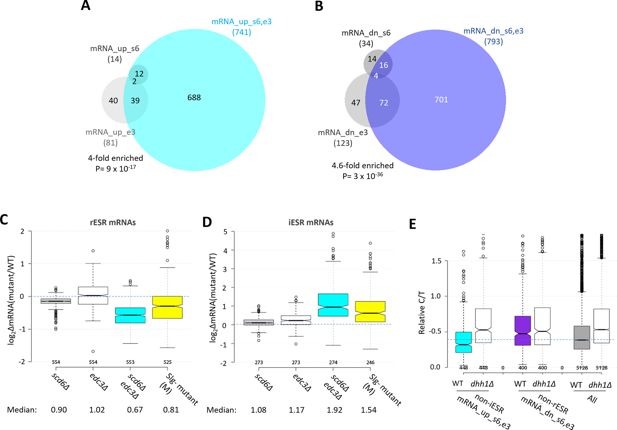
Functional redundancy between Scd6 and Edc3 in controlling mRNA abundance and mobilizing the environmental stress response (ESR).
(A–B) Venn diagrams of overlaps between all mRNA_up (A) and all mRNA_dn (B) groups identified in scd6Δ, edc3Δ, or scd6Δedc3Δ mutants vs. wild-type (WT), with fold-enrichments and p values from the hypergeometric distribution indicated for overlapping sets. (C–D) Notched box-plot analyses of log2 changes in mRNA abundance in mutant vs. WT for 585 rESR (C) and 283 iESR (D) mRNAs conferred by scd6Δ, edc3Δ, or scd6Δedc3Δ mutations and those observed for the slowest-growing yeast deletion mutants (M) analyzed previously (O’Duibhir et al., 2014). Each box depicts the interquartile range containing 50% of the data, intersected by the median; the notch indicates a 95% confidence interval (CI) around the median. Median changes (unlogged) are shown at the bottom. (E) Ratios of capped to total mRNA abundance in transcript numbers per million reads (TPMs) (Relative C/T) in WT or dhh1∆ cells plotted for all mRNAs, the 554 non-iESR mRNA_up_s6,e3, or 526 non-rESR mRNA_down_s6,e3 transcripts dysregulated in scd6Δedc3Δ vs. WT cells.
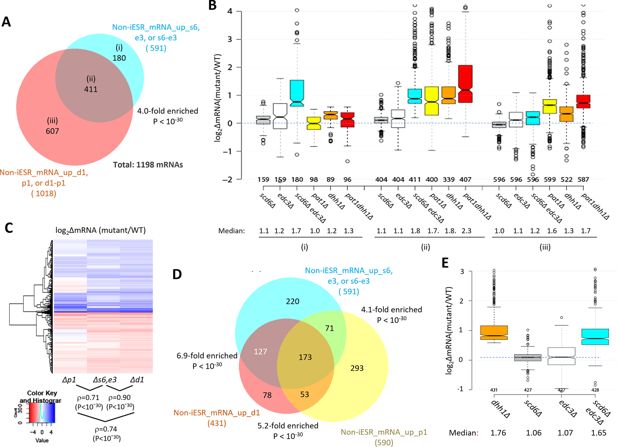
Most mRNAs up-regulated in the scd6∆edc3∆ mutant are also up-regulated by dhh1Δ and pat1Δ.
(A) Venn diagram of overlap between all 591 non-iESR mRNAs up-regulated in abundance by the scd6Δ, edc3Δ, or scd6Δedc3Δ mutations (from Figure 1B) and 1018 non-iESR mRNAs up-regulated by the dhh1Δ, pat1Δ, or pat1Δdhh1Δ mutations identified previously (Vijjamarri et al., 2023a). Fold enrichments and p value from the hypergeometric distribution are indicated for the overlap. (B) Notched box plots of log2∆mRNA between the indicated mutants vs. wild-type (WT) for mRNAs in the three sectors specified in (A). (C) Hierarchical clustering analysis of log2∆mRNA values conferred by the indicated mutations vs. WT for 784 of the 794 mRNAs up- or down-regulated in dhh1Δ vs. WT cells for which RNA-Seq data was obtained in all four strains and with log2∆mRNA values >-5 and <5, conducted with R heatmap.2 function from R ‘gplots’ library, using default hclust hierarchical clustering algorithm. Spearman coefficients (ρ) and associated p values are given for the indicated correlation analyses. (D) Venn diagram of overlaps between the 591 non-iESR mRNAs up-regulated by the scd6Δ/edc3Δ mutations vs. WT (from Figure 1B) and the indicated 431 and 590 non-iESR mRNAs up-regulated by dhh1Δ or pat1Δ vs. WT, respectively, identified previously (Vijjamarri et al., 2023a). The 220, 78, and 293 mRNAs found exclusively in only one of the three sets are indicated in the cyan, red-orange, and yellow sectors, respectively while the 127, 71, and 53 mRNAs shared by two of the three sets and the 173 mRNAs shared by all three sets are indicated in the corresponding regions of overlap. Fold enrichments and p values from the hypergeometric distribution are indicated for the overlaps. (E) Notched box plots of log2∆mRNA between the indicated mutants vs. WT for the 431 non-iESR mRNAs up-regulated by dhh1Δ vs. WT shown in panel (D). In panels A, B, D, and E, results for mRNAs up-regulated by the scd6∆, edc3∆, and scd6∆edc3∆ mutations are shown in gray, white, and cyan, respectively, while those for mRNAs up-regulated by the pat1∆, dhh1∆, and pat1∆dhh1∆ mutations are shown in yellow, orange, and red, respectively.
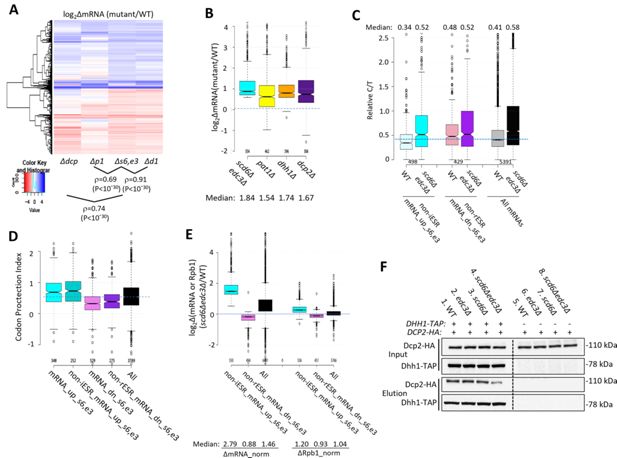
Evidence that impaired decapping not increased transcription, drives changes in mRNA abundance in scd6Δedc3Δ cells and that Edc3/Scd6 redundantly promote Dhh1 association with Dcp2.
(A) Hierarchical clustering analysis of log2∆mRNA values conferred by the indicated mutations vs. wild-type (WT) for 741 of the 1052 mRNAs up- or down-regulated in scd6Δedc3Δ vs. WT cells for which RNA-Seq data was obtained in all five strains and with log2∆mRNA values >-5 and <5, conducted as in Figure 2C, showing Spearman coefficients and p values for indicated correlations. (B) Notched box-plots of log2∆mRNA between the indicated mutants vs. WT for the 554 non-iESR mRNAs up-regulated by scd6Δedc3Δ vs. WT (shown in Figure 1B). (C) Ratios of capped to total mRNA abundance in transcript numbers per million reads (TPMs) (Relative C/T) in WT or scd6∆edc3∆ cells plotted for all 5391 mRNAs, the 498 non-iESR mRNA_up_s6,e3, or 429 non-rESR mRNA_down_s6,e3 transcripts dysregulated by scd6Δedc3Δ vs. WT. (D) Notched box plots of the codon-protection index (CPI) for all mRNAs or for the sets of mRNAs up- or down-regulated by scd6Δedc3Δ vs. WT, including or excluding ESR transcripts, as indicated. (E) Notched box-plots showing log2 changes in absolute mRNA abundance from External RNA Controls Consortium (ERCC) spike-in normalized RNA-Seq (left) or absolute Rpb1 occupancies averaged over the coding sequences (CDSs) from S. pombe chromatin spike-in normalized Rpb1 ChIP-Seq (right) in scd6Δedc3Δ vs. WT cells for all mRNAs or the 554 or 526 non-ESR mRNAs up- or down-regulated, respectively, by scd6Δedc3Δ vs. WT. (F) Co-immunoprecipitation analysis of Dhh1-Dcp2 association in yeast cell extracts. Transformants of DHH1-TAP strains H5695 (WT), H5696 (edc3Δ), H5697 (scd6Δ), and H5698 (edc3Δ scd6Δ) and the parental untagged strains all harboring single-copy plasmid pAK133 expressing HA-tagged Dcp2 were cultured in SC-Ura at 30°C and whole cell extracts were incubated with IgG sepharose beads to purify Dhh1-TAP and associated proteins. Washed beads were eluted by boiling in SDS loading buffer and eluates and input extracts were resolved in parallel by SDS-PAGE and subjected to immunoblot analysis to detect the tagged proteins. Immune complexes were visualized with enhanced chemiluminescence.
-
Figure 3—source data 1
Relative and spike-in normalized Rpb1 occupancies from ChIP-seq analysis.
Sheets 1–2 labeled ‘Relative occs._replicates’ and ‘Relative occs._ Reps._averaged’ contain the processed data from ChIP-Seq analysis of Rpb1 in three biological replicates of wild-type (WT) and scd6∆edc3∆ strains and the averaged data from combining the replicates, respectively, listing the relative occupancies averaged over the coding sequences for each expressed gene normalized to the average occupancy on each chromosome. Sheet ‘S. pombe norm. factor calcs,’ lists the calculations of normalization factors obtained from total numbers of reads aligned to the S. pombe genome for each chromatin sample spiked-in with equal aliquots of S. pombe chromatin prior to immunoprecipitation with Rpb1 antibodies. Factors are calculated for each individual replicate (col. E) or for the combined replicates for each strain (col. G). Sheets ‘Normalized occs._replicates’ and ‘Normalized occs._reps._avged’ list the spike-in normalized Rpb1 occupancies calculated for each replicate or the combined replicates for each strain, respectively, calculated using the respective normalization factors for individual or combined replicates determined in the previous two sheets.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig3-data1-v1.xlsx
-
Figure 3—source data 2
External RNA Controls Consortium (ERCC) spike-in normalized RNA-Seq data.
Sheet 1 lists the numbers of reads for each ERCC molecule identified in each RNA sample and calculations of the normalization factors for each sample. Sheet 2 lists the un-normalized reads for each yeast gene in each RNA sample, the ERCC-normalized reads for each sample, and the density of normalized reads for each gene calculated by normalizing for coding sequences (CDS) lengths. Sheets 2–4 list processed data from DESeq2, including log2Δ mRNA, p-value, and adj. p-value determined for each gene in the indicated mutant vs. wild-type (WT) strain, obtained by setting the size factor to unity.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig3-data2-v1.xlsx
-
Figure 3—source data 3
Figures of the uncropped blots with the relevant bands labelled used to prepare Figure 3F.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig3-data3-v1.zip
-
Figure 3—source data 4
Original images of the full uncropped, unedited blots used to prepare Figure 3F.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig3-data4-v1.zip
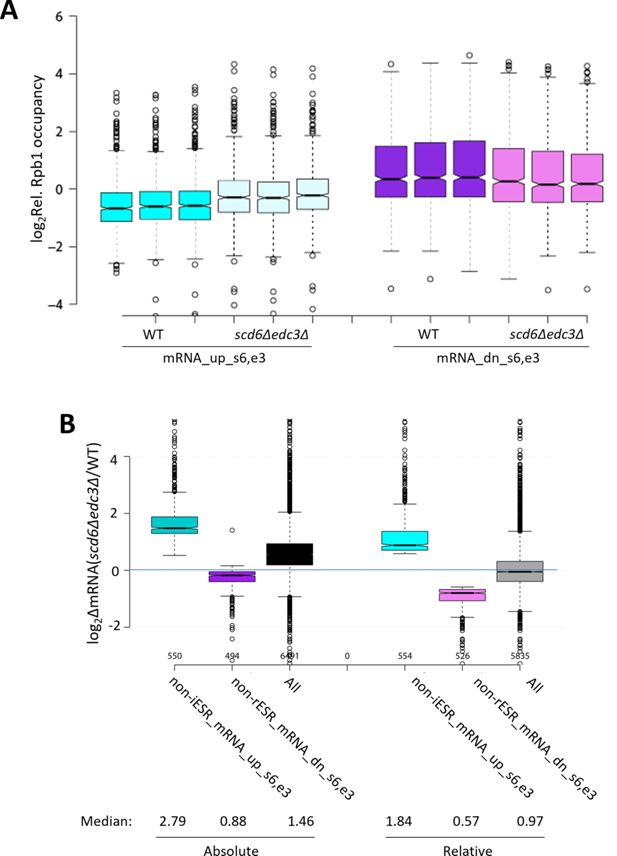
Supporting information for measurements of transcription and abundance of transcripts dysregulated in the scd6∆edc3∆ double mutant.
(A) Reproducibility among biological replicates of Rpb1 ChIP-seq data. Log2 values of relative Rpb1 occupancies averaged over the coding sequences (CDSs) for three replicates of wild-type (WT) or scd6Δedc3Δ cells for the mRNAs up- or down-regulated by scd6Δedc3Δ vs. WT, without excluding environmental stress response (ESR) transcripts. (B) Notched box-plots showing log2 changes in absolute mRNA abundance from External RNA Controls Consortium (ERCC) spike-in normalized RNA-Seq (left) or relative mRNA abundance determined by DESeq2 analysis of RNA-Seq results (right) in scd6Δedc3Δ vs. WT cells for all mRNAs or the 554 or 526 non-ESR mRNAs up- or down-regulated, respectively, by scd6Δedc3Δ vs. WT.
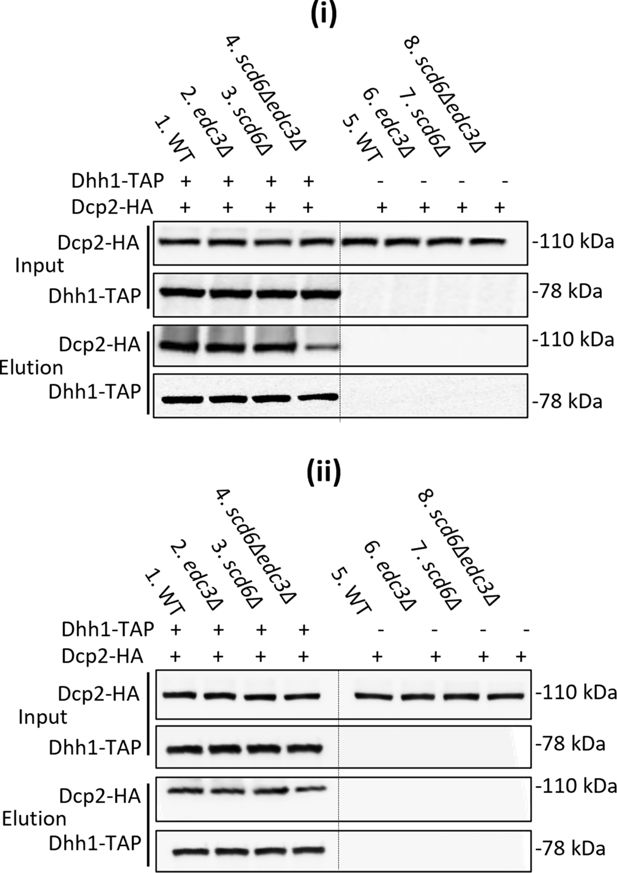
Biological replicates of co-immunoprecipitation analysis of Dhh1-Dcp2 association in yeast cell extracts.
(i-ii) Results for two of three biological replicates of the experiment described in Figure 3F.
-
Figure 3—figure supplement 2—source data 1
Figures of the uncropped blots with the relevant bands labelled used to prepare Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig3-figsupp2-data1-v1.zip
-
Figure 3—figure supplement 2—source data 2
Original images of the full uncropped, unedited blots used to prepare Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig3-figsupp2-data2-v1.zip
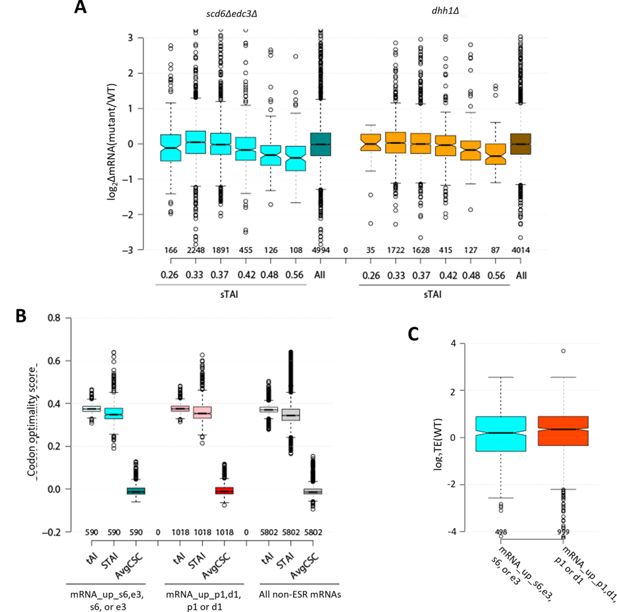
Average median codon optimality scores and average median translational efficiency (TE) values in wild-type (WT) cells for mRNAs repressed in abundance by Scd6/Edc3 or Dhh1/Pat1.
(A) Notched box-plots of log2∆mRNA between scd6Δedc3Δ (left, cyan) or dhh1Δ (right, orange) vs. WT for six bins of all non-environmental stress response (ESR) mRNAs sorted according to stAI values, designated by the median stAI value for each bin, or for all non-ESR mRNAs (All). (B) tAI, stAI, and average CSC values for the 591 non-iESR mRNAs derepressed in abundance by the scd6Δ, edc3Δ, or scd6Δedc3Δ mutations, the 1018 non-ESR mRNAs up-regulated by the dhh1Δ, pat1Δ, or pat1Δdhh1Δ mutations, or all 5802 non-ESR mRNAs. (C) Log2 values of TE determined in WT cells for the same groups of non-iESR mRNAs derepressed by the scd6Δ, edc3Δ, or scd6Δedc3Δ mutations or by the dhh1Δ, pat1Δ, or pat1Δdhh1Δ mutations analyzed in (A).
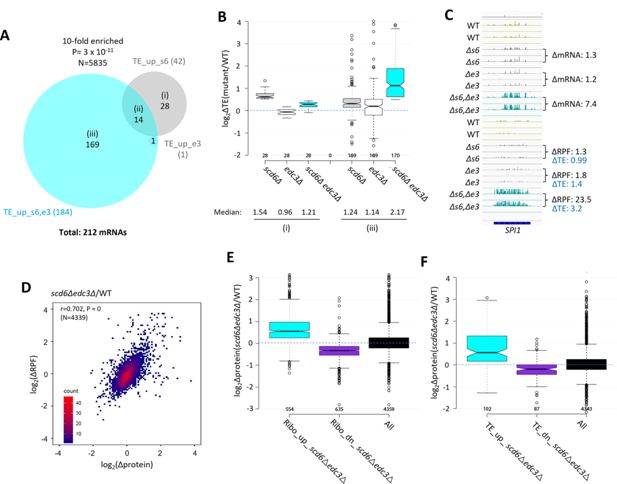
Most mRNAs translationally up-regulated in scd6∆edc3∆ cells exhibit Scd6/Edc3 functional redundancy for repressing translational efficiency (TE) and show correlated changes in TE and protein abundance.
(A) Venn diagram of overlap between the 184 and 42 mRNAs in the TE_up groups identified in the scd6Δedc3Δ vs. wild-type (WT) or scd6Δ vs. WT comparisons, respectively. Fold-enrichment and p value from the hypergeometric distribution are indicated for the overlap. (B) Notched box-plots of log2∆TE values between the indicated mutants vs. WT for the mRNAs belonging to sectors (i) or (iii) of the diagram in (A). (C) Gene browser image for SPI1 presented as in Figure 1D, except also giving the TE changes for each mutant vs. WT on the lower right. (D) Density scatterplot of log2∆RPF values measured by ribosome profiling vs. log2∆protein values measured by TMT mass spectrometry (TMT-MS) for 4339 mRNAs for which data were obtained in both analyses, indicating the Pearson correlation coefficient (r) and p-value of the correlation. (E–F) Notched box-plots of log2∆protein values from TMT-MS analysis between the scd6Δedc3Δ mutant vs. WT for the 843 and 839 mRNAs belonging to the Ribo_up or Ribo_down groups, respectively (D), or the 184 and 152 TE_up or TE_down mRNA groups (E) determined for the scd6Δedc3Δ mutant vs. WT, or for all mRNAs, for which TMT-MS data was obtained.
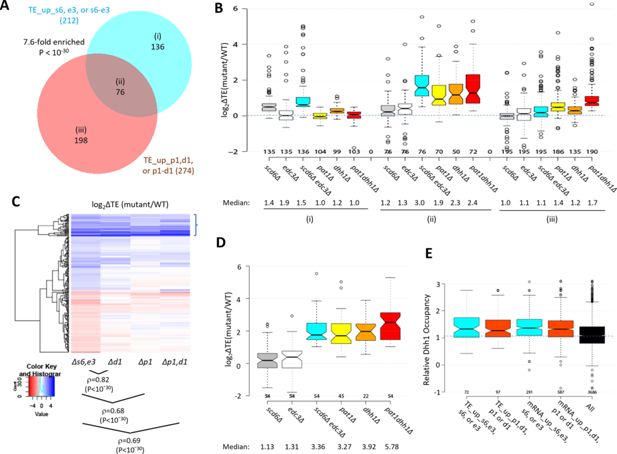
Most mRNAs translationally up-regulated in the scd6∆edc3∆ mutant are also translationally up-regulated by dhh1Δ or pat1Δ.
(A) Venn diagram of overlap between all 212 mRNAs translationally up-regulated by scd6Δ, edc3Δ, or scd6Δedc3Δ (defined in Figure 4) or all 274 mRNAs translationally up-regulated by dhh1Δ, pat1Δ, or pat1Δdhh1Δ vs. wild-type (WT) identified previously (Vijjamarri et al., 2023a). Fold-enrichment and p value from the hypergeometric distribution are indicated for the overlap. (B) Notched box plots of log2∆TE values between the indicated mutants vs. WT for the mRNAs belonging to the specified sectors of the diagram in (A). (C) Hierarchical clustering analysis of log2∆TE values conferred by the indicated mutations vs. WT for 222 of the 336 mRNAs translationally up- or down-regulated in scd6Δedc3Δ vs. WT cells for which RNA-Seq and Ribo-Seq data were obtained in all five strains and with log2∆TE values >-5 and <5 conducted as in Figure 2C, including the Spearman coefficients (ρ) and p values for the indicated correlations. (D) Notched box-plots of log2∆TE values between the indicated mutants vs. WT for the 54 mRNAs showing >2 fold translational efficiency (TE) increases conferred by both scd6Δedc3Δ and pat1Δdhh1Δ mutations vs. WT. (E) Relative Dhh1 occupancies from the Dhh1 RIP-seq experiments of Miller et al., 2018 for the 212 and 274 mRNAs identified as TE_up in the scd6Δ, edc3Δ, or scd6Δedc3Δ mutants, or the dhh1Δ, pat1Δ, or pat1Δdhh1Δ mutants, vs. WT, respectively (cols 1–2), or for the 591 and 1018 mRNAs identified as mRNA_up in either the scd6Δ, edc3Δ, or scd6Δedc3Δ mutants, or the dhh1Δ, pat1Δ, or pat1Δdhh1Δ mutants, vs. WT, respectively (cols. 3–4).
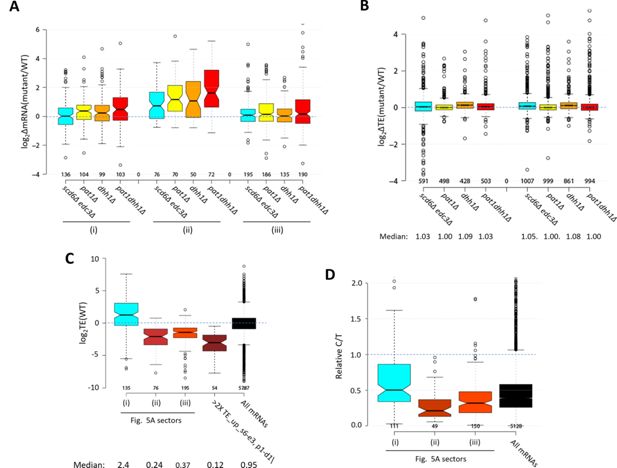
Properties of mRNAs translationally repressed by Scd6/Edc3 or Dhh1/Pat1.
(A) Notched box-plots of log2∆mRNA values between the indicated mutants vs. wild-type (WT) for the translationally up-regulated mRNAs belonging to the specified sectors of the diagram in Figure 5A. (B) Notched box plots of log2∆TE values between the indicated mutants vs. WT for the mRNAs belonging to the 591 non-iESR mRNAs derepressed in abundance by scd6Δ, edc3Δ, or scd6Δedc3Δ vs. WT (cols. 1–4) or the 1018 non-ESR mRNAs up-regulated by dhh1Δ, pat1Δ, or pat1Δdhh1Δ vs. WT (cols. 5–8). (C) Box plot of log2 values of TE determined in WT cells for the translationally up-regulated mRNAs belonging to the specified sectors of the diagram in Figure 5A, the 54 mRNAs showing > twofold translational efficiency (TE) increases conferred by both scd6Δedc3Δ and pat1Δdhh1Δ mutations vs. WT, or for all mRNAs. (D) Box-plot of ratios of capped to total mRNA abundance in transcript numbers per million reads (TPMs) (Relative C/T) in WT cells for the translationally up-regulated mRNAs belonging to the specified sectors of the diagram in Figure 5A, or for all mRNAs.
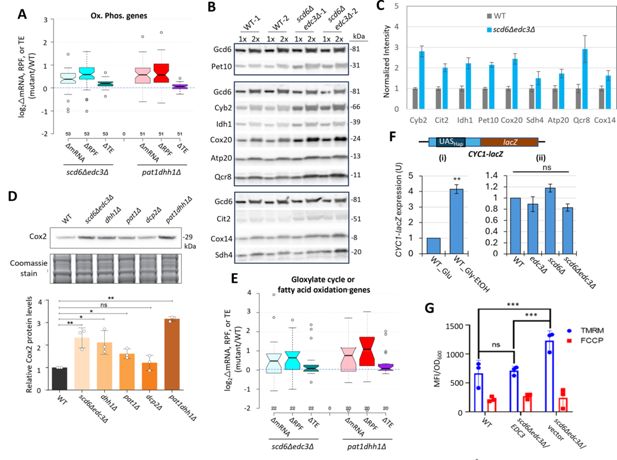
Scd6/Edc3 post-transcriptionally repress proteins involved in respiration and suppress mitochondrial membrane potential in rich medium.
(A) Log2 changes in mRNA, ribosome-protected fragments (RPFs), or translational efficiency (TE) conferred by the indicated double mutations vs. wild-type (WT) for 53 nuclear genes encoding mitochondrial proteins involved directly in oxidative phosphorylation. (B–C) Western blot analysis of nine mitochondrial proteins and Gcd6 (examined as loading control) in WT and scd6Δedc3Δ strains, cultured in duplicate in YPD medium to OD600 of ~0.6–0.8. WCEs were extracted under denaturing conditions and aliquots corresponding to 1 X or 2 X amounts of WCE were loaded in successive lanes for the two biological replicate cultures. Immune complexes were visualized with enhanced chemiluminescence (B). Signals for each protein were quantified, normalized to the corresponding signals for Gcd6 in the same extract and expressed relative to the resulting values for WT cells. Mean values and standard errors are plotted (C). (D) Western blot analysis of Cox2 in strains of the indicated genotypes in cells cultured as in (B). Cox2 signal intensity was normalized to total Coomassie-stained protein and the resulting relative Cox2 protein levels from three biological replicates were averaged and plotted. P-values from student’s t-test indicated as **,<0.01; *,<0.05; ns, not significant. (E) Log2 changes in mRNA, RPFs, or TE conferred by the indicated double mutations vs. WT for 22 genes encoding enzymes of the glyoxylate cycle or fatty acid metabolism. (F) Expression of the CYC1-lacZ reporter on plasmid pLG265, lacking UAS1 and containing the optimized version of UAS2, UAS2UP1, in the WT strain grown on SC-Ura medium containing either 2% glucose or 3% glycerol/2% ethanol as carbon sources (i), or in WT and the indicated mutant strains on SC-Ura with 2% glucose (ii). β-galactosidase activity (nmoles of o-nitrophenyl-β-D-galactopyranoside (ONPG) cleaved per min per mg of total protein) was measured in whole cell extracts for three biological replicates of each strain and the mean values were normalized to the mean activity measured in WT grown with glucose as carbon source. **p-value <0.01 from student’s t-test; ns, not significant. (G) Measurements of mitochondrial membrane potential. WT cells or transformants of the scd6Δedc3Δ mutant containing the EDC3 plasmid pLfz614-7 or empty vector were cultured in SC-Ura to mid-log phase. Tetramethylrhodamine (TMRM) (500 nM) was added and incubated for 30 min before samples were collected and washed once with deionized water. ∆Ψm was determined by measuring TMRM fluorescence intensity using flow cytometry. Data are presented in arbitrary fluorescence intensity units per OD600. Two-way ANOVA was used for statistical analysis and data are given as mean values ± SD (n=3) (****p<0.0001).
-
Figure 6—source data 1
Western blot analysis and lacZ reporter analysis of scd6∆edc3∆ vs. wild-type (WT) strains.
Sheet ‘Figure 6B–C Western analysis’ lists averages of band intensities normalized to the loading control (Gcd6) on the same blots and the corresponding S.E.M. values calculated from the replicates for each protein analyzed in Figure 6B–C. Sheet ‘Figure 6D Western analysis’ lists the Cox2 band intensities normalized to total stained proteins and the ratios of normalized Cox2 for each mutant vs. WT. Sheet ‘Figure 6F’ lists the specific activities of β-galactosidase determined from three biological replicates of each strain harboring the CYC1-lacZ reporter analyzed in Figure 6F. Sheet ‘Figure 6—figure supplement 2C’ lists the specific activities of β-galactosidase determined from three biological replicates of each strain harboring the ADH2-lacZ reporter analyzed in Figure 6—figure supplement 2C.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig6-data1-v1.xlsx
-
Figure 6—source data 2
Figures of the uncropped blots with the relevant bands labelled used to prepare Figure 6B.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig6-data2-v1.zip
-
Figure 6—source data 3
Original images of the full uncropped, unedited blots used to prepare Figure 6B.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig6-data3-v1.zip
-
Figure 6—source data 4
Figures of the uncropped blots with the relevant bands labelled used to prepare Figure 6D.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig6-data4-v1.zip
-
Figure 6—source data 5
Original images of the full uncropped, unedited blots used to prepare Figure 6D.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig6-data5-v1.zip

mRNAs repressed in abundance or translation by Scd6/Edc3 or Dhh1/Pat1 are enriched for common functional categories, including Ox. Phos. proteins and cell wall components.
(A–B) Functional categories showing enrichment for genes encoding the 591 non-iESR mRNAs derepressed in abundance by scd6Δ, edc3Δ, or scd6Δedc3Δ vs. wild-type (WT) or the 1018 non-ESR mRNAs up-regulated by dhh1Δ, pat1Δ, or pat1Δdhh1Δ vs. WT, using color-coding to indicate related functions or cellular components, conducted using the Web-based tool FunSpec and applying the Bonferroni correction and p<0.05 cutoff. (C–D) Functional categories showing enrichment for genes encoding the 853 mRNAs with RPFs up-regulated by scd6Δ, edc3Δ, or scd6Δedc3Δ vs. WT or the 1350 mRNAs with ribosome-protected fragments (RPFs) up-regulated by dhh1Δ, pat1Δ, or pat1Δdhh1Δ vs. WT, analyzed as in (A-B).
-
Figure 6—figure supplement 1—source data 1
Source table for Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig6-figsupp1-data1-v1.xlsx
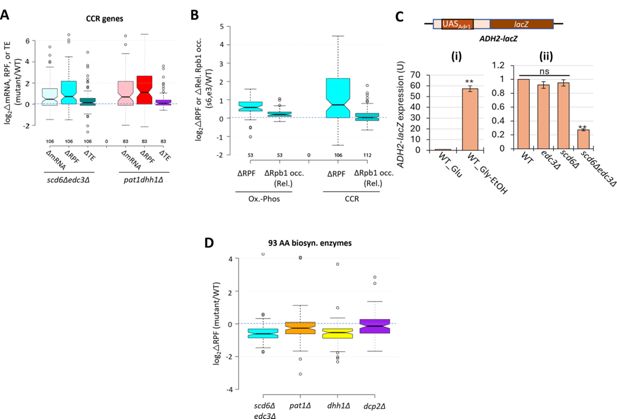
Scd6/Edc3 post-transcriptionally repress carbon-catabolite-repressed (CCR) genes in rich medium.
(A) Log2 changes in mRNA, ribosome-protected fragments (RPFs), or translational efficiency (TE) conferred by the indicated double mutations vs. wild-type (WT) for 106 genes subject to carbon catabolite repression or activated by transcription factors Adr1 or Cat8. (B) Notched box-plots showing log2∆RPFs or log2∆Rpb1 relative occupancies averaged over the coding sequences (CDSs) in scd6Δedc3Δ vs. WT cells for the same Ox-Phos (left) or CCR genes (right) analyzed in Figures 6A and 7A, respectively. (C) Expression of the ADH2-lacZ reporter on plasmid pLGADH2, containing the entire ADH2 5’ non-coding region, in the WT strain grown on SC-Ura medium containing either 2% glucose or 3% glycerol/2% ethanol as carbon sources (i), or in WT and the indicated mutant strains on SC-Ura cultured with 2% glucose (ii). For (ii), mean values of β-galactosidase activity measured for three biological replicates of each strain were normalized to the mean value measured for WT cells. **p-value <0.01 from student’s t-test; ns, not significant. (D) Log2 changes in RPFs for 93 genes encoding amino acid biosynthetic enzymes in the indicated mutants vs. WT.
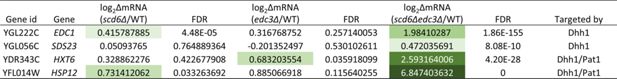
Synthetic genetic up-regulation of four Dhh1 target mRNAs on combining scd6∆ and edc3∆ mutations.
RNA-seq results obtained here for the four Dhh1 target mRNAs, two of which were designated as Pat1 targets as well (last column), identified by He et al., 2022, in the scd6∆, edc3∆, and scd6∆edc3∆ mutants vs. wild-type (WT). The log2∆mRNA values for each of the three mutants vs. WTs are listed together with the false discovery rate (FDR) values and color-coded according to derepression ratios.
-
Figure 6—figure supplement 3—source data 1
Source table for Figure 6—figure supplement 3.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig6-figsupp3-data1-v1.xlsx
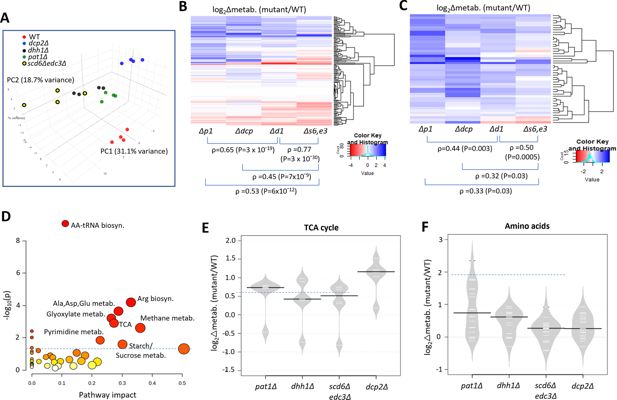
Eliminating decapping activators or decapping enzyme confers similar changes in polar metabolites.
(A) Principal component analysis of the levels of 147 metabolites in biological replicates of each strain. (B–C) Hierarchical clustering analysis of log2 changes in all 147 metabolites analyzed (B) or the 46 metabolites up-regulated in any two of the four mutants (C) conferred by the indicated mutations vs. wild-type (WT), including the Spearman coefficients (ρ) and p values for the indicated correlations. (D) Results of pathway analysis of the 46 up-regulated metabolites described in (C), conducted at https://www.metaboanalyst.ca/MetaboAnalyst/. Red ovals depict groups of metabolites significantly enriched among the set of 46 compounds, with p-value <0.05. (E–F) Log2 changes in levels of tricarboxylic acid (TCA) cycle intermediates (E) or amino acids (F) conferred by the indicated mutations vs. WT.
-
Figure 7—source data 1
Metabolomics of polar compounds of intermediary metabolism.
Sheets 1–4 list the statistical analyses of changes in metabolite concentrations in four biological replicates of the indicated four mutants vs. the wild-type (WT) determined in parallel for all 20 samples of metabolite extracts. Sheet 5 summarizes the log2 fold changes in metabolite levels and corresponding p-values for all 147 metabolites detected in for each of the four mutants. Sheet 6 lists the 46 metabolites up-regulated in any two of the four mutants (analyzed in Figure 7C), TCA cycle intermediates, and amino acids. Sheet 7 lists 93 amino acid biosynthetic genes interrogated in Figure 8D.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig7-data1-v1.xlsx
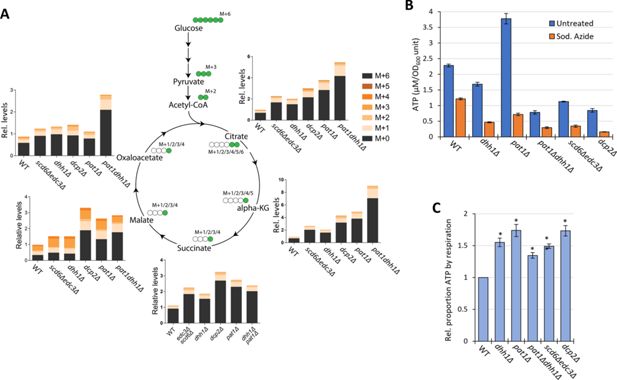
Elimination of decapping activators or decapping enzyme up-regulates respiration, increasing flux from glucose into TCA cycle intermediates and proportion of ATP produced by Ox. Phos.
(A) Three biological replicates of cells of each indicated genotype were cultured in YP with 2% unlabeled glucose, shifted to YP with 1% unlabeled glucose for 20 min, and pulsed with 13C6 labeled glucose (at final concentration of 1%) for 8 min, followed by extraction of metabolites and quantification of the indicated TCA cycle intermediates by mass spectrometry. In the diagrams, green circles signify the labeled carbon atom and the notation M+1, M+2, etc., indicates the mass increase in the molecules due to the labeled carbon. The depicted labeling pattern of metabolites reflects one cycle of the TCA cycle, resulting in mass additions of M+1 and M+2; however, across multiple cycles, a broader range of metabolite species with different mass additions will emerge. The metabolite signal intensities in all samples are expressed relative to that determined for the first replicate of the wild-type (WT) strain. (B–C) Measurements of ATP levels and proportions of total ATP impaired by azide inhibition of ETC activity. Cells cultured in YPD were treated or untreated with sodium azide for 30 min prior to harvesting. ATP levels were determined in extracts and normalized to OD600 units of cells for three biological replicates each of treated and untreated cell aliquots. Mean values for each strain are plotted in (B) and relative fractions of ATP in untreated samples retained following azide treatment (ATP_untreated)-(ATP_Azide)/(ATP_untreated), normalized to the values determined for WT are plotted in (C) with results from a student’s t-test indicated with asterisks: **p<0.005; *p<0.05.
-
Figure 8—source data 1
Glucose flux analysis.
In Sheet 2 ‘raw intensity,’ the numbers indicate the signal intensities (areas under the peaks from mass spectrometry) of the metabolite listed in column A in the three biological replicates of the indicated mutant or wild-type (WT) strains. In the subsequent sheets, raw data from Sheet 2 is collated for the different labeled isoforms of the indicated metabolite in the upper eight rows, and the proportion of the metabolite comprised of each isoform is given in the lower rows for the different samples. The ‘_N’ labeling indicates the label addition in the metabolite, i.e., _1 indicates mass addition of 1 labeled carbon, _2 indicates mass addition of 2 labeled carbons. Please let me know if you have any doubts regarding this.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig8-data1-v1.xlsx
-
Figure 8—source data 2
Measurements of cellular ATP content.
Sheets 2–3 provide source data for measurements of ATP content in three biological replicates of the indicated five mutants vs. wild-type (WT) and the fraction of total ATP content that is eliminated by inhibiting respiration by sodium azide treatment. Sheet 4 shows the standard curve produced using pure ATP employed to determine the ATP content in cell extracts.
- https://cdn.elifesciences.org/articles/102287/elife-102287-fig8-data2-v1.xlsx
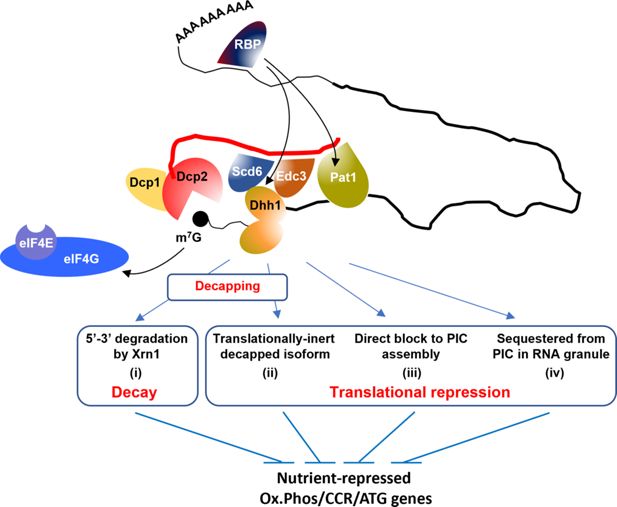
Hypothetical model to explain concerted repression of mRNA abundance or translation of particular mRNAs by decapping activators Scd6, Edc3, Dhh1, and Pat1.
A complex of Dcp1:Dcp2 containing Scd6/Edc3, Pat1/Dhh1, or different combinations of these factors is recruited to target mRNAs, possibly by a sequence-specific RNA-binding protein (RBP) binding to the 3’UTR. In agreement with a recent proposal (He et al., 2022), the recruitment of Dhh1 is enhanced by its redundant interactions with Scd6 or Edc3, which interact with the same segment of the Dcp2 CTT, whereas Pat1 is recruited independently to a distinct region of the C-terminal tail (CTT). One outcome of association of the decapping complex with an mRNA is activation of decapping with attendant 5’–3’ degradation by Xrn1 occurring without detectable repression of translation (i). A different outcome is translational repression by decapping wherein degradation by Xrn1 is inefficient and decapped intermediates accumulate that cannot bind the cap-binding initiation factors eIF4E and eIF4G (depicted as subunits of eIF4F excluded from the m7G mRNA cap) and thus persist as translationally inert isoforms (ii). Alternatively, decapping may not occur and the decapping complex competes with the cap-binding initiation factors to selectively inhibit translation initiation (iii), a fate that could be favored by sequestration of the transcript in RNA granules in a manner facilitated by the decapping activator proteins (iv).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Chemical compound, drug | Cycloheximide | Sigma | Cat # C7698 | |
| Chemical compound, drug | 5-fluoro-orotic acid | US Biological | Cat # F5050 | |
| Chemical compound, drug | ONPG (o-nitrophenyl-β-D-galactopyranoside) | Sigma | Cat # N1127 | |
| Chemical compound, drug | TCA (Trichloroacetic acid) solution | Sigma | Cat # T0699 | |
| Chemical compound, drug | G418 Sulfate (Geneticin) | US Biological | Cat # G1000 | |
| Chemical compound, drug | IgG sepharose | Cytiva | Cat # 17-0969-01 | |
| Commercial assay or kit | NEBuilder HiFi DNA assembly | New England Biolabs | Cat # E2621S | |
| Commercial assay or kit | RNase I | Ambion | Cat # AM2294 | |
| Commercial assay or kit | QIAzol Lysis reagent | Qiagen | Cat. # 79306 | |
| Commercial assay or kit | miRNeasy Mini Kit | Qiagen | Cat. # 217004 | |
| Commercial assay or kit | RNA Clean and Concentrator kit | Zymo Research | Cat # R1018 | |
| Commercial assay or kit | T4 Polynucleotide kinase | New England Biolabs | Cat # M0201L | |
| Commercial assay or kit | T4 Rnl2(tr) K227Q | New England Biolabs | Cat # M0351S | |
| Commercial assay or kit | 5’ deadenylase/RecJ exonuclease | Epicentre | Cat # RJ411250 | |
| Commercial assay or kit | Oligo Clean and Concentrator column | Zymo Research | Cat # D4060 | |
| Commercial assay or kit | Protoscript II | New England Biolabs | Cat # M0368L | |
| Commercial assay or kit | CircLigase ssDNA Ligase | Epicenter | Cat # CL4111K | |
| Commercial assay or kit | Phusion polymerase | New England Biolabs | Cat # M0530S | |
| Commercial assay or kit | High Sensitivity DNA Kit | Agilent | Cat # 5067–4,626 | |
| Commercial assay or kit | Fragmentation Reagent | Ambion | Cat # AM8740 | |
| Commercial assay or kit | Stop Solution | Ambion | Cat # AM8740 | |
| Commercial assay or kit | Ribo-Zero Gold rRNA Removal Kit | Illumina | Cat # MRZ11124C | |
| Commercial assay or kit | RNA 6000 Nano kit | Agilent | Cat # 5067–1511 | |
| Commercial assay or kit | ERCC ExFold RNA spike-In Mixes | Ambion | Cat # 4456739 | |
| Commercial assay or kit | DNA Library Prep Kit | New England Biolabs | Cat # E7370L | |
| Commercial assay or kit | DNase I | Roche | Cat # 4716728001 | |
| Commercial assay or kit | Luciferase Control RNA | Promega | Cat # L4561 | |
| Commercial assay or kit | Superscript III First-Strand synthesis kit | Invitrogen | Cat # 18080051 | |
| Commercial assay or kit | 25 mM triethylammonium-bicarbonate | Thermo Scientific | Cat # 90114 | |
| Commercial assay or kit | GelCode Blue Stain | Thermo Scientific | Cat # 24592 | |
| Commercial assay or kit | Pierce BCA Protein Assay Kit | Thermo Scientific | Cat # 23225 | |
| Commercial assay or kit | protease inhibitor cocktail | Roche | Cat # 5056489001 | |
| Commercial assay or kit | enhanced chemiluminescence (ECL) | Cytiva | Cat # RPN2109 | |
| Commercial assay or kit | Nano-Glo substrate | Promega | Cat # N1120 | |
| Commercial assay or kit | Bradford reagent | BioRad | Cat # 5000006 | |
| Commercial assay or kit | ATP Determination Kit | Thermo-Fisher Scientific | Cat # A22066 | |
| Commercial assay or kit | In-Fusion Snap Assembly Master Mix | Takara | Cat # 638949 | |
| Antibody | Anti-Gcd6, rabbit polyclonal | In house; Bushman et al., 1993 | Dilution: 1:2,000 (WB) | |
| Antibody | Anti-Pet10, rabbit polyclonal | Dr. Nikolaus Pfanner | Dilution: 1:1,000 (WB) | |
| Antibody | Anti-Cyb2, rabbit polyclonal | Dr. Thomas Fox | Dilution: 1:2,000 (WB) | |
| Antibody | anti-Idh1, goat polyclonal | Abnova | Cat # PAB19472 | Dilution: 1:500 (WB) |
| Antibody | Anti-Cox20, rabbit polyclonal | Dr. Thomas Fox | Dilution: 1:500 (WB) | |
| Antibody | Anti-Atp20, rabbit polyclonal | Dr. Nikolaus Pfanner | Dilution: 1:500 (WB) | |
| Antibody | Anti-Qcr8, rabbit polyclonal | Dr. Nikolaus Pfanner | Dilution: 1:500 (WB) | |
| Antibody | anti-Cit2, rabbit polyclonal | https://www.antibodies-online.com/ | Cat # ABIN4889057 | Dilution: 1:250 (WB) |
| Antibody | Anti-Cox14, rabbit polyclonal | Dr. Nikolaus Pfanner | Dilution: 1:500 (WB) | |
| Antibody | Anti-Sdh4, rabbit polyclonal | Dr. Nikolaus Pfanner | Dilution: 1:500 (WB) | |
| Antibody | anti-TAP, rabbit polyclonal | Thermo-Fisher Scientific | Cat # CAB1001 | Dilution: 1:2000 (WB) |
| Antibody | anti-HA (12CA5) mouse monoclonal | Roche | Cat # 10522600 | Dilution: 1:4000 (WB) |
| Antibody | anti-mouse IgG (HRP-conjugated), mouse polyclonal | GE HealthCare | Cat # NA931V | Dilution: 1:10000 (WB) |
| Antibody | anti-goat IgG (HRP-conjugated), chicken polyclonal | Abnova | Cat # PAB29101 | Dilution: 1:10000 (WB) |
| Antibody | anti-Rpb1 8WG16, mouse monoclonal | Biolegend | Cat # 664906; RRID:AB_2565554 | 4 µl used in Rpb1-ChIP |
| Strain, strain background (Saccharomyces cerevisiae) | Yeast strains used | This paper | RRID:NCBITaxon_2305205 | Table 1 |
| Genetic reagent (plasmid) | Plasmids used | This paper | Table 2 | |
| Sequence-based reagent | Primers used | This paper | Table 3 | |
| Software, algorithm | Notched box-plots | http://shiny.chemgrid.org/boxplotr/ | RRID:SCR_015629 | |
| Software, algorithm | Venn diagrams | https://www.biovenn.nl/ | RRID:SCR_026853 | |
| Software, algorithm | Hypergeometric distribution | https://systems.crump.ucla.edu/hypergeometric/index.php | ||
| Software, algorithm | Volcano plots | https://huygens.science.uva.nl/VolcaNoseR/ | RRID:SCR_025419 | |
| Software, algorithm | Gene ontology (GO) | http://funspec.med.utoronto.ca/ | RRID:SCR_006952 | |
| Software, algorithm | DESeq2 analysis | Zhang, 2023 | ||
| Software, algorithm | Integrative Genomics Viewer | http://software.broadinstitute.org/software/igv/ | RRID:SCR_011793 | IGV 2.4.14 |
| Software, algorithm | Genome-wide occupancy profiles for Rpb1 (ChIP) | Zhang, 2022 | ||
| Software, algorithm | Image Lab 6.0.1 program | https://www.bio-rad.com/en-us/product/image-lab-software?ID=KRE6P5E8Z | RRID:SCR_014210 | |
| Software, algorithm | SwissProt Yeast database | https://www.uniprot.org/proteomes/UP000002311 | ||
| Software, algorithm | Proteome Discoverer 2.4 | Thermo SCR_002798 | RRID:SCR_014477 | |
| Software, algorithm | GraphPad Prism 9 | GraphPad Software, San Diego, CA | RRID:SCR_002798 | 9.4.1 |
Yeast strains employed.
| Strain | Genotype | Source |
|---|---|---|
| 255 | MATa his3∆1 leu2∆0 met15∆0 ura3∆0 edc3∆::kanMX4 | Research Genetics |
| HFY114 (W303) | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3, 112 can1-100 | He et al., 2003 |
| SYY2352 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3, 112 can1-100 scd6∆::kanMX6 | He and Jacobson, 2015 |
| FZY855 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3, 112 can1-100 scd6∆::hphMX4 | This study |
| FZY858 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3, 112 can1-100 scd6∆::hphMX4 edc3∆::kanMX4 | This study |
| FZY862 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3, 112 can1-100 edc3∆::kanMX4 | This study |
| H5217/QZY126 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 dhh1∆::kanMX | Zeidan et al., 2018 |
| F2181/BSY3037 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 pat1∆::HIS3 | Charenton et al., 2017 |
| F2182/YFW168 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 pat1∆::HIS3 dhh1∆::kanMX | Charenton et al., 2017 |
| CFY1016 | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 dcp2::HIS3 | He et al., 2003 |
| F2262 | MATa his3-Δ1 leu2-Δ0 met15-Δ0 ura3-Δ0 DHH1::TAP::HIS3MX | GE Healthcare Dharmacon |
| H5695 | MATa ade2-1 ura3-1 his3-11,15 leu2-3,112 can1-100 DHH1- TAP::HIS3MX | This study |
| H5696 | MATa ade2-1 ura3-1 his3-11,15 leu2-3,112 can1-100 edc3∆::kanMX4 DHH1-TAP::HIS3MX | This study |
| H5697 | MATa ade2-1 ura3-1 his3-11,15 leu2-3,112 can1-100 scd6∆::kanMX4 DHH1-TAP::HIS3MX | This study |
| H5698 | MATa ade2-1 ura3-1 his3-11,15 leu2-3,112 can1-100 scd6∆::hphMX4 edc3∆::kanMX4 DHH1-TAP::HIS3MX | This study |
Plasmids employed.
| Plasmid | Description | Source |
|---|---|---|
| YCplac33 | s.c. URA3 vector | Gietz and Sugino, 1988 |
| YCplac111 | s.c. LEU2 vector | Gietz and Sugino, 1988 |
| pLfz614-7 | EDC3 in YCplac33 | This study |
| pLfz615-5 | SCD6 in YCplac33 | This study |
| pLfz635-5 | EDC3 in YCplac111 | This study (in case used) |
| pLfz636-1 | SCD6 in YCplac111 | This study (in case used) |
| pLGADH2 | ADH2 5’ non-coding region fused to lacZ | Sloan et al., 1999 |
| pLG265 | CYC1-lacZ reporter lacking UAS1 and containing the optimized version of UAS2, UAS2UP1 | Forsburg and Guarente, 1989 |
| pRK4 | UASGATA-CYC1-lacZ reporter containing the UAS from MEP2 modified to contain additional GATA sequences | Vijjamarri et al., 2023a |
| pAK133 | DCP2-3XHA cloned in YCplac33 under its native promoter. | This study |
Primers employed.
| Primer | Sequence (5’ to 3’) |
|---|---|
| AKV224 | CAAGCCGTTAATGTCGTTATCAATTTCGAT |
| AKV225 | TTCATCTTGTCAGTTGAAATGAATAGTTTA |
| AKV372 | CGACTCTAGAGGATCAAAGAACAATGAACTCTAGAGCATC |
| AKV373 | TCCTGCATAGTCCGGGACGTCATAGGGATAGCCCGCATAGTCAGGAACATCGTATGGGTAAACGGCCGCCTTCCTATGCAAAATGCTTAATAATT |
| AKV374 | TATCCCTATGACGTCCCGGACTATGCAGGATCCTATCCATATGACGTTCCAGATTACGCTCCGGCCGCCTGAAAGAATAAGTGTTATACGTTTTA |
| AKV375 | CGGTACCCGGGGATCAATATCGACAGTTTTAAGAACCGC |
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/102287/elife-102287-mdarchecklist1-v1.docx
-
Source data 1
Parallel RNA-Seq and Ribo-Seq analysis of decapping mutants, gene groups dysregulated in decapping mutants or belonging to specific functional categories, and capped/total mRNA ratios, codon optimality scores, codon protection indices, and Dhh1 occupancy values for all mRNAs.
Sheets 1-7 labeled ‘mutant vs WT all comp’ contain the processed data from Ribo-seq and RNA-seq analysis of two or three biological replicates of each strain listing the fold changes in mRNA, ribosome-protected fragments (RPFs), or translational efficiency (TE) between the relevant mutant vs. WT with the p-values and adjusted p-values (FDRs) assigned by DESeq2 analysis. The data for dhh1∆, dcp2∆ (Zeidan et al., 2018), pat1∆, and pat1∆dhh1∆ mutants (Vijjamarri et al., 2023a) were reported previously. Sheet ‘Gene groups_mRNA analysis’ contains the lists of mRNAs (identified by systematic gene name) that are up-regulated or down-regulated in abundance by ≥1.5-fold at FDR < 0.05 in the scd6∆, edc3∆, or scd6∆edc3∆ mutants vs. WT, either including or excluding iESR mRNAs for up-regulated transcripts or rESR mRNAs for down-regulated transcripts, as indicated. It also lists the transcripts belonging to the three sectors of Figs. 1B-C, the iESR and rESR transcripts defined previously (Gasch et al., 2000), and all of the non-iESR mRNAs up-regulated by ≥1.5-fold at FDR < 0.05 in dhh1∆, pat1∆, or pat1∆dhh1∆ mutants, as well as those equally up-regulated by the dcp2∆ mutation. Sheet ‘Gene groups_TE analysis’ contains the lists of mRNAs that are up-regulated or down-regulated in TE by ≥1.41-fold at FDR <0.10 in the different mutants vs. WT. Sheet ‘Gene groups_RPF analysis’ contains the lists of mRNAs that are up-regulated or down-regulated in RPFs by ≥1.5-fold at FDR <0.05 in the different mutants vs. WT. Sheet ‘Pathway gene groups’ lists the genes involved in specific pathways (e.g. Ox. Phos.) analyzed for changes in expression or translation. Sheet ‘Codon Opt Scores nonESR mRNAs’ lists the tRNA adaptation index (tAI), species-specific tRNA adaptation index (stAI), and the average codon stabilization coefficient (AvgCSC) for all non-ESR yeast genes (Presnyak et al., 2015; Radhakrishnan et al., 2016). Sheet ‘Codon Protection Indices’ lists the CPI values for all genes determined previously (Pelechano et al., 2015). Sheet ‘Capped to Total RNA ratios_s6,e3’ lists the ratios of transcript numbers per million reads (TPMs) determined by CAGE sequencing of capped mRNAs (C) to TPMs determined by parallel RNA-seq of the same total RNA samples for 5393 genes, determined for scd6∆edc3∆ and WT cells. Sheet ‘Capped to Total RNA ratios_dhh1’ lists the ratios of transcript numbers per million reads (TPMs) determined by CAGE sequencing of capped mRNAs (C) to TPMs determined by parallel RNA-seq of the same total RNA samples for 5129 genes, determined previously for dhh1∆ and WT cells (Vijjamarri et al., 2023a). Sheet ‘Dhh1 occ enrich scores’ lists the relative Dhh1 occupancies (enrichment scores) determined globally for yeast mRNAs by RIP-seq analysis (Miller et al., 2018) for the 3686 transcripts for which both Dhh1 enrichment score and Ribo-seq and RNA-seq data from the dhh1∆ vs. WT comparison (Zeidan et al., 2018) are available. Sheet ‘IGV tracks’ lists the results of ribosome profiling of the scd6∆, edc3∆, and scd6∆edc3∆ mutants vs. WT for the genes selected for gene-browser depictions.
- https://cdn.elifesciences.org/articles/102287/elife-102287-data1-v1.xlsx





