Distinct functions of three Wnt proteins control mirror-symmetric organogenesis in the C. elegans gonad
Figures
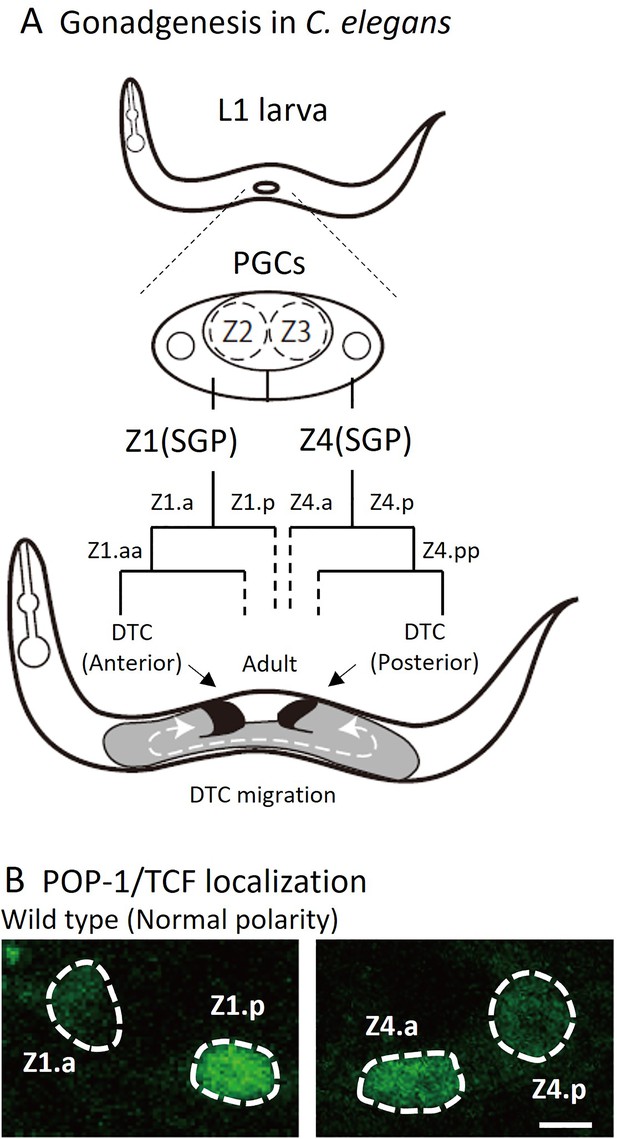
Cell lineage of somatic gonad and asymmetric localizations of POP-1/TCF between somatic gonadal precursor (SGP) daughter cells.
(A) After hatching, L1 animals have two SGPs (Z1 and Z4) and primordium germ cells (Z2 and Z3). SGPs undergo mirror-symmetric divisions along the proximal-distal axis, and their distal granddaughters Z1.aa and Z4.pp become distal tip cells (DTCs) that migrate anteriorly and posteriorly, respectively. The U-shape of gonad arms is established through the migration of DTCs. (B) Examples of POP-1 localizations in wild-type animals using GFP::POP-1 (qIs74). Animals were cultured at 22.5°C. Anterior is to the left. Scale bars indicate 2 μm.
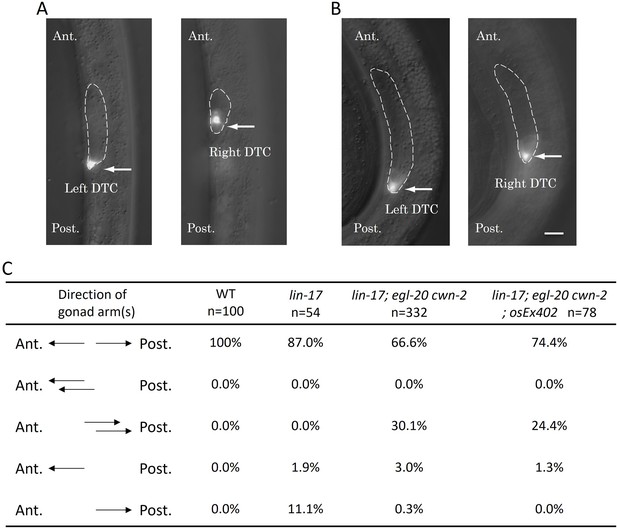
The Dpd phenotype of lin-17; egl-20 cwn-2 animals.
Merged images of DIC and GFP of lin-17; egl-20 cwn-2 animals at the L2 stage (A) and the L3 stage (B) are presented. Arrows highlight Z4-derived left and Z1-derived right distal tip cells (DTCs) expressing mig-24::Venus. The gonad is delineated with dashed lines. The anterior is up. Left and right images in each panel are correspond to the same area with different focal planes in the same animals. (C) The table compiles the direction of gonad arms extension. These experiments were conducted with animals grown at 15°C. Ant. and Post. indicate anterior and posterior, respectively. Scale bars indicate 10 μm.
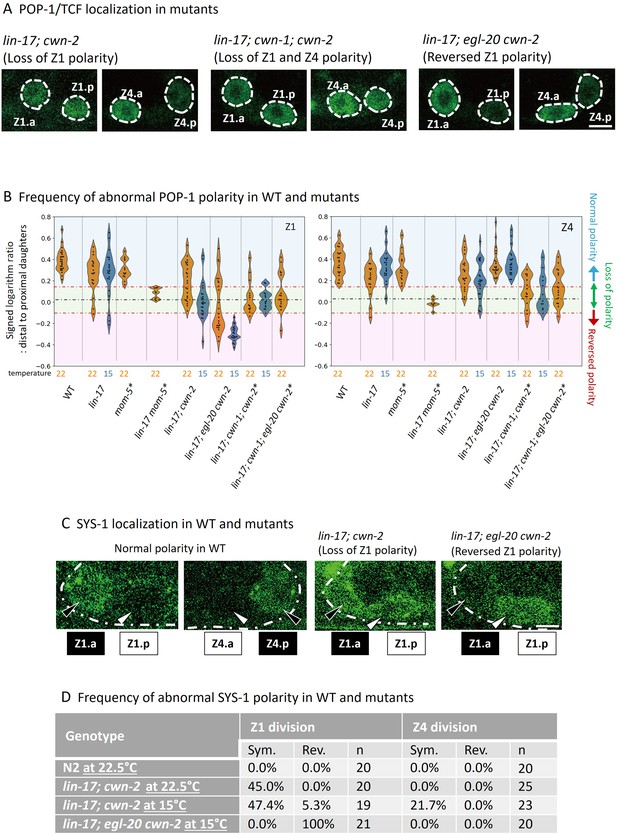
POP-1 and SYS-1 asymmetry is redundantly regulated by lin-17 and multiple Wnts.
(A) Examples of sys-1p::GFP::POP-1 (qIs74) localizations in the indicated genotypes. Animals were cultured at 22.5°C, except for lin-17; egl-20 cwn-2, which was cultured at 15°C. (B) Violin plots illustrate the distribution of signed ratios on a logarithmic scale of GFP::POP-1 signals proximal to distal daughters. The violin plots show the distribution of experimental data at two temperatures 22.5°C (22°C in the figure) and 15°C, represented on the left and right halves of each violin, respectively. The black dashed lines represent the zero residual lines (where the predicted values equal the observed values), and the red dashed lines indicate the 95% confidence interval (CI) calculated from signals of symmetrically localizing sys-1p::GFP(NLS). Values within the 95% CI (between the red lines; light green regions) indicate symmetric localization. Values below the lower red line (light blue regions) indicate reversed localization, while values above the upper red line (light red regions) indicate normal localization. See Materials and methods for details. For the strains indicated by asterisks, homozygous progeny from balanced heterozygotes (see Supplementary file 1) was analyzed. (C) Examples of SYS-1 localizations in the indicated genotypes were observed using VENUS::SYS-1 (qIs95). Animals were cultured at 22.5°C, except for lin-17; egl-20 cwn-2, which was cultured at 15°C. (D) Abnormal SYS-1 localization in compound mutants. SYS-1 localizations were analyzed using qIs95 (Venus::SYS-1). Sym: the fluorescence was observed in both daughter cells. Rev: the fluorescence was observed in the proximal daughter cells. n: number of animals scored. Since somatic gonadal precursor (SGP) daughter cells are often present at distinct focal planes, we normalized the depth effects on fluorescence intensities (see Materials and methods for details) for the quantification shown in (B). The images in (A) and (C) are from animals with SGP daughters at similar depths. Scale bars indicate 2 μm. Source data is available (Figure 3—source data 1).
-
Figure 3—source data 1
Data utilized to generate the graph in Figure 3B and D.
- https://cdn.elifesciences.org/articles/103035/elife-103035-fig3-data1-v2.xlsx

Examples of POP-1 localizations in the indicated genotypes observed using sys-1p::GFP::POP-1 (qIs74).
Asterisk indicates germ cell. Animals were cultured at 22.5°C. Scale bars indicate 5 μm.

Comparison of absolute differences in POP-1 asymmetry regulated by lin-17 and multiple Wnts.
Violin plots illustrate the distribution of absolute ratios on a logarithmic scale of GFP::POP-1 signals between proximal and distal daughters. The violin plots show the distribution of experimental data at two temperatures 22.5°C (22°C in the figure) and 15°C, represented on the left and right halves of each violin, respectively. The black dashed lines represent the zero residual lines (where the predicted values equal the observed values), and the red dashed line indicates the 95% confidence interval (CI) calculated from signals of symmetrically localizing sys-1p::GFP(NLS). Values between the black lines and the red lines indicate symmetric localization (light green regions), while values upper than the 95% CI indicate polarization (light blue regions). See Materials and methods for details. For the strains indicated by asterisks, homozygous progeny from balanced heterozygotes (see Supplementary file 1) was analyzed. Source data is available (Figure 3—source data 1).

CWN-1 expression is not affected in lin-17; egl-20 cwn-2 animals.
Merged confocal images of L1 animals expression cwn-1 promoter::CWN-1::Venus (osIs22) are shown. The animals were grown at 15°C. osIs22 is an UV-induced integration of an extrachromosomal array containing cwn-1 promoter::CWN-1::Venus (Yamamoto et al., 2011). The fluorescence was observed by confocal microscopy (Zeiss LSM510).
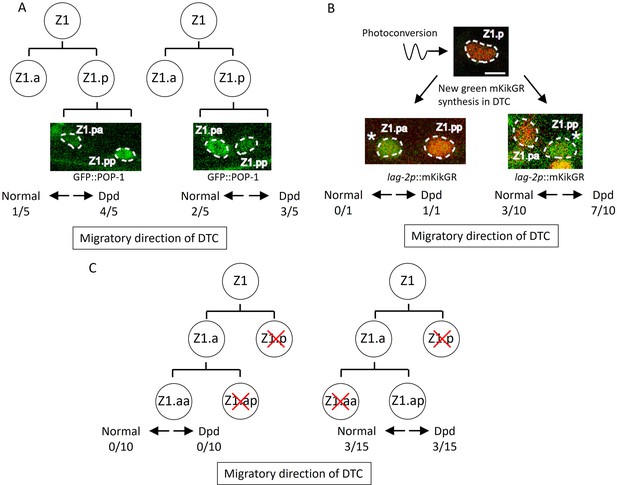
Abnormal positions of distal tip cells (DTCs) in lin-17; cwn-2 egl-20 mutant cause the Dpd phenotype.
(A) POP-1 asymmetry in Z1.p daughter cells was observed at the late L1 stage. LH (normal) POP-1 polarity (left panel) and HL (reversed) POP-1 polarity (right panel) indicate that Z1.pa and Z1.pp become DTCs, respectively. After the recovery and growth of the animals, the migratory directions of DTCs were evaluated at the L4 stage by extending gonad arms. (B) Green fluorescence of lag-2p::mkikGR (osIs168) in Z1.p of lin-17; cwn-2 egl-20 animals was photoconverted to red by irradiation with a 405 nm light laser. After the recovery and 10 hr of growth of the animals, Z1.p daughter cells were observed for the presence of newly synthesized green mkikGR fluorescence indicating the DTC fate. Subsequently, following the recovery and growth of the animals, the Dpd phenotype was assessed at the L3-L4 stages based on the positions of DTCs expressing green mkikGR fluorescence. (C) The Z1.p cells of lin-17; egl-20 cwn-2; tkIs12 animals were laser-ablated at the late L1 stage. After the recovery and growth of the animals, either Z1.ap (left) or Z1.aa (right) cell was laser ablated. The migratory directions of DTCs derived from Z1.aa (left) or Z1.ap (right) were evaluated at the L4 stage by the positions of DTCs expressing mig-24::Venus. The animals were grown at 15°C (A–B) or 22.5°C (C). Scale bars indicate 2 μm. Source data is available (Figure 4—source data 1).
-
Figure 4—source data 1
Source data for abnormal migration of distal tip cells (DTCs) in lin-17; cwn-2 egl-20 mutants in Figure 4.
- https://cdn.elifesciences.org/articles/103035/elife-103035-fig4-data1-v2.xlsx
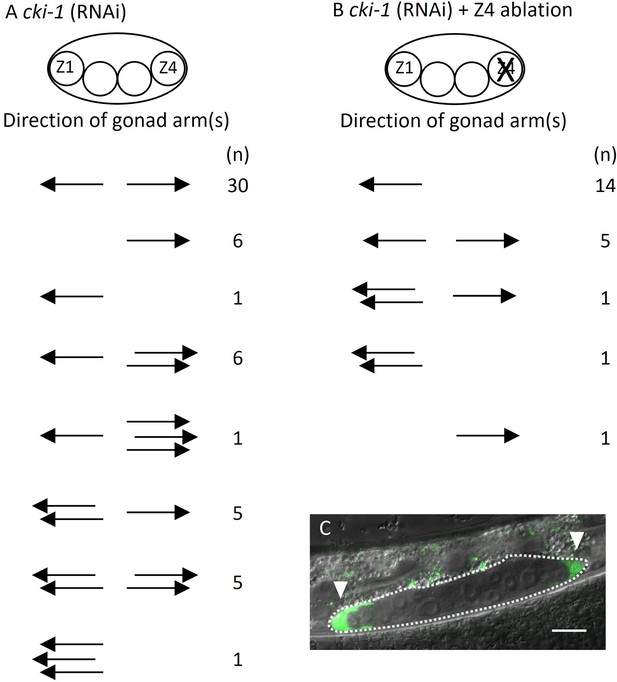
Ectopic positions of distal tip cells (DTCs) cause the Dpd phenotype.
Migratory directions and numbers of DTCs in cki-1(RNAi) (A) and Z4 cell-ablated cki-1(RNAi) (B) animals grown at 15°C judged by mig-24::Venus at the L4 or young adult stages. Each arrow represents the migratory direction of an individual DTC. (C) An example of Z4 cell-ablated cki-1(RNAi) animal carrying anteriorly and posteriorly migrated DTCs (arrow heads) expressing mig-24::Venus. Scale bars indicate 10 μm.
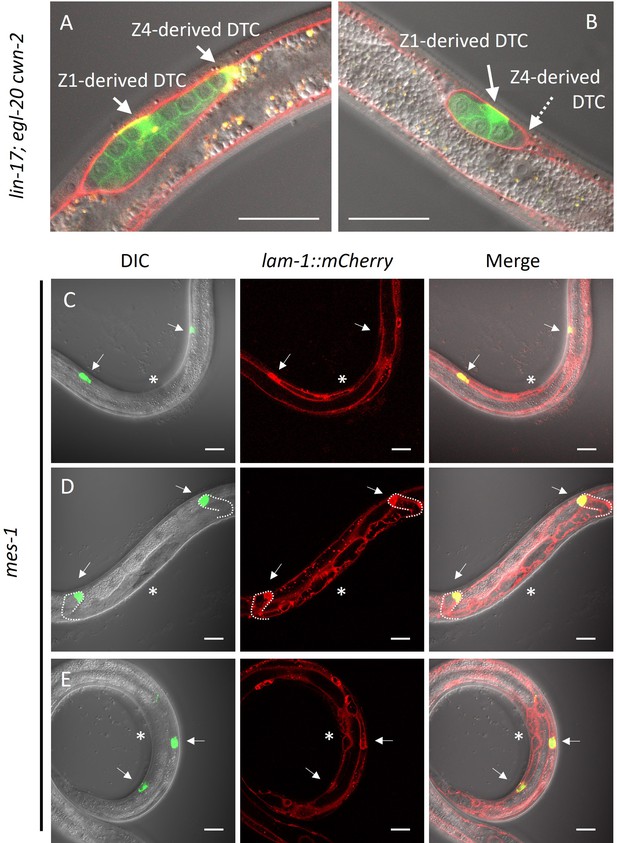
Germ cell-independent migration of distal tip cells (DTCs).
(A–B) lin-17; egl-20 cwn-2 and (C–E) germless mes-1 animals carrying lam-1::mCherry (qyIs127) and mex-5::GFP::PH (xnSi1) and mig-24::Venus (tkIs12 in (A) and osEx283 in (B–E)) were grown at 15°C (A–B) or 22.5°C (C–E). The signals of mex-5::GFP::PH and mig-24::Venus can be distinguished by membrane and cytoplasmic fluorescence, respectively. Germless mes-1 phenotype was confirmed by the absence of the mex-5::GFP::PH signal in the gonad. Merged images are shown in (A–B). In (C–E), merged images of DIC and green channels, red channel, and those of all channels are shown. In (E), merged images of two different focal planes are shown. Scale bars indicate 20 μm. Arrows indicate DTCs, and a dotted arrow in (B) indicates a DTC out of focus. Asterisks in (C–E) indicate positions of vulval investigation.
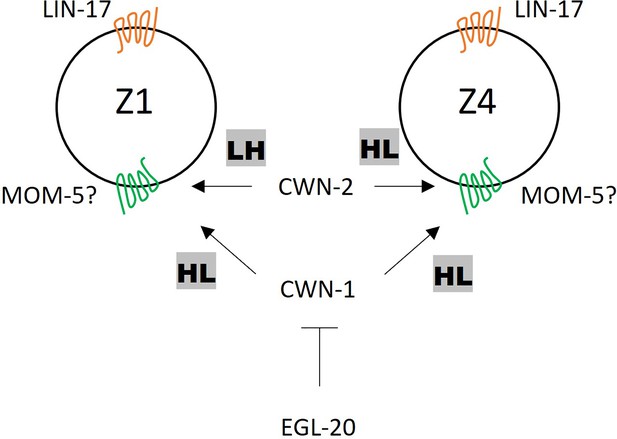
A model of somatic gonadal precursor (SGP) polarity regulation.
LIN-17 regulates SGP polarity in a Wnt-independent manner. CWN-2 promotes LH and HL polarity in Z1 and Z4, respectively, while CWN-1 promotes HL polarity in both Z1 and Z4. EGL-20 inhibits the function of CWN-1. MOM-5 might serve as the receptor for both CWN-1 and CWN-2.
Tables
Missing distal tip cell (DTC) phenotype of compound mutants.
| Genotype | Anterior | Posterior | n | |
|---|---|---|---|---|
| WT | N2 | 0.0% | 0.0% | 100 |
| Wnt receptors | lin-17(n3091)* | 4.7% | 2.8% | 107 |
| lin-17 at 15°C* | 11.1% | 1.9% | 54 | |
| mom-5§ | 0.0% | 0.0% | 54 | |
| lin-17 mom-5§ | 100% | 100% | 30 | |
| +osIs113 (ΔCRD-LIN-17)*, § | 0.0% | 0.0% | 34 | |
| lin-17(os2) mom-5§ | 2.0% | 1.0% | 100 | |
| lin-17(mn589) mom-5§ | 0.0% | 0.0% | 50 | |
| mig-1 lin-17(n671) | 9.0% | 9.0% | 67 | |
| lin-17; cfz-2 | 9.4% | 4.7% | 64 | |
| lin-17; lin-18§ | 7.6% | 3.8% | 53 | |
| lin-17; cam-1† | 5.7% | 0.0% | 300 | |
| Fz+Wnt | lin-17; cwn-1† | 7.2% | 0.9% | 111 |
| lin-17; cwn-2* | 45.8% | 4.3% | 94 | |
| +osIs113 (ΔCRD-LIN-17)* | 0.0% | 0.0% | 40 | |
| lin-17; cwn-2 at 15°C* | 88.5% | 54.1% | 61 | |
| +osIs113 (ΔCRD-LIN-17) at 15°C* | 0.0% | 0.0% | 45 | |
| lin-17(n671); cwn-2* | 48.8% | 7.3% | 41 | |
| lin-17; egl-20† | 4.4% | 0.0% | 340 | |
| lin-17; egl-20 cwn-2*, ‡ | 2.9% | 0.0% | 70 | |
| lin-17; egl-20 cwn-2 at 15°C*, ‡ | 0.3% | 3.0% | 332 | |
| lin-17; cwn-1; egl-20* | 5.8% | 1.5% | 69 | |
| lin-17; cwn-1; egl-20 at 15°C* | 1.3% | 1.3% | 78 | |
| lin-17; cwn-1; cwn-2*, § | 89.1% | 78.3% | 46 | |
| lin-17; cwn-1; cwn-2 at 15 °C*, § | 100% | 97.6% | 42 | |
| lin-17; cwn-1; egl-20 cwn-2*, § | 86.8% | 84.9% | 53 | |
| lin-17; cwn-1; egl-20 cwn-2 at 15°C*, § | 97.8% | 95.7% | 46 | |
| +osEx395 (ceh-22p::CWN-1::Venus) at 15°C*, § | 96.0% | 100% | 25 | |
| +osIs93 (egl-20p::CWN-2::Venus) at 15°C*, § | 88.9% | 74.1% | 27 | |
| mom-5; cwn-1; egl-20 cwn-2†, § | 0.0% | 0.0% | 22 |
-
n3091 was used as a lin-17 mutation unless otherwise indicated.
-
The strains were grown at 22.5°C unless otherwise indicated.
-
*
The strains had tkIs12 encoding the mig-24p::Venus that expresses in DTCs.
-
†
The strain had vpIs1 encoding elt-3::GFP that was used to observe hypodermal defects.
-
‡
Dpd animals were considered to have both anterior and posterior DTCs.
-
§
Homozygous progeny from balanced heterozygotes (see Supplementary file 1).
-
n: number of animals scored.
Additional files
-
Supplementary file 1
List of strains used for experiments.
- https://cdn.elifesciences.org/articles/103035/elife-103035-supp1-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/103035/elife-103035-mdarchecklist1-v2.pdf





