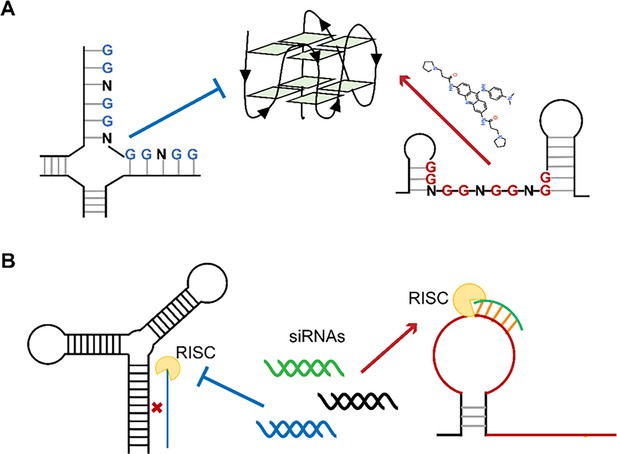
Exploiting functional regions in the viral RNA genome as druggable entities
Figures
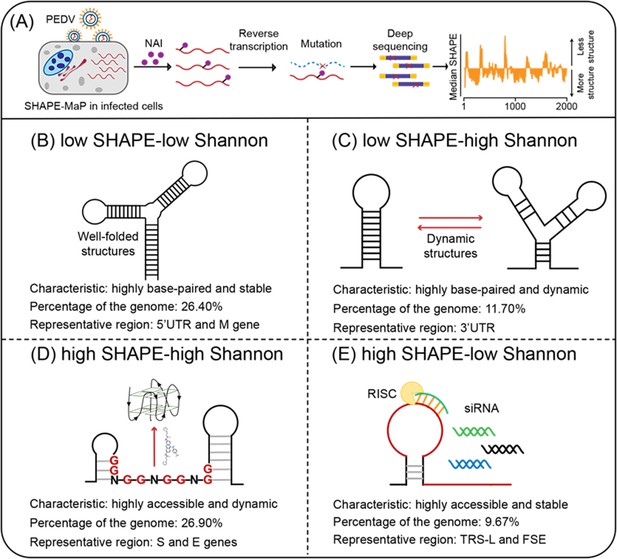
Four regions with different characteristics in the porcine epidemic diarrhea virus (PEDV) genome.
(A) Schematic of SHAPE-MaP for probing the RNA structure of the PEDV genome in situ. (B) Well-folded regions (low SHAPE reactivity and low Shannon entropy; 26.40% of genome). These regions represent stably folded RNA structures with minimal conformational flexibility, serving as structural scaffolds or functional elements in viral replication. (C) Dynamic structured regions (low SHAPE reactivity and high Shannon entropy; 11.70% of genome). These conformationally plastic domains mediate regulatory switches between alternative secondary structures during infection. (D) Dynamic unpaired regions (high SHAPE reactivity and high Shannon entropy; 26.90% of genome). These regions are prone to form non-canonical nucleic acid structures (e.g., G-quadruplexes), which can be stabilized by small-molecule ligands to inhibit viral replication. (E) Persistent unpaired regions (high SHAPE reactivity and low Shannon entropy; 9.67% of genome). These regions are more accessible for siRNA binding, facilitating recruitment of Argonaute proteins and Dicer to form the RNA-induced silencing complex (RISC) for targeted cleavage.
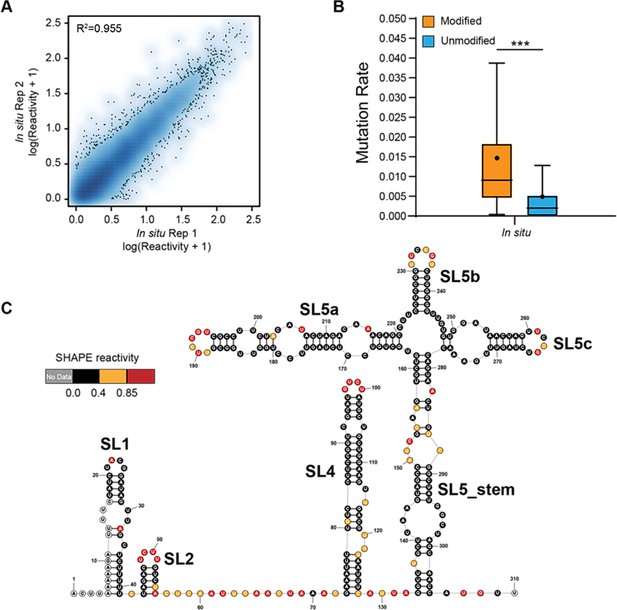
Genome-wide SHAPE-MaP analysis of porcine epidemic diarrhea virus (PEDV).
(A) Heat scatter plot of the SHAPE reactivities across two biological replicates for the ex vivo datasets. (B) Reverse transcription mutation rates (median indicated by line, average indicated by ‘.’) for two ex vivo biological replicates: NAI-treated (modified) vs. the DMSO-treated (unmodified). A significant increase in the reverse transcription mutation rate was observed in the NAI-treated group compared to the DMSO-treated group, suggesting that NAIs are effective at modifying viral genomic RNA ex vivo. (C) SHAPE secondary structure modeling of the PEDV 5’ UTR. ***p<0.001 by equal variance unpaired Student’s t-test.
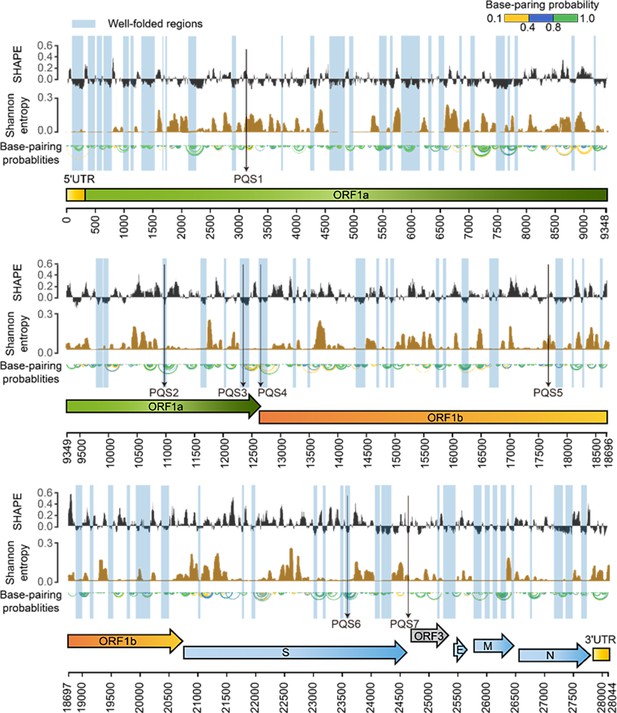
SHAPE structural map of the porcine epidemic diarrhea virus (PEDV) genome in infected cells.
From top to bottom: SHAPE reactivity, Shannon entropy, base-pairing probabilities, translation reading frames are indicated by arrows, and genomic coordinates are marked at the bottom. Well-folded regions with low SHAPE reactivity and low Shannon entropy are shaded in blue. Potential G4 forming sequences (PQSs) are marked with a black arrow.
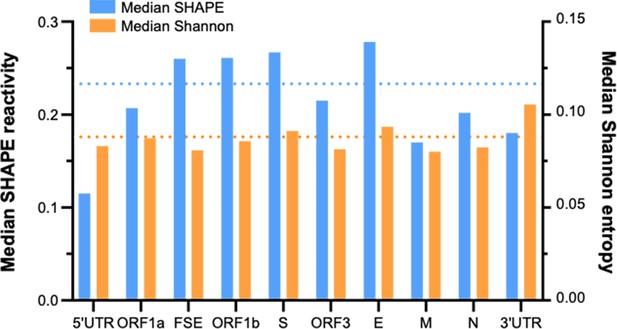
Folding characteristics of known functional regions and coding areas in the porcine epidemic diarrhea virus (PEDV) genome.
The length of ORF1ab is 20,345 nt, accounting for 72.5% of the total length of the PEDV genome (28,044 nt) (a major contributor to the global median). The blue dashed line and the orange dashed line represent the global median SHAPE reactivity and the global median Shannon entropy, respectively.
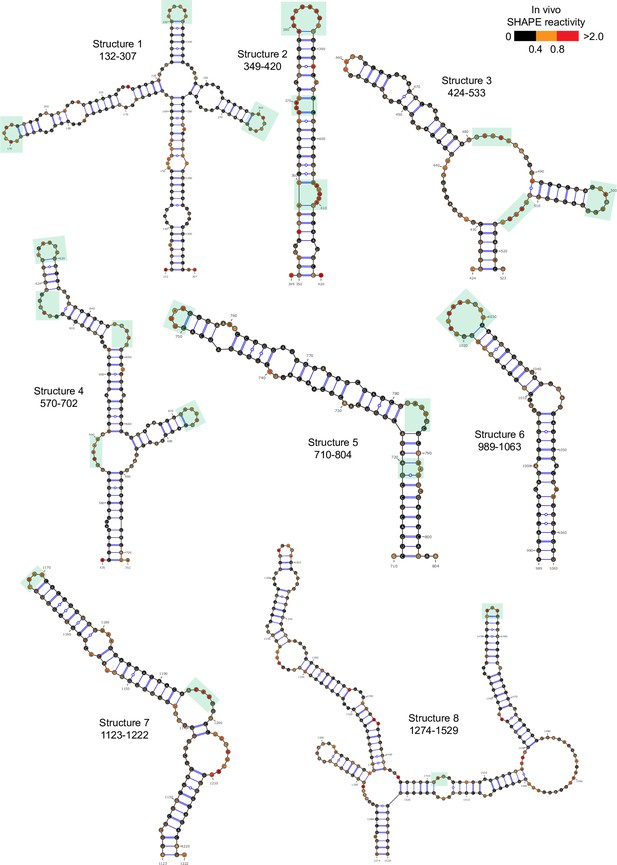
Well-folded structures 1–8 in porcine epidemic diarrhea virus (PEDV) genome.
Secondary structure predicted by SHAPE reactivity as a constraint. Green shading indicates high accessibility windows.
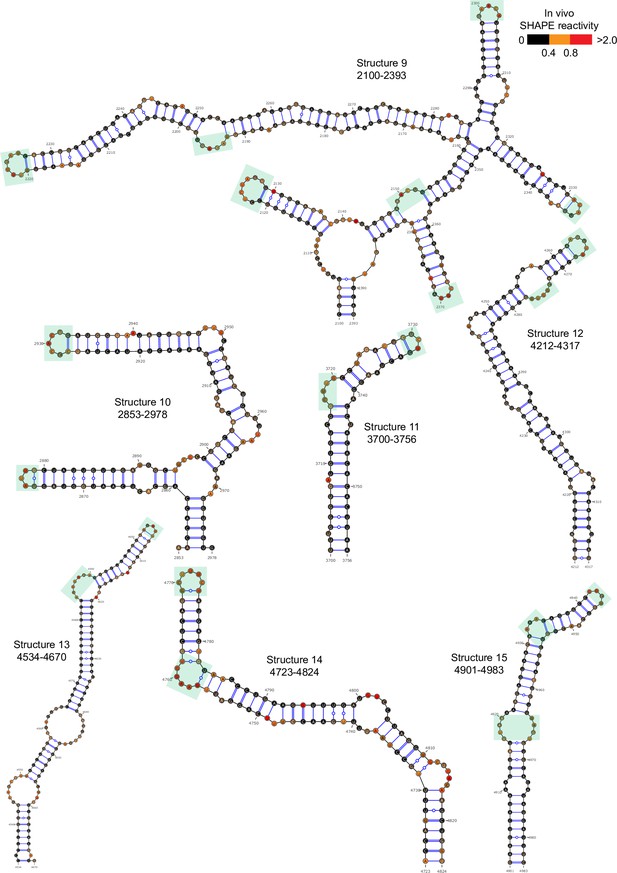
Well-folded structures 9–15 in porcine epidemic diarrhea virus (PEDV) genome.
Secondary structure predicted by SHAPE reactivity as a constraint. Green shading indicates high accessibility windows.
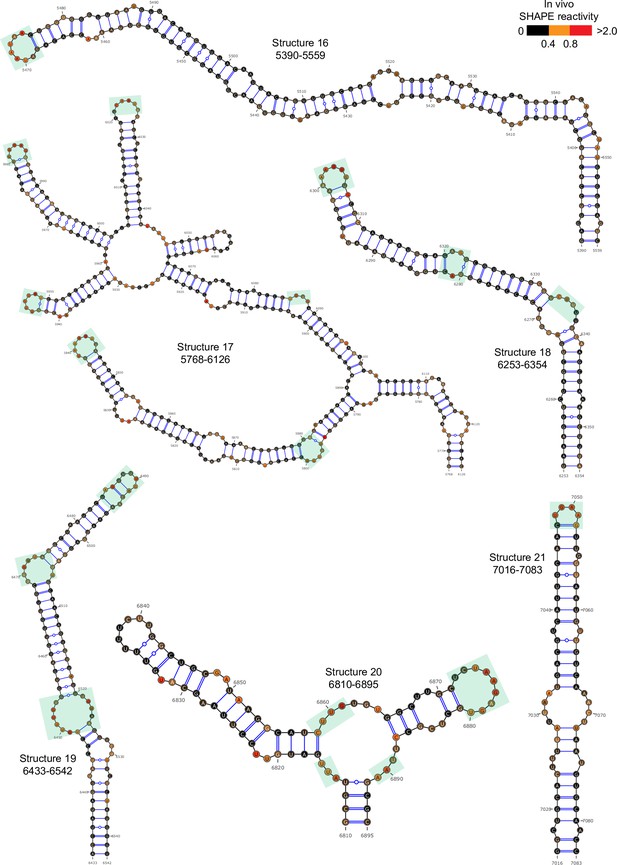
Well-folded structures 10–21 in porcine epidemic diarrhea virus (PEDV) genome.
Secondary structure predicted by SHAPE reactivity as a constraint. Green shading indicates high accessibility windows.
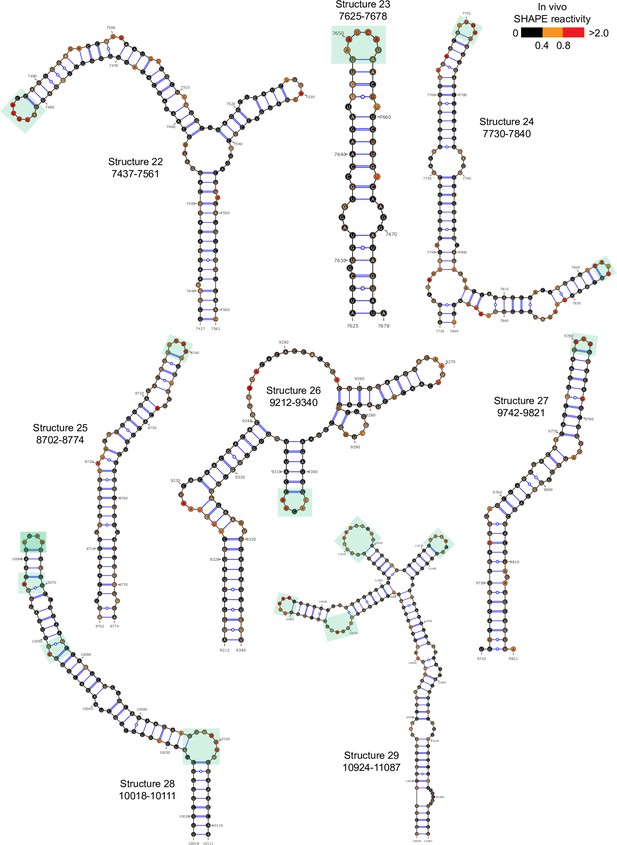
Well-folded structures 22–29 in porcine epidemic diarrhea virus (PEDV) genome.
Secondary structure predicted by SHAPE reactivity as a constraint. Green shading indicates high accessibility windows.
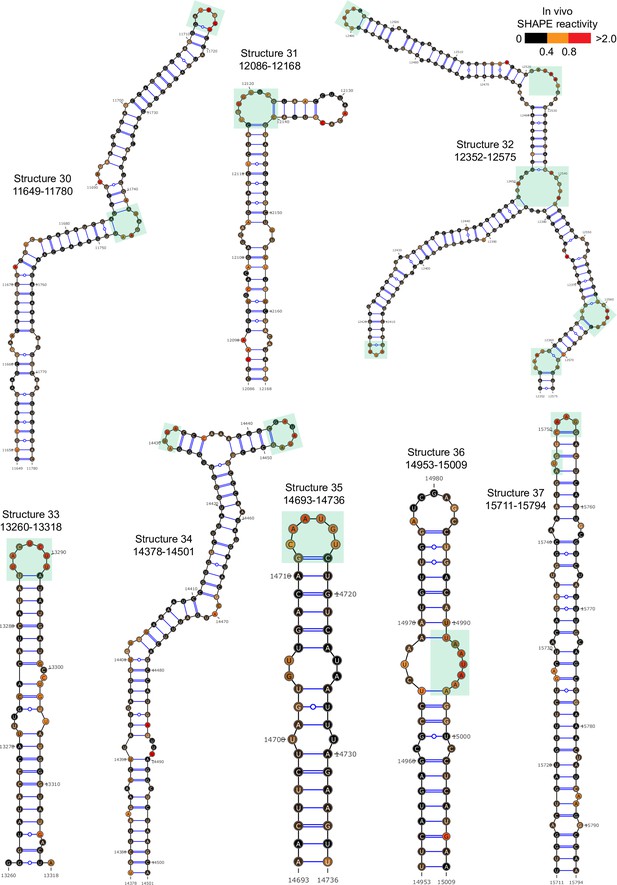
Well-folded structures 30–37 in porcine epidemic diarrhea virus (PEDV) genome.
Secondary structure predicted by SHAPE reactivity as a constraint. Green shading indicates high accessibility windows.
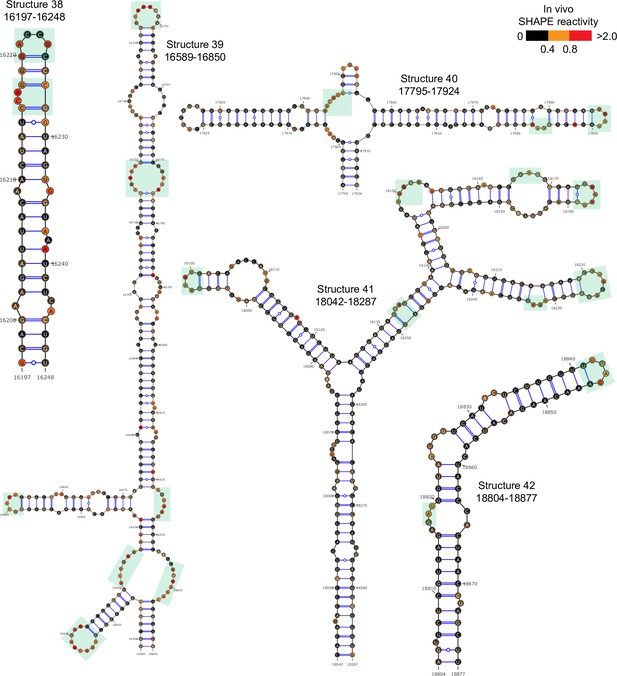
Well-folded structures 38–42 in porcine epidemic diarrhea virus (PEDV) genome.
Secondary structure predicted by SHAPE reactivity as a constraint. Green shading indicates high accessibility windows.
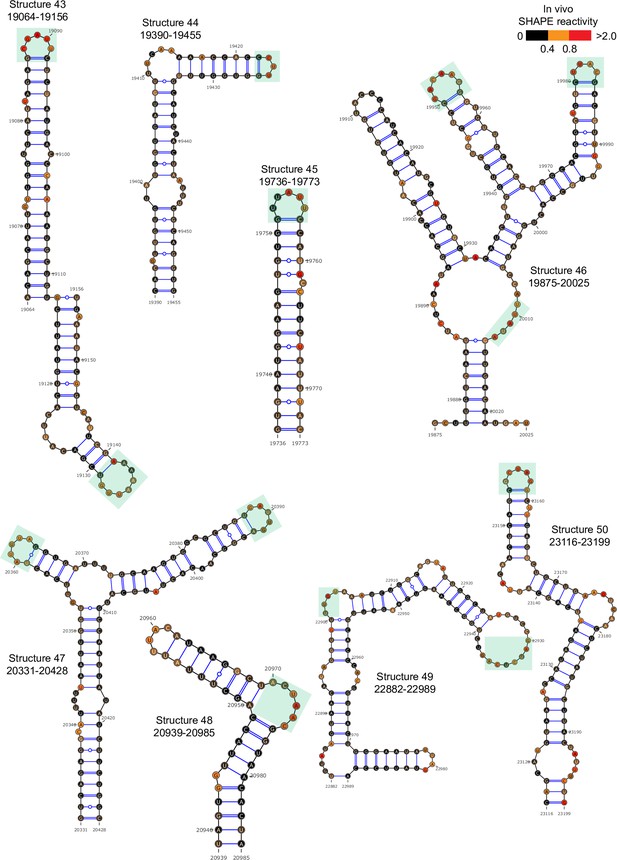
Well-folded structures 43–50 in porcine epidemic diarrhea virus (PEDV) genome.
Secondary structure predicted by SHAPE reactivity as a constraint.Green shading indicates high accessibility windows.
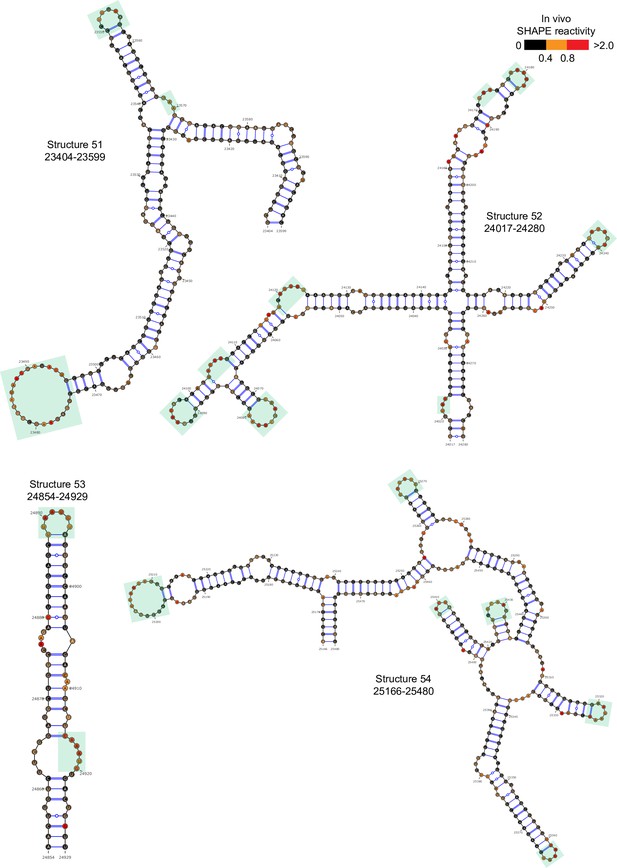
Well-folded structures 51–54 in porcine epidemic diarrhea virus (PEDV) genome.
Secondary structure predicted by SHAPE reactivity as a constraint. Green shading indicates high accessibility windows.
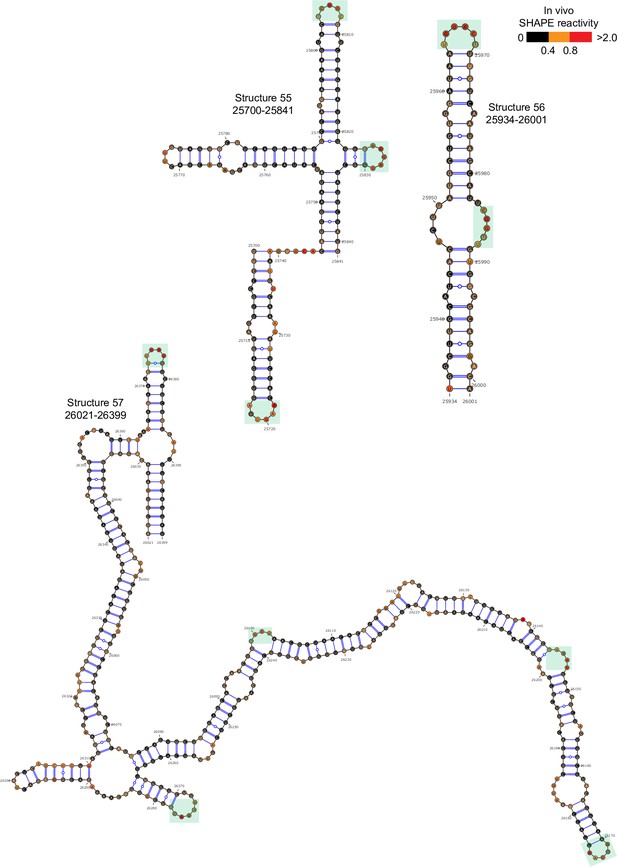
Well-folded structures 55–57 in porcine epidemic diarrhea virus (PEDV) genome.
Secondary structure predicted by SHAPE reactivity as a constraint. Green shading indicates high accessibility windows.
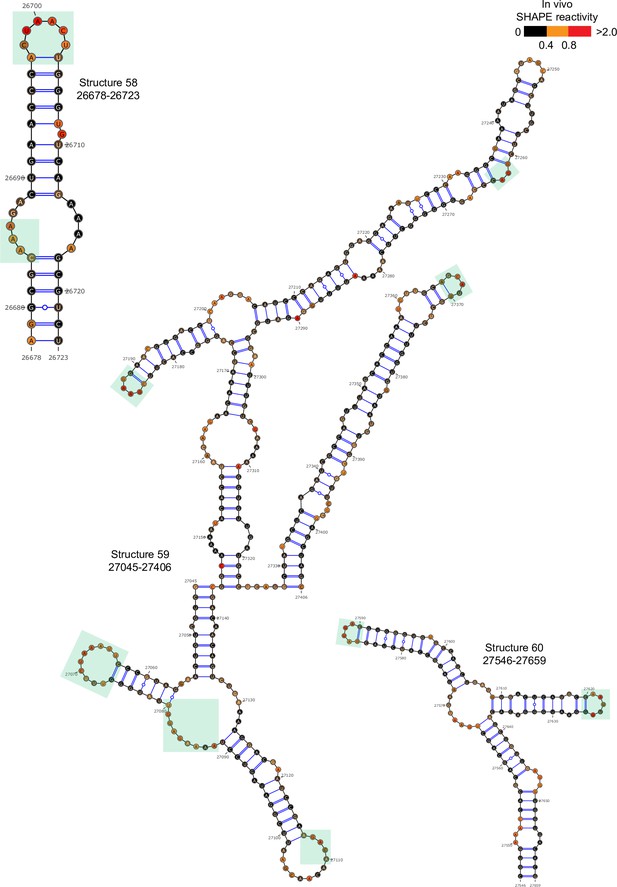
Well-folded structures 58–60 in porcine epidemic diarrhea virus (PEDV) genome.
Secondary structure predicted by SHAPE reactivity as a constraint. Green shading indicates high accessibility windows.
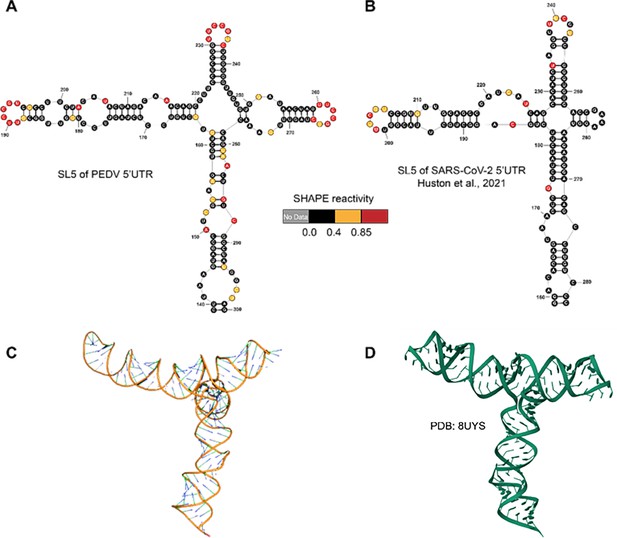
Structures of 5’UTR-SL5 in the genomes of porcine epidemic diarrhea virus (PEDV) and SARS-CoV-2.
(A) Secondary structure of 5’UTR-SL5 in the PEDV genome predicted by SHAPE reactivity as a constraint. (B) Secondary structure of 5’UTR-SL5 in the SARS-CoV-2 genome (Huston et al., 2021). (C) The three-dimensional structural model of PEDV 5’UTR-SL5 based on secondary structure was predicted using FARFAR2. (D) Three-dimensional of 5’UTR-SL5 in the SARS-CoV-2 genome (PDB id: 8UYS).
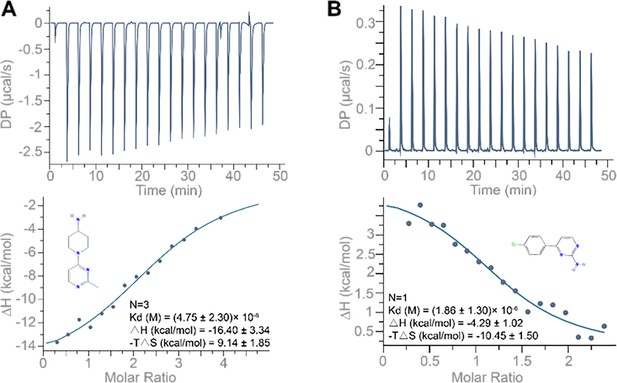
ITC binding profile of (A) compound 1 (1-(2-methylpyrimidin-4-yl) piperidin-4-amine; 250 µM) and (B) compound 4 (4-(4-chlorophenyl) pyrimidin-2-amine; 250 µM) titrated into the 10 µM 5’UTR-SL5 of porcine epidemic diarrhea virus (PEDV).
Buffer solution: 100 mM HEPES (pH 8.0), 100 mM NaCl, and 10 mM MgCl2. Temperature: 25°C.
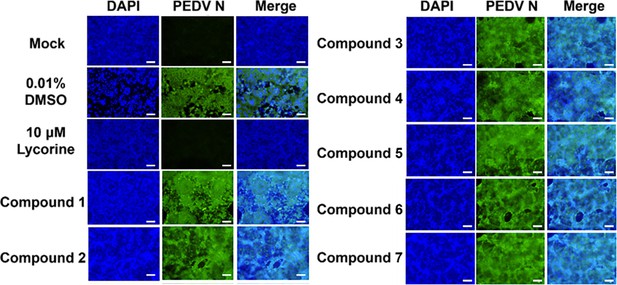
Antiviral activity of compounds interacting with the porcine epidemic diarrhea virus (PEDV) 5’UTR-SL5 in vitro.
Lycorine is a PEDV-positive inhibitor. Confocal fluorescence microphotographs of the same cells with DAPI (left), N protein of PEDV (middle) or Merge (right) were demonstrated (scale bar = 50 µm).
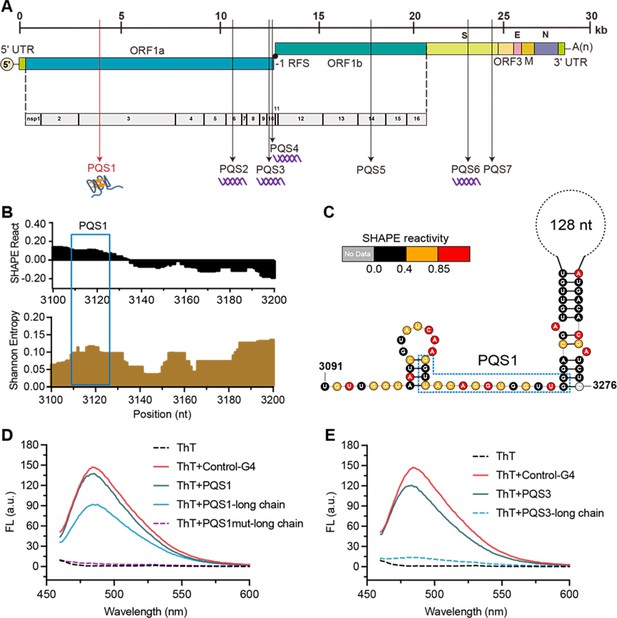
The PQS1 within high SHAPE-high Shannon regions has the potential to form G4 structure in the porcine epidemic diarrhea virus (PEDV) genome.
(A) Distribution of PQSs in the PEDV genome. (B) SHAPE reactivity and Shannon entropy for the regions containing PQS1. (C) Local secondary structure of the region containing PQS1 predicted with SHAPE reactivity constraints. PQS1 is marked with a blue dashed box. (D) Fluorescence turn-on assays of ThT (1 µM) in the presence of PQS1 (0.5 µM), PQS1-long chain (0.5 µM), and PQS1mut-long chain (0.5 µM). (E) Fluorescence turn-on assays of ThT (1 µM) in the presence of PQS3 (0.5 µM) and PQS3-long chain (0.5 µM). Excitation wavelength was 442 nm. PRRSV-G4 RNA was used as control.
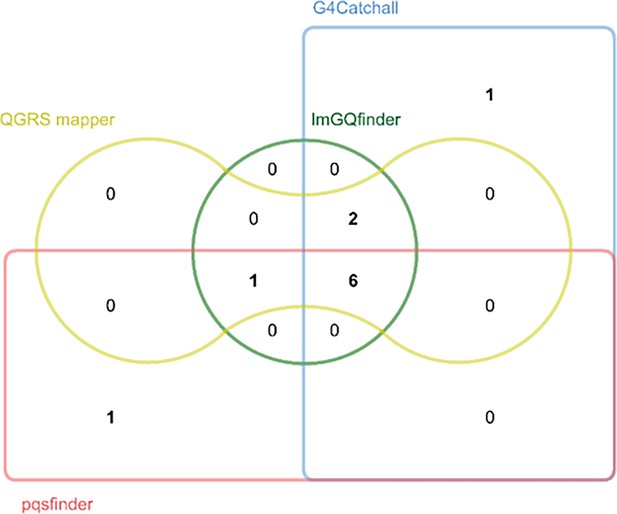
Prediction of potential G-quadruplex forming sequences (PQSs) in the porcine epidemic diarrhea virus (PEDV) genome by different prediction tools.
Edwards’ Venn diagram showing the overlap of the putative PQSs predicted by G4Catchall, QGRS mapper, ImGQfinder and pqsfinder.
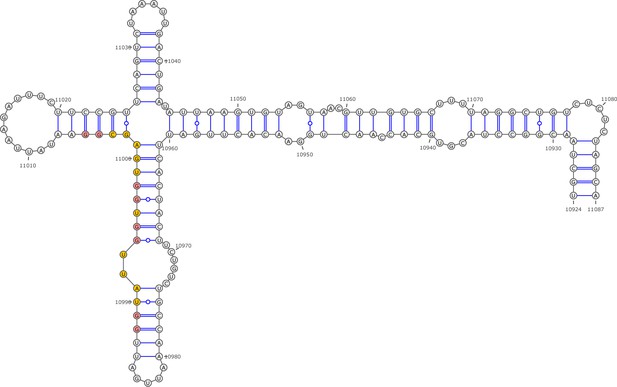
Secondary structure of the local region containing PQS2 predicted by SHAPE reactivity as a constraint.
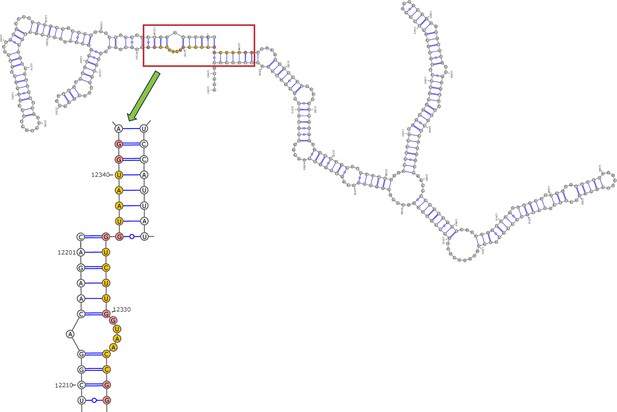
Secondary structure of the local region containing PQS3 predicted by SHAPE reactivity as a constraint.
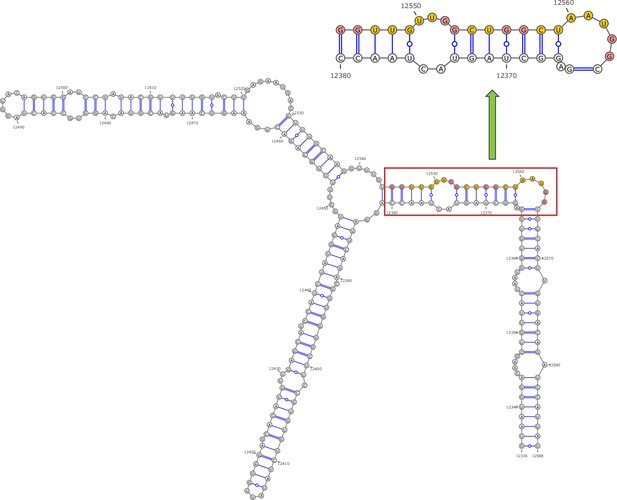
Secondary structure of the local region containing PQS4 predicted by SHAPE reactivity as a constraint.
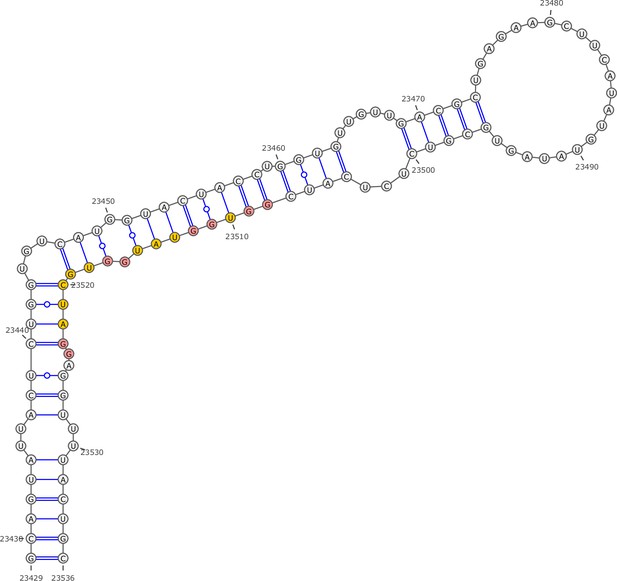
Secondary structure of the local region containing PQS5 predicted by SHAPE reactivity as a constraint.
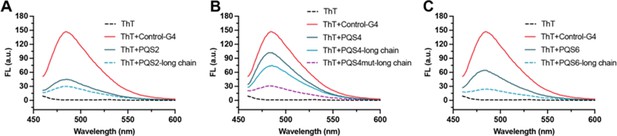
PQSs in well-folded regions are resistant to G4 formation.
(A) Fluorescence turn-on assays of ThT (1 µM) in the presence of PQS2 (0.5 µM) and PQS2-long chain (0.5 µM). (B) Fluorescence turn-on assays of ThT (1 µM) in the presence of PQS4 (0.5 µM), PQS4-long chain (0.5 µM), and PQS4mut-long chain (0.5 µM). (C) Fluorescence turn-on assays of ThT (1 µM) in the presence of PQS6 (0.5 µM) and PQS6-long chain (0.5 µM). Excitation wavelength was 442 nm. PRRSV-G4 RNA was used as control.
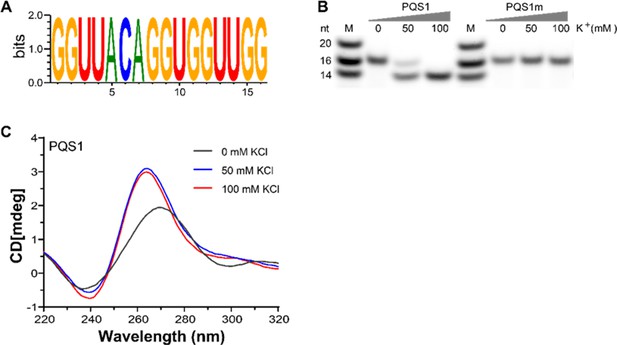
G-quadruplex structure folded by PQS1.
(A) WebLogo representation nucleotide conservation of PQS1 among different porcine epidemic diarrhea virus (PEDV) strains. The height of the letters indicates the relative frequency of occurrence of a particular nucleotide at that position. (B) Native-PAGE analysis of the migration of G4 RNAs (PQS1) and G4 mutant RNAs (PQS1m) was performed under different KCl concentrations. Lanes 1 and 5, RNA ladder; lanes 2, 3, 4, PQS1; lanes 6, 7, 8, PQS1m. (C) CD spectroscopy of PQS1 in the presence of different concentrations of KCl.
-
Figure 3—figure supplement 7—source data 1
Original native-PAGE gel for Figure 3—figure supplement 7B, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/103923/elife-103923-fig3-figsupp7-data1-v2.zip
-
Figure 3—figure supplement 7—source data 2
Original files for native-PAGE gel displayed in Figure 3—figure supplement 7B.
- https://cdn.elifesciences.org/articles/103923/elife-103923-fig3-figsupp7-data2-v2.zip
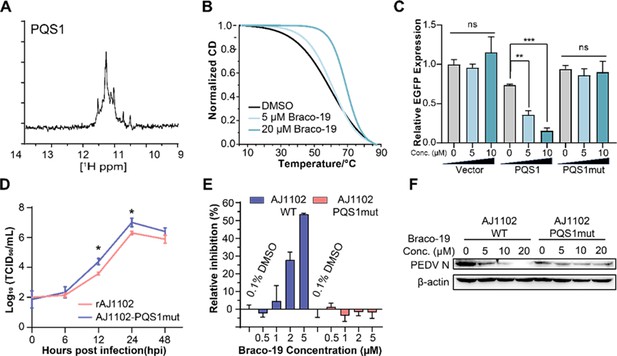
The G-quadruplex structure, biological functions of PQS1, and antiviral effects of Braco-19.
(A) 1H nuclear magnetic resonance (NMR) analysis of PQS1. (B) Circular dichroism (CD) melting profiles of PQS1. (C) Quantitative fluorescence signal using the corrected total cell fluorescence method for EGFP in cells transfected with plasmids containing the empty vector, PQS1 and PQS1mut. (D) Proliferation curve of the PEDV wild type (WT) strain and PQS1 mutant strain. (E) The relative inhibition rates of Braco-19 against AJ1102-WT and AJ1102-PQS1mut. (F) Western blot analysis of the effects of Braco-19 on the viral N protein expression of AJ1102-WT and AJ1102-PQS1mut.
-
Figure 4—source data 1
Original western blots for Figure 4F, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/103923/elife-103923-fig4-data1-v2.zip
-
Figure 4—source data 2
Original files for western blot analysis displayed in Figure 4F.
- https://cdn.elifesciences.org/articles/103923/elife-103923-fig4-data2-v2.zip
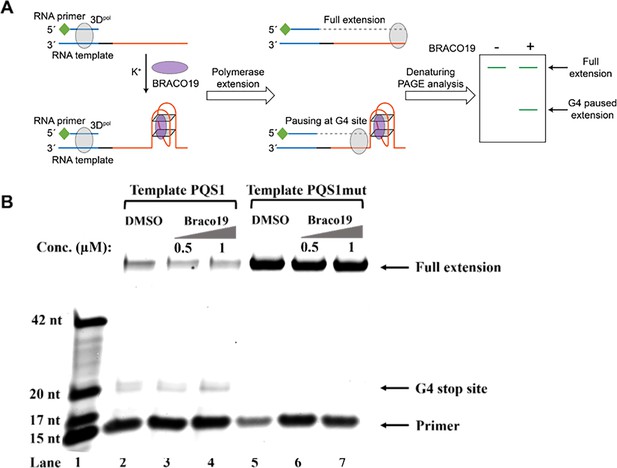
G4 structure of PQS1 inhibits RNA-dependent RNA synthesis.
(A) Schematic diagram of the RNA stop assay. The PQS1 sequence (orange part) was integrated into the 5’ end of the RNA template, and a 5’ FAM-tagged RNA primer P15 (green and blue parts of the RNA primer) was designed to target the 3’ end of the template (blue part of the RNA blue part of the template) to initiate RNA synthesis. When G4 blocks RNA synthesis, there are fewer full-length elongated bands and truncated bands at the site of G4 formation. (B) Denaturing-PAGE analysis. Black arrows indicate the positions of the full-length product, G4 paused product, and free primer. The fully extended product and the template RNA and the polymerase form a stable complex that moves much more slowly than the ssRNA. Partial full-length extended products and stopped products (due to G-quadruplex fold) were observed along template PQS1 (lane 2), but only fully extended products were observed along G4-mutated template PQS1mut (lane 5). When increasing amounts of compound Braco-19 were incubated with template PQS1, a gradual decrease in fully extended products was observed (lanes 2–4). On the contrary, the fully extended products of template PQS1mut were not affected by the addition of Braco-19, and the G4-specific termination event was not characterized (lanes 5–7). Lane 1, RNA ladder (p15, m17, m20, and m42 in Supplementary file 1); lanes 2, no Braco19 control; lanes 3 and 4, 0.5 and 1 µM compounds inhibit RNA extension; lanes 5, no Braco19 control; lanes 6 and 7, compounds do not inhibit RNA extension.
-
Figure 4—figure supplement 1—source data 1
Original denaturing-PAGE gel for Figure 4—figure supplement 1B, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/103923/elife-103923-fig4-figsupp1-data1-v2.zip
-
Figure 4—figure supplement 1—source data 2
Original files for denaturing-PAGE gel displayed in Figure 4—figure supplement 1B.
- https://cdn.elifesciences.org/articles/103923/elife-103923-fig4-figsupp1-data2-v2.zip
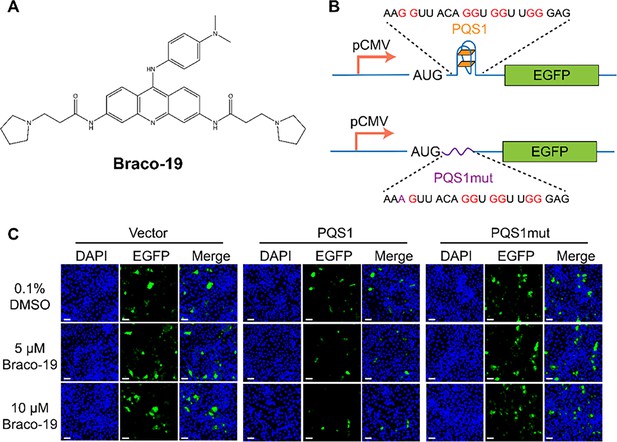
PQS1 inhibits green fluorescent protein (GFP) expression.
(A) Structure of compound Braco-19. (B) Schematic representation of the pEGFP-C1 plasmids. The empty vector carries the wild-type EGFP sequence, while the engineered plasmids contain the porcine epidemic diarrhea virus (PEDV) PQS1 sequence and the PQS1mut sequence. (C) Plasmids containing wild-type EGFP, PQS1, and PQS1mut were transfected into Vero cells, which were then treated with 0, 5 or 10 µM Braco-19 for 24 h. EGFP expression levels were measured via confocal fluorescence microscopy. Compared with DMSO treatment, the addition of Braco-19 led to a significant decrease in PQS1 EGFP fluorescence intensity. In contrast, no difference was observed in the EGFP expression of the PQS1mut vector and the empty vector. Scale bar = 50 µm.
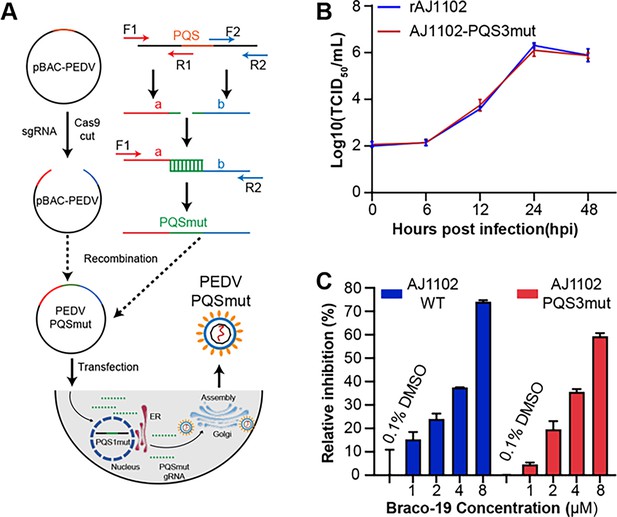
The PQS3 in the well-folded region lacks biological function.
(A) Schematic diagram of the process used to obtain the PQSs mutant recombinant strains. (B) Proliferation curve of the PQS3 mutant strain. (C) The relative inhibition rates of Braco-19 against AJ1102-WT and AJ1102-PQS3mut.
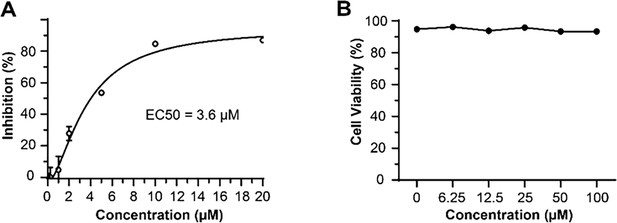
Antiviral activity and cytotoxicity of Braco-19.
(A) The median effective inhibitory concentration (EC50) of Braco-19 against PEDV AJ1102 in Vero cells and (B) the cytotoxicity of Braco-19 for Vero cells.
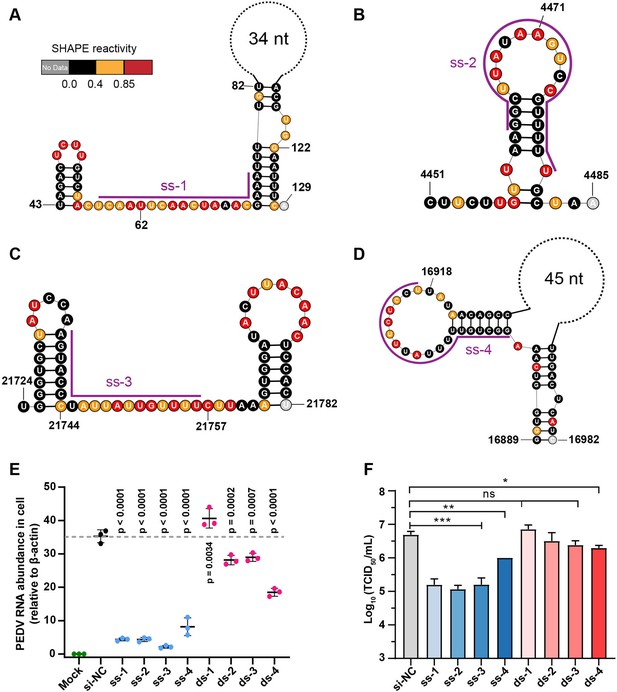
Secondary structure of target regions and antiviral effects of siRNAs.
(A–D) Local secondary structures of the stable single-stranded regions targeted by siRNAs predicted by SHAPE reactivity as a constraint; (A) ss-1; (B) ss-2; (C) ss-3; (D) ss-4. (E) qPCR showing the relative abundance of the porcine epidemic diarrhea virus (PEDV) RNA genome in infected Vero cells. The four siRNAs targeting the high SHAPE-low Shannon regions and the four siRNAs targeting the duplex regions are labeled ss-1 to ss-4 and ds-1 to ds-4, respectively. ss (single-stranded targeting siRNAs); ds (dual-stranded targeting siRNAs). si-NC was a control siRNA that did not target any viral or host sequences, and the mock group was not inoculated with virus. (F) TCID50 assays for detecting virus titers. The presented results represent the means and standard deviations of data from three independent experiments. ns: no significant difference. *p<0.05; **p<0.01; ***p<0.001, Duncan’s multiple comparison test.
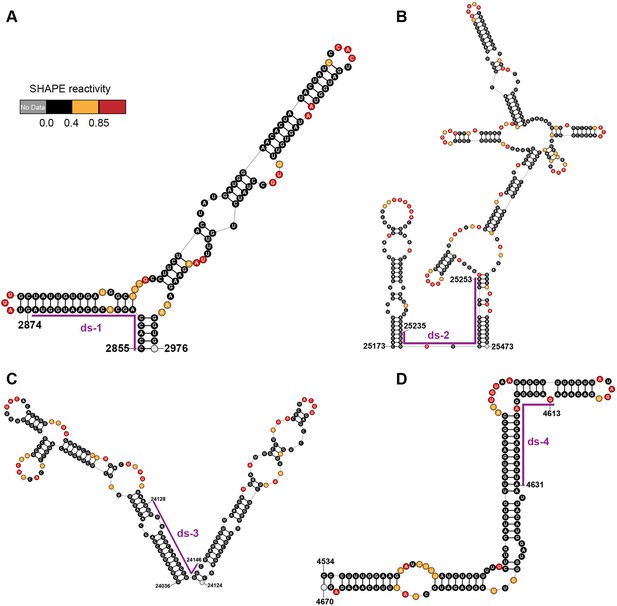
Local secondary structures of the duplex regions targeted by siRNAs.
Secondary structure of the (A) ds-1; (B) ds-2; (C) ds-3; (D) ds-4 target regions predicted by SHAPE reactivity as a constraint. Ds: dual-stranded targeting siRNAs.
Cytopathic effects (CPE) of siRNA targeting regions of duplex or single-strand on porcine epidemic diarrhea virus (PEDV)-infected Vero cells.
Scale bar = 50 µm.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/103923/elife-103923-mdarchecklist1-v2.docx
-
Supplementary file 1
Oligos used in this study.
- https://cdn.elifesciences.org/articles/103923/elife-103923-supp1-v2.docx
-
Supplementary file 2
Characteristics of regions with different SHAPE reactivity and Shannon entropy.
- https://cdn.elifesciences.org/articles/103923/elife-103923-supp2-v2.docx
-
Supplementary file 3
Location of regions with different SHAPE reactivity and Shannon entropy.
- https://cdn.elifesciences.org/articles/103923/elife-103923-supp3-v2.docx
-
Supplementary file 4
Binding affinities of Compounds with 5'UTR-SL5 in the PEDV genome.
- https://cdn.elifesciences.org/articles/103923/elife-103923-supp4-v2.docx
-
Supplementary file 5
PQSs with high conservation in the PEDV genome.
- https://cdn.elifesciences.org/articles/103923/elife-103923-supp5-v2.docx
-
Supplementary file 6
Genomic locations and structural features of PQS-long chain regions.
- https://cdn.elifesciences.org/articles/103923/elife-103923-supp6-v2.docx
-
Supplementary file 7
Sequence and characterization of target regions of siRNAs.
- https://cdn.elifesciences.org/articles/103923/elife-103923-supp7-v2.docx
-
Supplementary file 8
Structural features of anti-SARS-CoV-2 siRNA target regions.
- https://cdn.elifesciences.org/articles/103923/elife-103923-supp8-v2.docx