Trained immunity: A new player in cancer immunotherapy
Figures
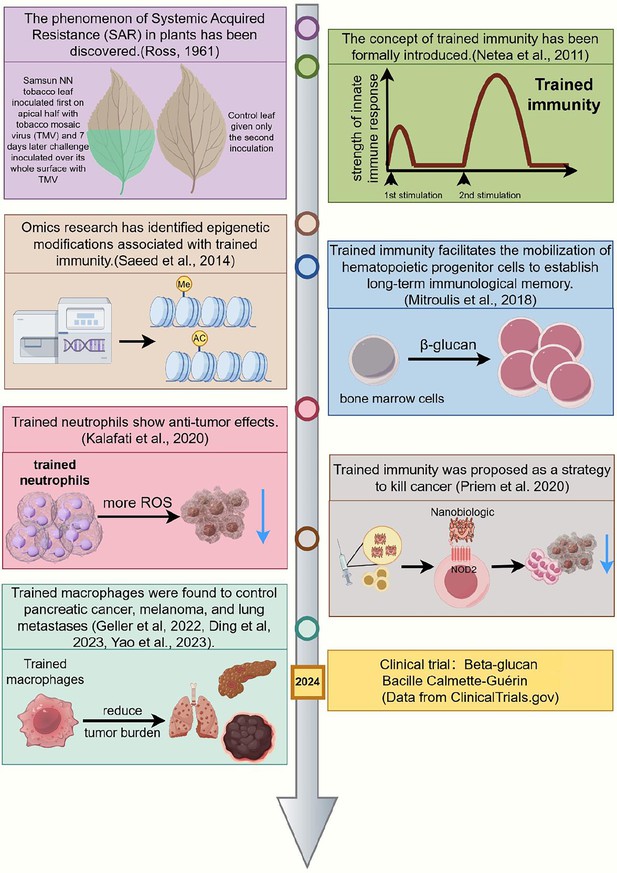
Chronology of significant milestones in research on trained immunity and cancer treatment.
This timeline highlights major developments in trained immunity from 1961 to 2024. The concept originated with the discovery of Systemic Acquired Resistance (SAR) in plants (118) and was formally introduced as ‘trained immunity’ in 2011 (Netea et al., 2011). In 2014, omics studies identified key epigenetic modifications associated with trained immunity (Saeed et al., 2014). By 2018, research demonstrated that trained immunity could mobilize hematopoietic progenitors to establish long-term innate immune memory (Mitroulis et al., 2018). In 2020, trained neutrophils were shown to exhibit antitumor effects (Kalafati et al., 2020), and trained immunity was proposed as a strategy for cancer treatment (Kalafati et al., 2020; Priem et al., 2020). Subsequently, trained macrophages were found to control pancreatic cancer, melanoma, and lung metastases (Geller et al., 2022; Wang et al., 2023; Ding et al., 2023). Most recently, in 2024, clinical trials have explored the use of β-glucan and Bacillus Calmette-Guerin (BCG) vaccines to induce trained immunity (ClinicalTrials.gov). This figure illustrates the evolution of trained immunity research and its potential applications in cancer therapy. This figure was created using FigDraw.
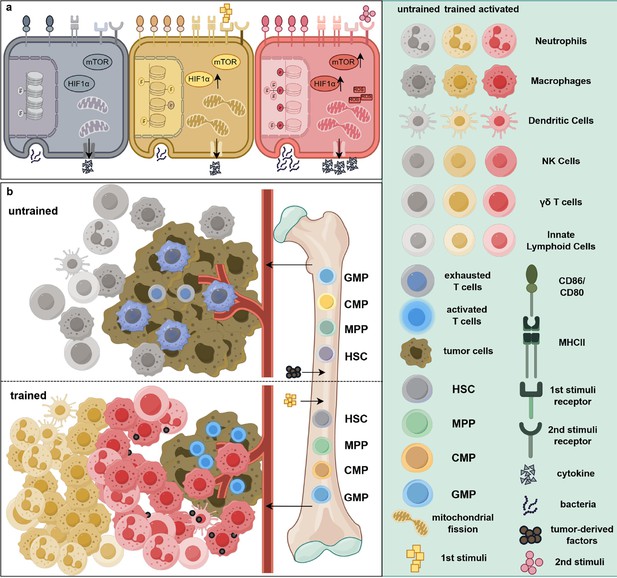
Epigenetic and metabolic reprogramming associated with trained immunity in cancer treatment.
(a) Intracellular pathway changes associated with trained immunity. Compared to resting cells (gray), immune cells exposed to an initial stimulus exhibit augmented epigenetic modifications, elevated expression levels of mTOR and HIF1α, and increased mitochondrial fission (yellow). In addition, surface markers such as CD80, CD86, and MHC II are upregulated, indicating a pre-activated state. Notably, at this stage, the cells’ ability to secrete cytokines and phagocytose bacteria remains unaltered. Upon exposure to a secondary stimulus, these cells (red) transition into a fully activated state, characterized by increased production of reactive oxygen species (ROS) and cytokines, thereby enhancing their tumoricidal efficacy. (b) The differential effects of tumor-associated factors on hematopoiesis. In the absence of trained immunity-inducing stimuli, tumor-derived factors promote the differentiation of immunosuppressive myeloid cells including neutrophils and macrophages from bone marrow progenitors. These cells infiltrate the tumor microenvironment, leading to immune cell suppression, exhaustion, or dormancy. In contrast, exposure to trained immunity-inducing agents induces myelopoiesis, facilitating the mobilization of a greater number of trained monocytes and/or neutrophils into the peripheral circulation. Consequently, more activated immune cells accumulate within and around the tumor, collectively suppressing tumor progression and metastasis. Abbreviations: CMP, common myeloid progenitors; GMP, granulocyte–macrophage progenitors; HIF1α, hypoxia-inducible factor 1 alpha; HSC, hematopoietic stem cells; mTOR, mammalian target of rapamycin; MPP, multipotent progenitors. This figure was created using FigDraw.
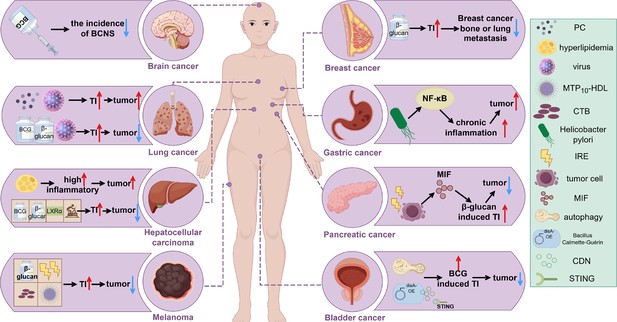
Induction of trained immunity in different tumors.
The tumor-promoting or tumor-suppressing roles of various trained immunity inducers, such as BCG and β-glucan, across different cancer types are illustrated. An upward red arrow indicates an enhanced effect or tumor-promoting activity, while a downward blue arrow denotes inhibition of tumor progression. Abbreviations: BCNS, brain and central nervous system tumors; CDN, cyclic di-nucleotide; CTB, cholera toxin B; IRE, irreversible electroporation; MIF, macrophage migration inhibitory factor; MTP10-HDL, a new nanobiologic candidate; PC, phosphatidylcholine; TI, trained immunity. This figure was created using FigDraw.
Tables
Preclinical investigations of diverse trained immunity inducers across various tumor types.
| Cancer type | Innate immune cell type | Primary stimuli | Secondary stimuli | Administration route | Ref. |
|---|---|---|---|---|---|
| Lung cancer | Alveolar macrophages | Influenza A virus | Tumor-derived factors | Intranasally (i.n.) | Wang et al., 2023 |
| Lung cancer and melanoma | Interstitial macrophages | Yeast-derived particulate β-glucan | Tumor-derived factors e.g. MIF | Intraperitoneally (i.p.) | Ding et al., 2023 |
| Lung cancer and melanoma | Neutrophils | β-glucan from Trametes versicolor | Unknown | i.p. | Kalafati et al., 2020 |
| Pancreatic cancer | Macrophages | Yeast-derived particulate β-glucan | Lipopolysaccharide (LPS) and tumor-derived factors e.g. MIF | i.p. or orally | Geller et al., 2022; Woeste et al., 2023 |
| Melanoma and bladder cancer | Monocytes | β-Glucan from S. cerevisiae | Tumor-derived factors | i.p. | Vuscan et al., 2024 |
| Melanoma | Dendritic cells (DCs) | Cholera toxin B | Cholera toxin B | Intradermally (i.d.) | Tepale-Segura et al., 2024 |
| Melanoma | Bone marrow | MTP10-HDL | LPS and tumor-derived factors | Intravenously (i.v.) | Priem et al., 2020 |
| Hepatocellular carcinoma | Unknown | Bacillus Calmette-Guérin (BCG) | LPS | Subcutaneously (s.c.) | Vaziri et al., 2024 |
| Bladder cancer | Monocytes | BCG | LPS | i.v. | Buffen et al., 2014 |
| Multiple tumors | Splenic CD11b+ cells | KK2DP7 | LPS | i.v. | Zhang et al., 2025 |
| Lung cancer | Macrophages | Macrophage membrane-camouflaged BCG | LPS | i.v. | Zhang et al., 2024 |
-
.
-
MIF: macrophage migration inhibitory factor; MTP10-HDL: a new nanobiologic candidate; KK2DP7: a dendrimer-structured peptide derived from the immunomodulatory antimicrobial peptide DP7 (VQWRIRVAVIRK).
Summary of β-glucan treatment effects in various tumor types.
| Trial type | Tumor type | β-Glucan form/ administration route | Treatment arm | Control arm | Main effects of β-glucan | Ref. |
|---|---|---|---|---|---|---|
| RCT Phase II | High-risk relapsed metastatic neuroblastoma | A gel formulation /P.O. | Ganglioside vaccine+β-glucan | Ganglioside vaccine | Enhanced seroconversion of anti-ganglioside IgG1 | Cheung et al., 2023 |
| RCT | Breast cancer | Soluble/P.O. | Chemo.+β-glucan | Chemo.+placebo | Decreased IL-4; increased IL-12; enhanced energy intake | Ostadrahimi et al., 2014 |
| RCT | Breast cancer | Soluble/P.O. | Chemo.+Lactobacillus rhamnosus strain Heriz I+β-glucan | Chemo.+placebo | Decreased IL-4 | Ostadrahimi et al., 2024 |
| SACT | Advanced breast cancer | Soluble/P.O. | β-Glucan | - | Increased peripheral monocyte count | Demir et al., 2007 |
| SACT | NSCLC | Particulate/P.O. | β-Glucan | - | Decreased MDSC | Albeituni et al., 2016 |
| RCT Phase II | Advanced NSCLC | Soluble/I.V. | Chemo.+Cetuximab+β-glucan | Chemo.+Cetuximab | Increased ORR | Thomas et al., 2017 |
| RCT | Advanced NSCLC | Soluble/I.V. | Chemo.+bevacizumab+β-glucan | Chemo.+bevacizumab | Relatively increased ORR | Liu et al., 2020 |
| CCT | Esophageal carcinoma | Soluble/I.V. | Chemo.+lentinan | Chemo. | Increased chemotherapeutic efficacy and pro-inflammatory ILs, decreased anti-inflammatory ILs | Wang et al., 2012 |
| SACT | Gastric cancer | Soluble/I.V. | Chemo.+lentinan | - | Increased quality-of-life scores | Kataoka et al., 2009 |
| SACT | Pancreatic cancer | Superfine dispersed/P.O. | Superfine dispersed lentinan | - | Increased quality-of-life scores | Kataoka et al., 2009 |
| SACT Phase II | Stage IV KRAS-mutant colorectal cancer | Soluble/I.V. | Cetuximab+β-glucan | Cetuximab | Compelling clinical activity | Segal et al., 2016 |
| RCT | ASCUS/LSIL | Soluble/TOP. | β-Glucan | No treatment | Increased disease clearance rates | Laccetta et al., 2015 |
| SACT Phase I/II | High-risk chronic lymphocytic leukemia | Soluble/I.V. | Alemtuzumab+rituximab+β-glucan | - | A high complete response rate | Zent et al., 2015 |
| RCT | Mixed | NS/P.O. | Hypercaloric diet enriched in β-glucan | Hypercaloric diet | Increased energy intake | Milla et al., 2024 |
| SACT Phase I/II | Mixed advanced | Soluble/P.O. | Chemo.+β-glucan | - | Relatively increased blood cell counts | Weitberg, 2008 |
-
RCT: randomized clinical trial; CCT: controlled clinical trial; SACT: single arm clinical trial; NSCLC: non-small cell lung cancer; ASCUS: atypical squamous cells of undetermined significance; LSIL: low-grade squamous intraepithelial lesions; NS: not specified; P.O.: Per os; I.V.: intravenous; TOP.: topical; Chemo.: chemotherapy; IL: interleukin; MDSC: myeloid-derived suppressor cells; ORR: objective response rates.





