Interleukin-4 induces CD11c+ microglia leading to amelioration of neuropathic pain in mice
Figures
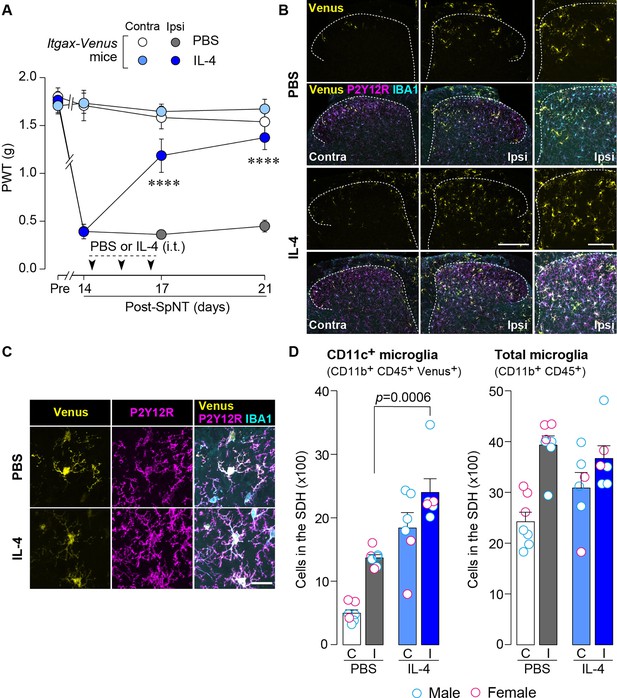
IL-4 increases CD11c+ microglia in the SDH and ameliorates pain hypersensitivity in the SpNT mice.
(A) Paw withdrawal threshold (PWT) of Itgax-Venus mice before (Pre) and after SpNT (n=7–8 mice). IL-4 or PBS was intrathecally administrated from day 14 to day 16 post-SpNT (once a day for 3 days). ****p<0.0001 versus the ipsilateral side of PBS-treated group, two-way ANOVA with post hoc Tukey multiple comparison test. (B) Venus fluorescence (yellow) and P2Y12R and IBA1 immunostaining (magenta and cyan, respectively) in the SDH of Itgax-Venus mice with SpNT 21 days after PBS or IL-4 treatment (from day 14–16). Scale bars, 200 µm (middle), 100 µm (right). (C) Colocalization of Venus, P2Y12R, and IBA1. Scale bar, 20 µm. (D) Flow cytometric quantification for the number of CD11c+ (CD11b+CD45+Venus+) and total (CD11b+CD45+) microglia in the 3–4th lumbar SDH contralateral (C) and ipsilateral (I) to SpNT (n=6–7 mice). One-way ANOVA with post hoc Tukey multiple comparison test. Data are shown as mean ± SEM.
-
Figure 1—source data 1
Raw numerical values for Figure 1 plots.
- https://cdn.elifesciences.org/articles/105087/elife-105087-fig1-data1-v1.xlsx
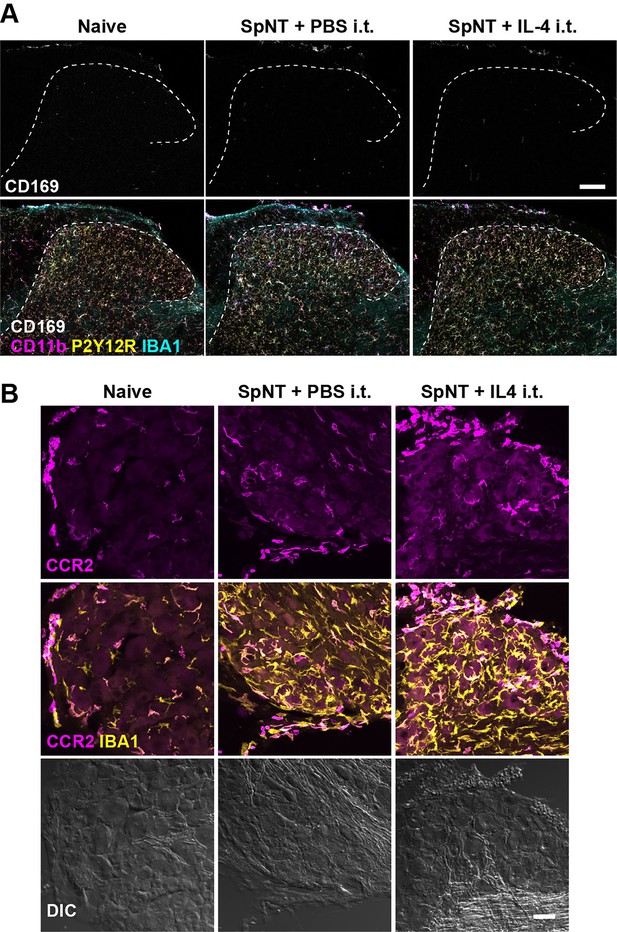
Monocytes/macrophages in the SDH and DRG after intrathecal administration of IL-4.
Immunohistochemical analysis of the SDH (A) and the DRG (B) of naive WT mice and SpNT-WT mice with intrathecal injection of PBS or IL-4 (from day 14–16 post-SpNT). Immunofluorescence of CD169, CD11b, P2Y12R, and IBA1 in the SDH (L4) ipsilateral to the SpNT (day 17) (A) and of CCR2 and IBA1 in the DRG (L4) ipsilateral to the SpNT (day 17) (B). Scale bars, 100 μm (A) and 40 μm (B).
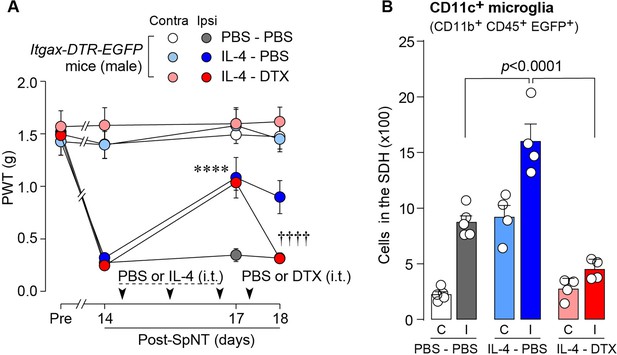
IL-4-induced remission of pain hypersensitivity requires spinal CD11c+ microglia.
(A) Paw withdrawal threshold (PWT) of Itgax-DTR-EGFP mice before (Pre) and after SpNT (n=6–7 mice). IL-4 or PBS was intrathecally administrated from days 14–16 post-SpNT (once a day for 3 days). On day 17, DTX (0.5 ng/mouse) or PBS was intrathecally injected. ****p<0.0001 versus the ipsilateral side of PBS-PBS group, ††††p<0.0001 versus the ipsilateral side of IL-4-PBS group, two-way ANOVA with post hoc Tukey multiple comparison test. (B) Flow cytometric quantification for the number of CD11c+ microglia (CD11b+CD45+EGFP+) in the 3–4th lumbar SDH contralateral (C) and ipsilateral (I) to SpNT in Itgax-DTR-EGFP mice treated with PBS/PBS, IL-4/PBS, or IL-4/DTX (n=4–5 mice). One-way ANOVA with post hoc Tukey multiple comparison test. Data are shown as mean ± SEM.
-
Figure 2—source data 1
Raw numerical values for Figure 2 plots.
- https://cdn.elifesciences.org/articles/105087/elife-105087-fig2-data1-v1.xlsx
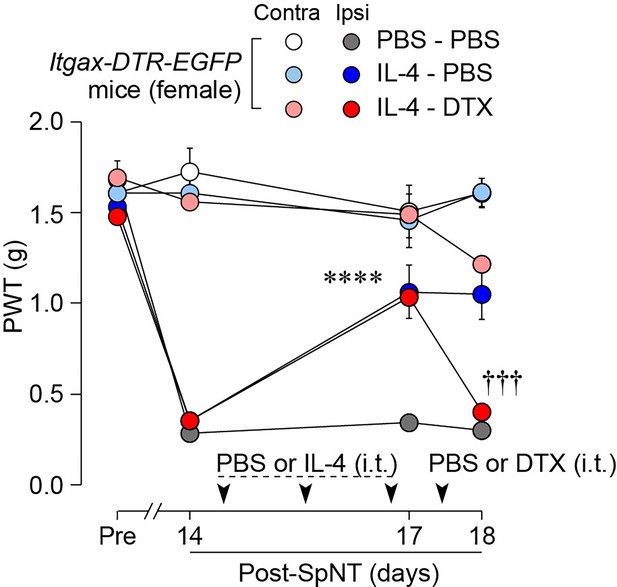
IL-4 alleviates pain hypersensitivity in female mice.
Paw withdrawal threshold (PWT) of Itgax-DTR-EGFP mice before (Pre) and after SpNT (n=6 female mice). IL-4 or PBS was intrathecally administrated from days 14–16 post-SpNT (once a day for 3 days). On day 17, DTX (0.5 ng/mouse) or PBS was intrathecally injected. ****p<0.0001 versus the ipsilateral side of PBS-PBS group, †††p<0.001 versus the ipsilateral side of IL-4-PBS group, two-way ANOVA with post hoc Tukey multiple comparison test. Data are shown as mean ± SEM.
-
Figure 3—source data 1
Raw numerical values for Figure 3 plots.
- https://cdn.elifesciences.org/articles/105087/elife-105087-fig3-data1-v1.xlsx
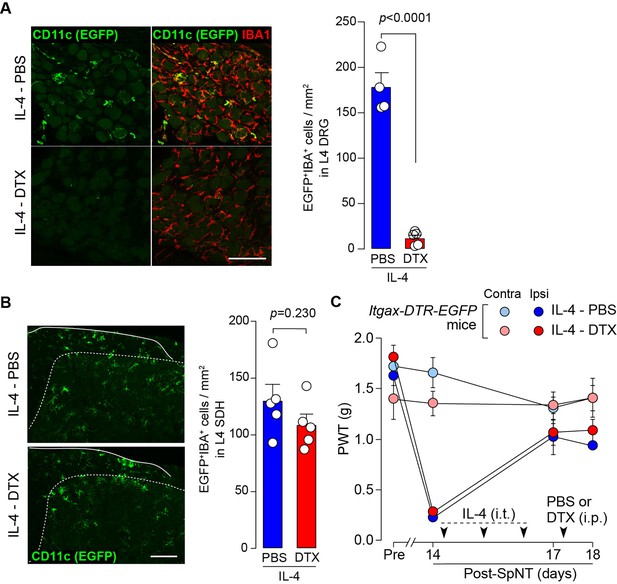
CD11c+ cells in the DRG are involved in IL-4-induced remission of pain hypersensitivity.
(A and B) Immunohistochemical analyses of CD11c+ cells (EGFP+ IBA1+) in the DRG (A) and SDH (B) on day 18 post-SpNT of Itgax-DTR-EGFP mice treated intrathecally with IL-4 (from days 14 to 16) (n=4–5 mice). DTX (2 ng/g) or PBS was intraperitoneally injected on day 17. The myeloid marker IBA1 was also stained in the SDH (B). Scale bars, 100 µm. Unpaired t-test. (C) Effect of DTX (2 ng/g) intraperitoneally injected (on day 17) on PWT of SpNT mice treated intrathecally with PBS or IL-4 (from days 14 to 16) (n=5 mice). Data are shown as mean ± SEM.
-
Figure 4—source data 1
Raw numerical values for Figure 4 plots.
- https://cdn.elifesciences.org/articles/105087/elife-105087-fig4-data1-v1.xlsx
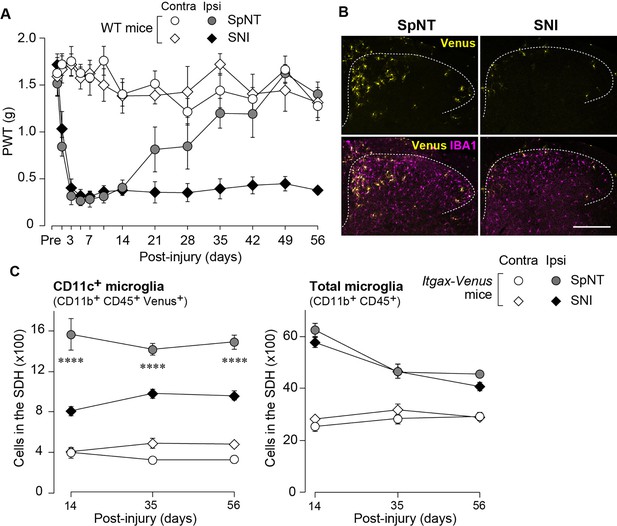
Blunted appearance of CD11c+ microglia in the SDH after SNI.
(A) PWT of wild-type (WT) mice before (Pre) and after SpNT and SNI (n=5 mice). (B) Venus (yellow) and IBA1 immunostaining (magenta) in the SDH of SpNT and SNI mice on day 14. Scale bar, 200 µm. (C) Flow cytometric quantification of the number of CD11c+ (CD11b+CD45+Venus+) and total (CD11b+CD45+) microglia in the 3–4th lumbar SDH of Itgax-Venus mice after SpNT and SNI (n=3–5 mice). ****p<0.0001 versus the ipsilateral side of the SNI group, two-way ANOVA with post hoc Tukey multiple comparison test. Data are shown as mean ± SEM.
-
Figure 5—source data 1
Raw numerical values for Figure 5 plots.
- https://cdn.elifesciences.org/articles/105087/elife-105087-fig5-data1-v1.xlsx
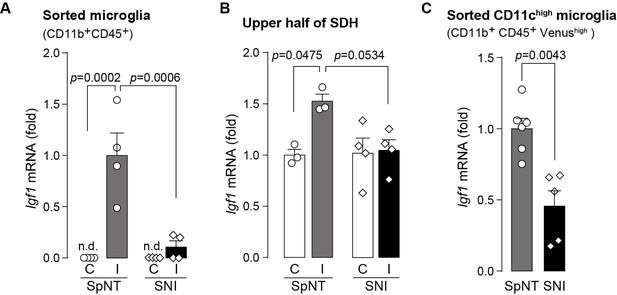
Expression of Igf1 is lower in the SNI model.
(A–C) Igf1 mRNA in total RNA extracted from sorted SDH microglia (A; n=4 mice), from tissue homogenate of the upper half of the SDH (B; n=3–4 mice), or from the sorted CD11chigh microglia (C; n=5–6 mice) in Itgax-Venus mice was quantified by qPCR on day 36 post-SpNT or SNI. Cells and tissues were collected from the contralateral (C) and ipsilateral (I) side to the SpNT or SNI. Values represent the relative ratio of Igf1 mRNA (normalized to the value for Actb mRNA) to the ipsilateral (A and C) or contralateral (B) side of SpNT mice. One-way ANOVA with post hoc Tukey multiple comparison test (A and B). Unpaired t-test (C). Data are shown as mean ± SEM.
-
Figure 6—source data 1
Raw numerical values for Figure 6 plots.
- https://cdn.elifesciences.org/articles/105087/elife-105087-fig6-data1-v1.xlsx
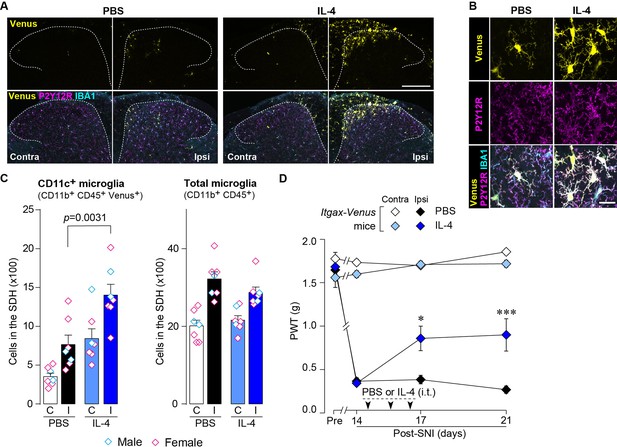
IL-4 increases CD11c+ microglia in the SDH and ameliorates pain hypersensitivity in the SNI mice.
(A) Venus fluorescence (yellow) and P2Y12R and IBA1 immunostaining (magenta and cyan, respectively) in the SDH of Itgax-Venus mice with SpNT after PBS or IL-4 treatment. Scale bar, 200 µm. (B) Colocalization of Venus, P2Y12R, and IBA1. Scale bar, 20 µm. (C) Flow cytometric quantification for the number of CD11c+ (CD11b+CD45+Venus+) and total (CD11b+CD45+) microglia in the 3–4th lumbar SDH contralateral (C) and ipsilateral (I) to SNI (n=7 mice). One-way ANOVA with post hoc Tukey multiple comparison test. (D) Paw withdrawal threshold (PWT) of Itgax-Venus mice before (Pre) and after SNI (n=6–8 mice). IL-4 or PBS was intrathecally administrated from days 14 to 16 post-SNI (once a day for 3 days). *p<0.05, ***p<0.001 versus the ipsilateral side of PBS group, two-way ANOVA with post hoc Tukey multiple comparison test. Data are shown as mean ± SEM.
-
Figure 7—source data 1
Raw numerical values for Figure 7 plots.
- https://cdn.elifesciences.org/articles/105087/elife-105087-fig7-data1-v1.xlsx
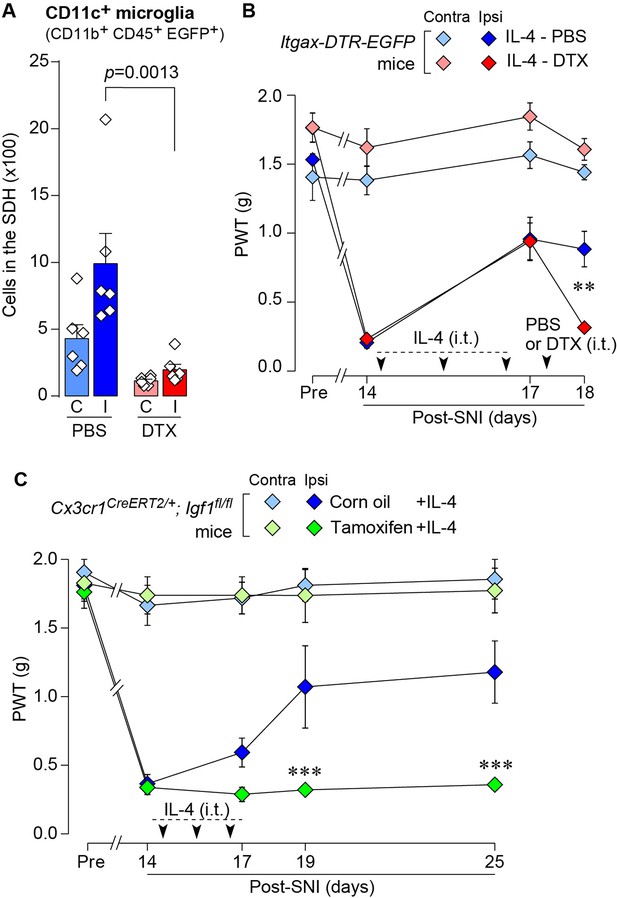
IL-4 alleviates SNI-induced pain hypersensitivity in CD11c+ microglia and IGF1-dependent manners.
(A) Flow cytometric quantification for the number of CD11c+ microglia (CD11b+CD45+EGFP+) in the 3–4th lumbar SDH contralateral (C) and ipsilateral (I) to SNI in Itgax-DTR-EGFP mice treated with IL-4/PBS or IL-4/DTX (n=6 mice). One-way ANOVA with post hoc Tukey multiple comparison test. (B) PWT of Itgax-DTR-EGFP mice before (Pre) and after SNI (n=6 mice). IL-4 was intrathecally administrated from days 14 to 16 post-SNI (once a day for 3 days). On day 17, DTX (0.5 ng/mouse) or PBS was intrathecally injected. **p<0.01 versus the ipsilateral side of the IL-4-PBS group, two-way ANOVA with post hoc Tukey multiple comparison test. (C) PWT of Cx3cr1CreERT2/+;Igf1flox/flox mice before (Pre) and after SNI (n=5–7 mice). Tamoxifen or vehicle was administered 4 weeks before SNI to induce recombination. IL-4 was intrathecally administered from days 14 to 16 post-SNI (once a day for 3 days). ***p<0.001 versus the ipsilateral side of Corn oil group, two-way ANOVA with post hoc Tukey multiple comparison test. Data are shown as mean ± SEM.
-
Figure 8—source data 1
Raw numerical values for Figure 8 plots.
- https://cdn.elifesciences.org/articles/105087/elife-105087-fig8-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (include species and sex here) | C57BL/6 J | The Jackson Laboratory Japan | NA | |
| Genetic reagent (include species here) | B6.Cg-Tg(Itgax-Venus)Mnz/J | Jackson Laboratory | RRID:IMSR_JAX:008829 | |
| Genetic reagent (include species here) | B6.FVB-1700016L21RikTg(Itgax-DTR/EGFP)57Lan/J | Jackson Laboratory | RRID:IMSR_JAX:004509 | |
| Genetic reagent (include species here) | B6.129P2(Cg)-Cx3cr1tm2.1(cre/ERT2)Litt/WganJ | Jackson Laboratory | RRID:IMSR_JAX:021160 | |
| Genetic reagent (include species here) | B6.129(FVB)-Igf1tm1Dlr/J | Jackson Laboratory | RRID:IMSR_JAX:016831 | |
| Antibody | Guinea pig anti-IBA1 | Synaptic Systems | RRID:AB_2924932 | 1:2000 |
| Antibody | Rabbit anti-P2Y12R | AnaSpec | RRID:AB_2298886 | 1:2000 |
| Antibody | Rabbit anti-GFP | MBL International | RRID:AB_591819 | 1:1000 |
| Antibody | APC anti-mouse CD169 | Biolegend | RRID:AB_2565640 | 1:500 |
| Antibody | Rabbit anti-CCR2 | Abcam | RRID:AB_2893307 | 1:500 |
| Antibody | Goat Anti-Guinea pig IgG H&L (Alexa Fluor 405) | Abcam | RRID:AB_2827755 | 1:1000 |
| Antibody | Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | RRID:AB_2576217 | 1:1000 |
| Antibody | Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 546 | Thermo Fisher Scientific | RRID:AB_2534093 | 1:1000 |
| Antibody | Rat Anti-CD16 /CD32 Monoclonal Antibody, Unconjugated, Clone 2.4G2 | BD Biosciences | RRID:AB_394657 | 1:200 |
| Antibody | Rat Anti-CD11b Monoclonal Antibody, Alexa Fluor 647 Conjugated, Clone M1/70 | BD Biosciences | RRID:AB_396796 | 1:1000 |
| Antibody | PE anti-mouse CD45 | BioLegend | RRID:AB_312971 | 1:1000 |
| Antibody | Brilliant Violet 785(TM) anti-mouse/human CD11b | BioLegend | RRID:AB_2561373 | 1:1000 |
| Antibody | APC anti-mouse CD206 (MMR) | BioLegend | RRID:AB_10900231 | 1:200 |
| Peptide, recombinant protein | Recombinant Murine IL-4 | PeproTech | 214–14 | |
| Commercial assay or kit | Myelin Removal Beads II | Miltenyi Biotec | 130-096-433 | |
| Commercial assay or kit | MACS LS column | Miltenyi Biotec | 130-042-401 | |
| Commercial assay or kit | Quick-RNA Micro-Prep kit | ZYMO | R1051 | |
| Commercial assay or kit | Trisure | Bioline | BIO-38032 | |
| Commercial assay or kit | Prime Script reverse transcriptase | Takara | 2680B | |
| Commercial assay or kit | Premix Ex Taq (Probe qPCR) | Takara | RR390B | |
| Chemical compound, drug | tamoxifen | SIGMA | T5648 | |
| Chemical compound, drug | Diphtheria Toxin Solution | Wako | 048–34371 | |
| Chemical compound, drug | collagenase D | Roche | 11088866001 | |
| Chemical compound, drug | dispase | GIBCO | 17105041 | |
| Chemical compound, drug | RNAlater | Invitrogen | AM7021 | |
| Chemical compound, drug | 7-aminoactinomycin D | Miltenyi Biotec | 170-081-088 | |
| Software, algorithm | LSM700 Imaging System | Carl Zeiss | NA | |
| Software, algorithm | CytoFLEX SRT | Beckman Coulter | NA | |
| Software, algorithm | FlowJo | BD | RRID:SCR_008520 | |
| Software, algorithm | FACSAria III | BD Biosciences | NA | |
| Software, algorithm | QuantStudio 3 | Applied Biosystems | NA | |
| Software, algorithm | Prism 7 | GraphPad | NA |





