The C-terminus of the multi-drug efflux pump EmrE prevents proton leak by gating transport
Figures
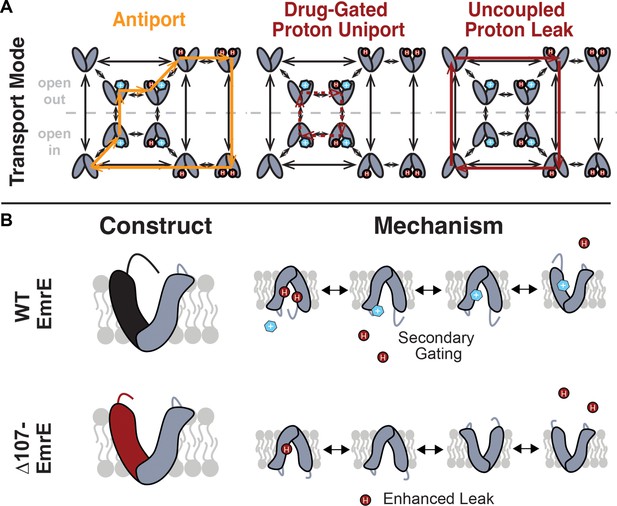
Model of coupled antiport and uncoupled proton leak through EmrE.
(A) All of the drug- and proton-bound states that are reasonably populated at near-physiological temperature and pH and the transitions between these states observed by NMR lead to a model for EmrE transport that allows for both coupled antiport (orange) and proton leak (red solid line). (B) In WT-EmrE, the C-terminal tail on the open face acts as a secondary gate (top), minimizing proton leak in the absence of substrate. Truncation of EmrE in ∆107-EmrE removes this gate (bottom). The drug binding to a secondary binding site near the tail opens the gate (top,right), allowing proton exit from the primary binding site near E14, and drug to progress to the primary binding site at E14. This leads to either coupled antiport (A, orange) as shown. If the substrate does not rapidly move into the primary binding site, only proton entry/exit occurs upon opening of the secondary gate, resulting in drug-gated proton leak (A, red dashed line). Truncation of the C-terminal tail in ∆107-EmrE (B,bottom) allows uncoupled proton leak in the absence of substrate (A, red solid line).
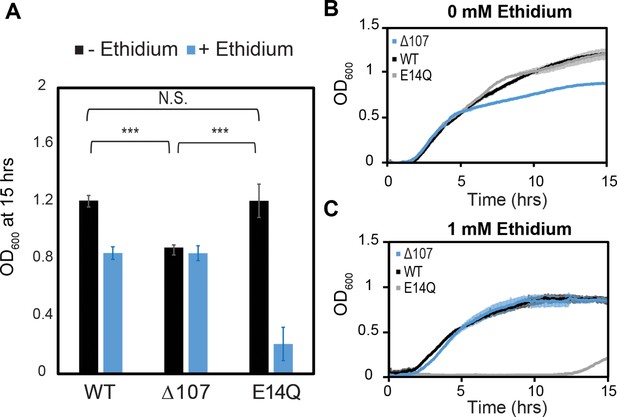
C-terminal tail truncation does not impair the ability of EmrE to confer resistance to toxic compounds.
(A–C) WT-, E14Q-, or ∆107-EmrE was heterologously expressed in MG1655-∆emre E. coli using a plasmid with p15 origin and pTrc promoter without induction to minimize any growth defect due to expression. In vivo growth assays were monitored by OD700 to allow consistent monitoring in the absence (B) or presence of (C) ethidium bromide. Growth at 15 hr. (A) shows identical growth for WT-EmrE and ∆107-EmrE in the presence of ethidium, while E14Q-EmrE is severely impaired (A, B). There is a 20% reduction in growth for ∆107-EmrE relative to WT-EmrE or non-functional EmrE (p < 0.001), but this does not prevent the mutant from transporting ethidium out of the cell and thus conferring resistance (A, C). The error bars show the standard deviation across six replicates (two biological replicates with three technical replicates each). All p-values were calculated from a two-sided t-test. ***p < 0.001.
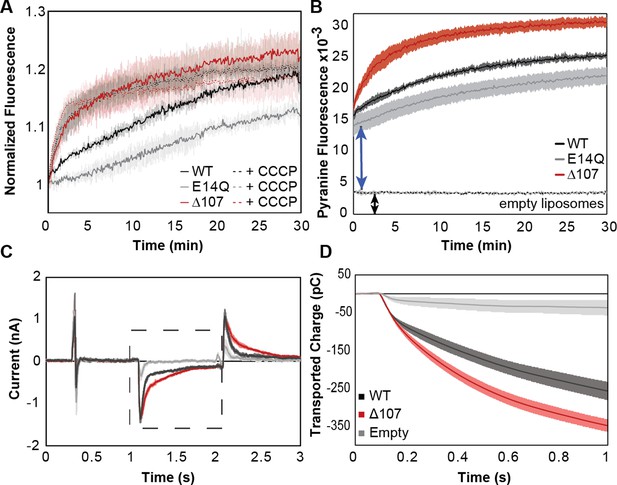
C-terminal tail truncation enhances proton leak.
(A, B) Pyranine fluorescence directly reports on proton leak through EmrE. (A) WT (black), ∆107 (red, to distinguish in vitro assays from the cellular assays of Figure 2), or E14Q-EmrE (gray) proteoliposomes with 1 mM internal pyranine and internal pH 6.5 were diluted 100-fold into pH 7.5 buffer (solid lines) or pH 7.5 buffer with CCCP (dashed lines) and fluorescence was normalized to time zero. CCCP is a protonophore, providing a positive control for maximal proton leak under these conditions. (B) Pyranine fluorescence normalized by subtracting the fluorescence of proteoliposomes diluted into pH 6.5 (no gradient, baseline) from the fluorescence of proteoliposomes diluted into pH 7.5 (transport) shows intraliposomal pH change with proteoliposomes in the lag time prior to initial fluorescence read and increased intraliposomal pH change for ∆107-EmrE than WT-EmrE or E14Q-EmrE. (C–D) Solid supported membrane electrophysiology data shows measurable charge movement through WT- and Δ107-EmrE proteoliposomes in the presence of a pH gradient alone, as compared to empty liposomes, with increased charge transport through ∆107-EmrE. (C) Current is recorded in real time as a matching pH internal buffer (pH 6.5) is flowed over the liposomes to establish baseline, then a higher pH (pH 7) buffer is flowed over the liposomes to create an outwardly directed proton gradient (dashed box), and finally, the initial buffer (pH 6.5) is flowed back over the liposomes to reverse the charge movement and return to baseline. (D) The recorded current during the period of the applied gradient (dashed box, C) is integrated to determine the transported charge during that time. In all cases, Δ107-EmrE shows increased proton leak compared to WT-EmrE and controls. The error bars show the standard deviation across three replicates or sensors.
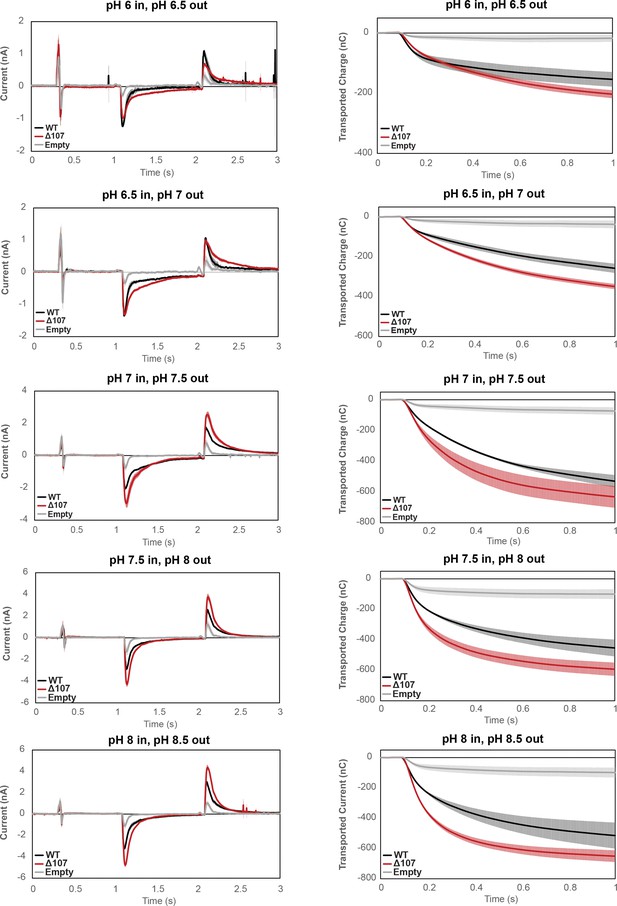
Averaged currents and integrated transport curves of WT-EmrE, ∆107-EmrE, and empty liposomes in the presence of different pH gradients.
Graphs represent the current upon formation of a pH gradient and then return to starting conditions (left) and integrated current upon formation of the pH gradient (right). The magnitude of the pH gradient is constant, but the absolute pH differs (top to bottom). Each curve is an average of three technical replicates of individually prepared sensors, and error bars represent the standard deviation from the mean. The kinetics of uncoupled proton leak between WT-EmrE and ∆107-EmrE is distinct at different absolute pH, but the overall transported charge by ∆107-EmrE is consistently higher.
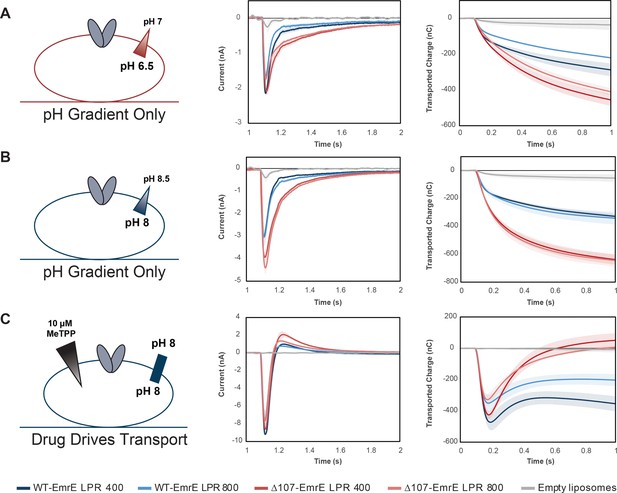
Averaged currents and integrated transport curves of WT-EmrE and ∆107-EmrE with different lipid to protein ratios (LPRs).
Comparing more than one LPR will alter the kinetics, but not the thermodynamics of transport. As such, comparing the peak currents for more than one LPR for a given set of gradient conditions allows us to ensure the signal is dominated by steady state transport rather than pre-steady state electrogenic partial reactions, such as substrate binding or proton release from the transporter. (A) The peak currents for WT-EmrE with different LPR do not match, and the peak current for ∆107-EmrE with different LPR do not match, indicating contributions from a pre-steady state process (left). Instead, the LPR 400 data for WT- and ∆107-EmrE peak currents match, and the LPR800 data for WT- and ∆107-EmrE match, suggesting that release of protons from the transporter from E14 when the external buffer is switched dominates the peak current in this pH range. No substrate is present and H110 is only present in one construct, so substrate binding or proton release from H110 are unlikely to contribute significantly to this signal. However, the slower kinetics of the off-rates for these currents are not consistent with a strictly pre-steady state process, indicating transport of protons, or leak, is also occurring for both constructs consistent with the initial jump in fluorescence followed by a slower leak over time that was seen in the pyranine assay. The similarity of the integrated currents (transported charge over time, right) for the different LPRs of each construct and the consistently higher signal in ∆107-EmrE across LPRs further signifies the predominance of uncoupled proton leak upon deletion of the C-terminal tail. If pre-steady state signals were dominant, we would expect WT-EmrE to have a higher signal due to the contribution of an additional titratable residue (H110). (B) With the same magnitude gradient at high pH, the transporters will be mostly de-protonated, such that we do not see peak currents that are dependent on the density of protein as in A. Instead, the peak currents and off-rates are dependent on the presence or absence of the tail (left), and the transported charge over time is the same for both LPRs of each construct (right). (C) In the absence of a pH gradient, addition of an infinite drug gradient to the outside of the liposomes containing de-protonated transporters reveals how coupled transport impacts the observed current. The infinite inward-directed drug gradient causes rapid coupled antiport of two protons out of the liposome for every one MeTPP+ molecule transported in (initial negative peak current). This creates both a negative inside potential that inhibits further transport and increases the external proton concentration. The resulting inward-directed proton gradient and negative-inside potential drive back transport of protons into the liposome (slower phase, positive current). This positive current is greater for Δ107-EmrE than for WT-EmrE, with the net transported charge returning to near baseline for Δ107-EmrE. There is only limited proton backflow through WT-EmrE, which maintains tighter coupling due to the C-terminal tail. Each curve is an average of at least three technical replicates of individually prepared sensors, and error bars represent the standard deviation from the mean.
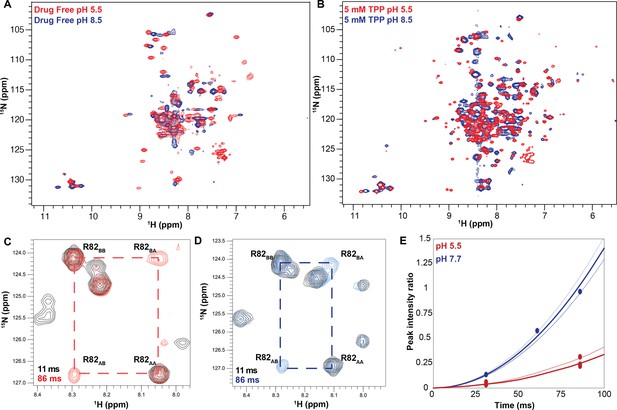
The pH dependence of alternating access in ∆107-EmrE is distinct from WT-EmrE.
TROSY-HSQC spectra of ∆107-EmrE in the absence (A) and presence (B) of the tight-binding ligand tetraphenylphosphonium (TPP+). While drug binding slows the dynamics of the protein at both low (red) and high (blue) pH, as evident by the better spectral quality in B, in both drug-free and drug-bound ∆107-EmrE, the dynamics of the mutant are highly sensitive to the pH conditions. ZZ-exchange spectroscopy of ∆107-EmrE bound to TPP+ was used to quantify the alternating-access rates at low and high pH. ZZ-exchange spectra with the indicated delays are shown for (C) pH 5.5 and (D) pH 7.7. (E) The composite peak intensity ratios for F78, G80, R82, L83, and R106 fit to an exchange rate of 4 ± 1 s–1 at pH 5.5. At pH 7.7, the composite peak intensity ratios for G80, R82, L83, and R106 fit to an exchange rate of 17 ± 3 s–1.
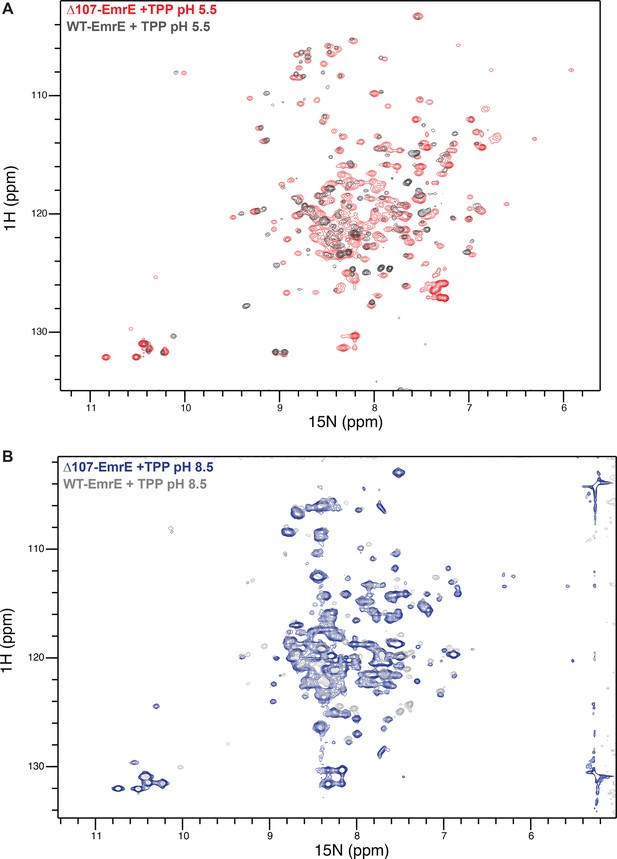
Overlay of drug-bound WT- and ∆107-EmrE at low and high pH.
1H-15N TROSY-HSQC spectra of WT- and ∆107-EmrE at pH 5.5 (A) and pH 8.5 (B) are highly similar, suggesting the C-terminally truncated ∆107-EmrE mutant is properly folded and has an intact binding site.
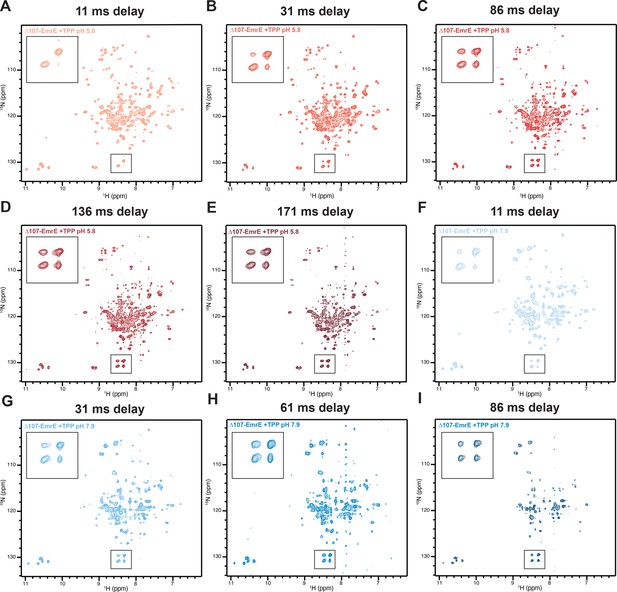
Full ZZ-exchange TROSY-HSQC spectra of the low and high pH ∆107-EmrE with TPP+.
Spectra shown were collected with the indicated delay time (A-I, header) between recording the 15N and 1H chemical shifts. The indicated boxes were enlarged to highlight the increasing intensity of the cross-peaks compared to the auto-peaks of R106, the new C-terminus of the truncated construct.
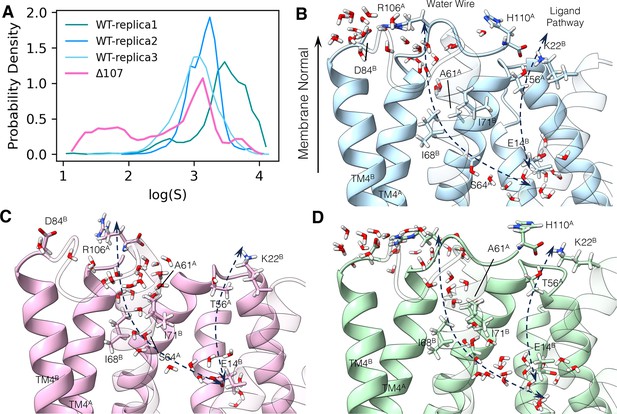
The C-terminus tail caps the water wire from the open side.
(A) Logarithm of the minimum water distance log(S) histogram. (B–D) The following panels illustrate a few snapshots in the simulation. The membrane normal vector points to the open side of EmrE. Two dashed arrows show the ligand pathway and the water chain, respectively. TM1 to TM3 in subunit B is shown transparently to better illustrate the interface between the two subunits. (B) Dry snapshot of WT-EmrE. (C) Wet snapshot of ∆107-EmrE. (D) A rare event snapshot when WT-EmrE is hydrated. The color codes are the same as in Figure 6. Yellow stars highlight the backbone of the C-terminal residue (R106 or H110), and the yellow arrowhead (B, D) highlights the backbone of R106 in the full-length construct to illustrate where the tail would terminate in ∆107.
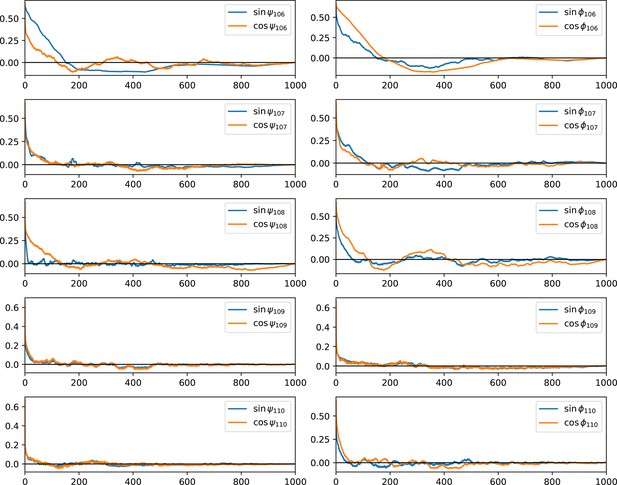
Autocorrelation function of dihedral angles of the tail.
The normalized autocorrelation function of psi- and phi-dihedral angle of the C-terminal tail backbone was plotted against time (unit: ns), averaged over three replicas. This shows the equilibration of the tail is fast except for residue 106 because of a potential salt bridge (with D84) and its proximity to helix 4, which has a stable secondary structure and a rigid backbone. The fast equilibration allows us to conclude the sampling of the tail structure is close to ergodic.
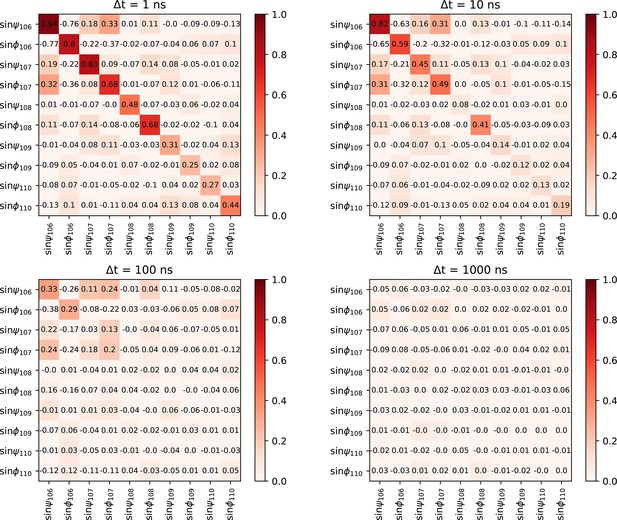
Cross-correlation function of dihedral angles of the tail at four given lag times.
The normalized cross-correlation function of psi- and phi-dihedral angle of the C-terminal tail backbone at four different timescales. Cross-correlation is minimal except for very close angles. The fast equilibration allows us to conclude the sampling of the tail structure is close to ergodic.
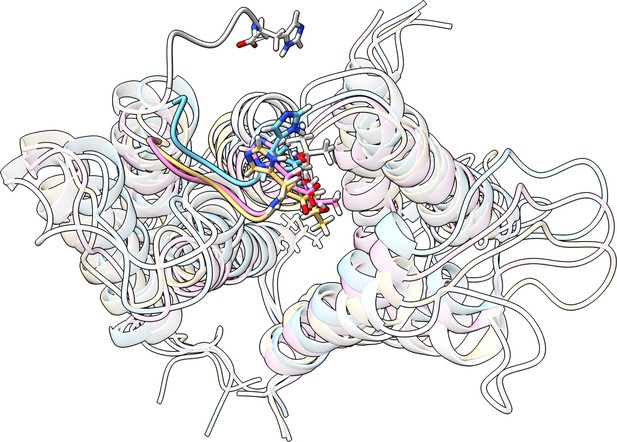
Overlay of initial and final structures from three replicates.
The starting structure is shown in white, and the last frame of three different replicas is shown in colors. In all three replicas, the tail folds onto the protein surface, supporting the robustness of this conformational preference.
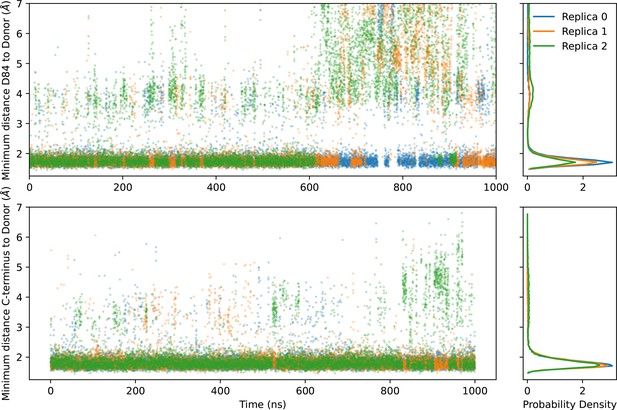
Hydrogen bonding of D84 and the C-terminal tail.
The hydrogen bonding of D84 and the carboxylate group of the C-terminal can be characterized by the distances between these oxygens and their nearest donor hydrogen. The left two panels show the time series of these distances, and the right two panels show the probability density. The donor for the C-terminus is the side chain of T56. The donor for D84 is primarily R106, but there is a small probability density of hydrogen bonding to S105 near 4 Å.
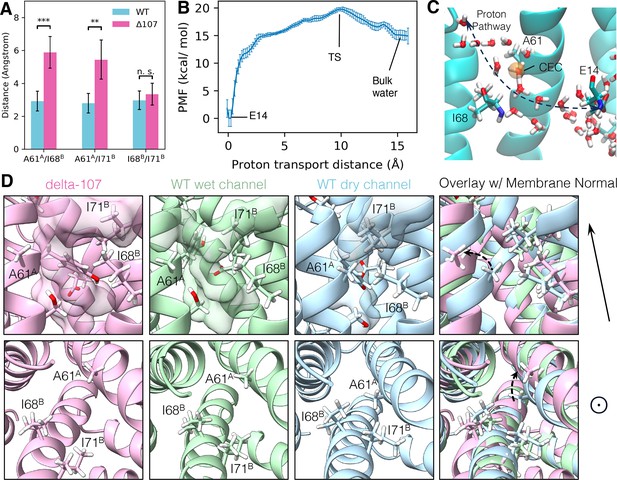
The structural basis of C-terminal gating.
(A) The minimum distance between side chain hydrogens for A61A, I68B, and I71B. In WT-EmrE, the side chain of A61A is significantly closer to I68B and I71B, while the distance between I68B and I71B does not change significantly. The error bars show the standard deviation along the trajectory. All p-values were calculated from a two-sided t-test, **p < 0.01, ***p < 0.001. (B) The proton transport potential of mean force (PMF), as a function of the distance between the center of the excess charge (CEC) and the donor (E14) on the direction of transport (see Equation 2 in Methods). Error bars show standard deviation from a block analysis of 5 blocks. (C) A snapshot of the transition state. The orange sphere is the proton CEC. (D) Conformations of the A61A, I68B, I71B triad from two different angles. The membrane normal shown at the right points to the open side of EmrE. The upper panels are from a side view, and the lower panels are looking top–down into the primary binding site from the open side. The transparent surface in the upper panels shows the water wire. Unlabeled residues shown as stick representation are E14B, Y60A, and S64A.
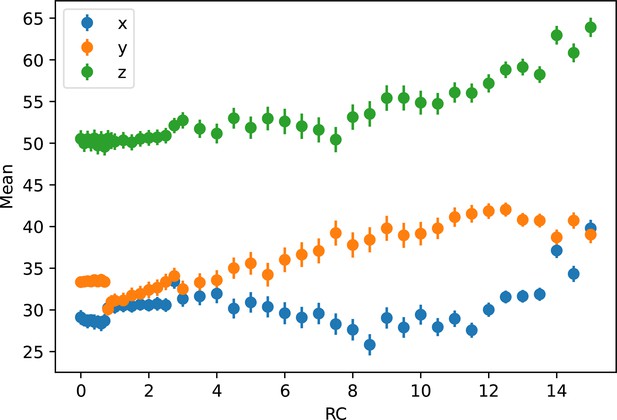
Average Cartesian coordinates of umbrella windows.
The Cartesian coordinates of each umbrella window by reaction coordinate (RC), along with the standard deviation, are plotted. This shows the continuity of the motion of the center of excess charge (CEC) when biasing the projected collective variable (CV). The jump at x ~ 1 Å corresponds to an E14 sidechain rotation event.
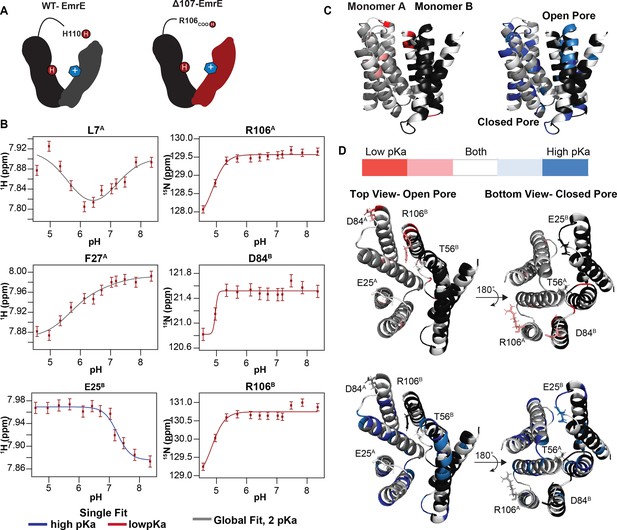
pH titration of TPP+-bound ∆107-EmrE supports the possibility of secondary gating.
(A) In WT-EmrE bound to TPP+, one E14 residue and one H110 residue are the only titratable sites (dark red circles labeled H+). In ∆107-EmrE, H110 is not present, suggesting that only one titratable group should remain (E14). (B) The proton and nitrogen chemical shifts (error bars reflect spectral resolution) for individual residues of TPP+-bound ∆107-EmrE were recorded as a function of pH. The resulting titration profiles do not show the expected single-pKa pattern. Some are curved, consistent with multiple pKa values, and others are consistent with a single pKa but at either high or low pH. All of the data can be globally fit to two pKa values, using either a 2-pKa fit (5.6 and 7.1, gray) or single pKa fit at the relevant value (5.6, red; 7.1, blue). (C) Residues sensitive to each pKa value are plotted on the faRM model (Vermaas et al., 2018) using the indicated color scale. (D) Residues strongly sensing the lower pKa value cluster around the C-terminus (R106) and 3–4 loop (includes residue D84) on both the open and closed face of the transporter, while the 1–2 loop (includes residue E25) and T56 on the open side of the pore sense both pKa values (left).
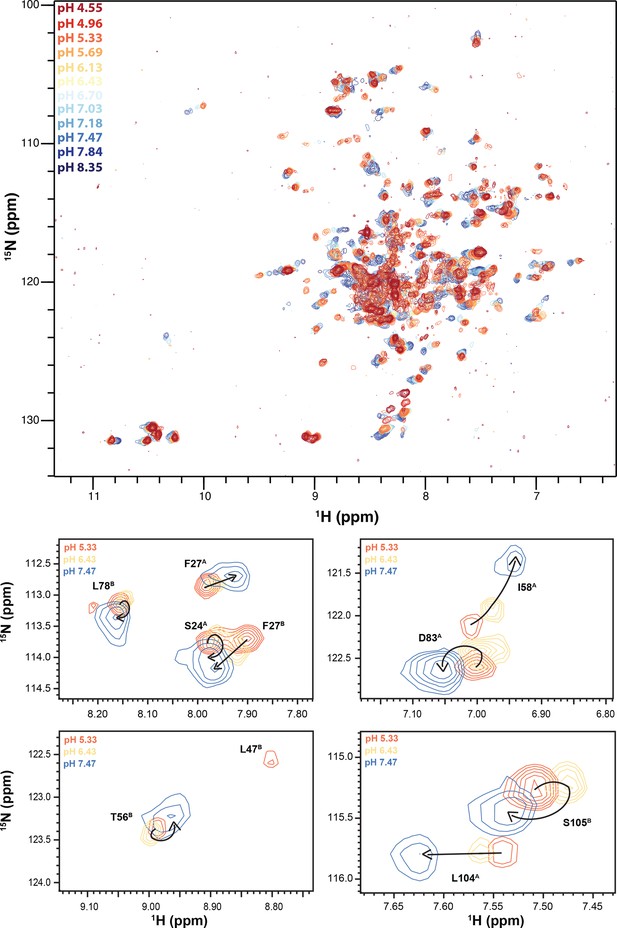
NMR pH titration of TPP+-bound ∆107-EmrE.
The pH titration of TPP+-bound ∆107-EmrE in isotropic bicelles was performed at 45°C and individual spectra were collected by mixing portions of a high and low pH sample in equivalent buffers to obtain the given pH and collecting 1H-15N TROSY HSQCs. While it appears the majority of titrating peaks are moving along a straight line, indicative of one protonation event, peaks can be seen that display curved titration paths and large jumps between pH points.
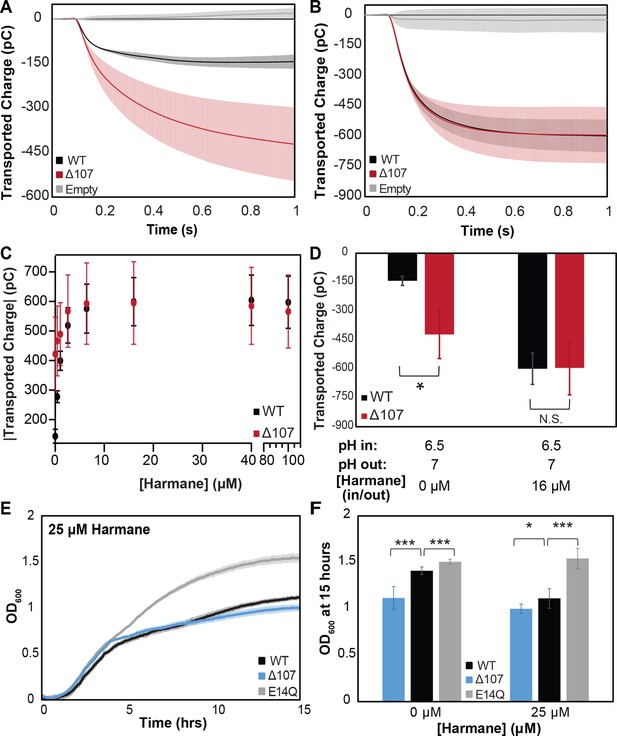
Intrinsic leak in ∆107-EmrE does not synergize with harmane-induced leak.
Solid supported membrane electrophysiology (SSME) traces of transported charge corresponding to proton leak in the absence (A) and presence (B) of harmane show that 16 µm harmane induces leak in WT-EmrE that is comparable to the leak observed through ∆107-EmrE in the absence of harmane. In the presence of increasing concentrations of harmane (C) the leak signal for WT-EmrE quickly converges to that of ∆107-EmrE. The leak observed for ∆107-EmrE is more variable, displaying larger standard deviations than WT-EmrE proteoliposomes (C) This could be due to greater variability in the unregulated transport activity of ∆107-EmrE compared to harmane-gated leak in WT-EmrE, and the impact of this unregulated behavior on the sensitivity of SSME to variation in the absolute number of proteoliposomes adsorbed on the surface sensor. (D) Bar graph of uncoupled proton leak through WT- and ∆107-EmrE proteoliposomes is significant (*) in the absence of drug, but these differences are abolished upon additon addition of 16 µM harmane. Growth assays in the absence of substrate (Figure 1A) show a clear growth defect for E. coli expressing ∆107-EmrE compared to WT-EmrE, which is nearly eliminated when cells are grown in the presence of 25 µM harmane (E, F). ∆107-EmrE data is shown in blue for cellular assays and red for in vitro assays to readily distinguish the assay type. The error bars show the standard deviation across three sensors for SSME or across six replicates for growth assays (two biological replicates with three technical replicates each). All p-values were calculated from a two-sided t-test, *p < 0.05, **p < 0.01, ***p < 0.001.
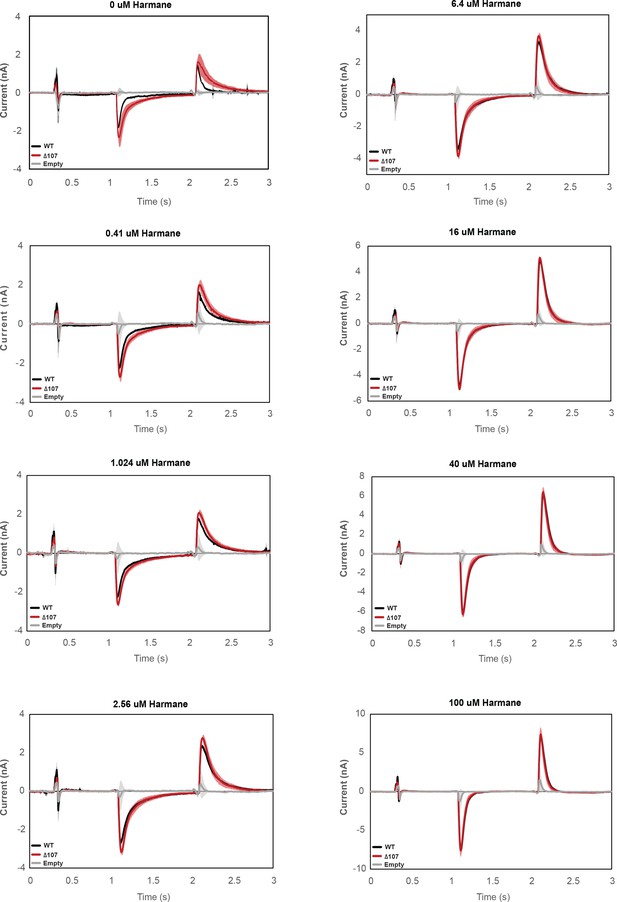
Averaged currents of WT-EmrE, Δ107-EmrE, and empty liposomes in the presence of different concentrations of harmane.
Graphs represent the current under a constant pH (pH 6.5 inside vs pH 7 outside) in the presence of varying harmane concentrations. Each curve is an average of three technical replicates of individually prepared sensors, and error bars are the standard deviation from the mean. The initial difference in the amount of uncoupled proton leak between WT-EmrE and Δ107-EmrE is abolished in the presence of higher concentrations of harmane.
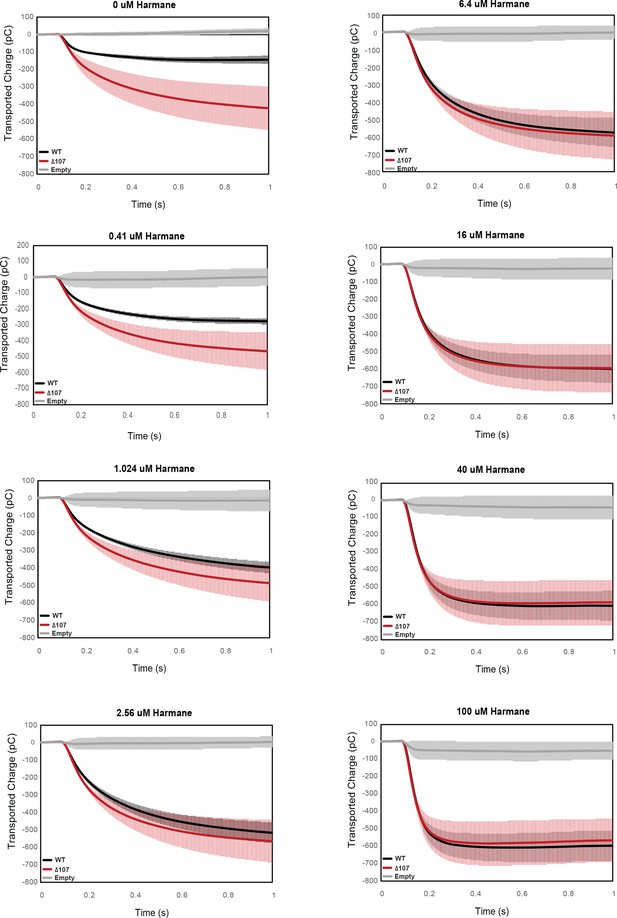
Integrated transport curves of WT-EmrE, Δ107-EmrE, and empty liposomes in the presence of different concentrations of harmane.
Graphs represent the transported charge under a constant pH (pH 6.5 inside vs pH 7 outside) in the presence of varying harmane concentrations. Each curve is an average of three technical replicates of individually prepared sensors, and error bars are the standard deviation from the mean. The initial difference in the amount of uncoupled proton leak between WT-EmrE and Δ107-EmrE is abolished in the presence of higher concentrations of harmane.
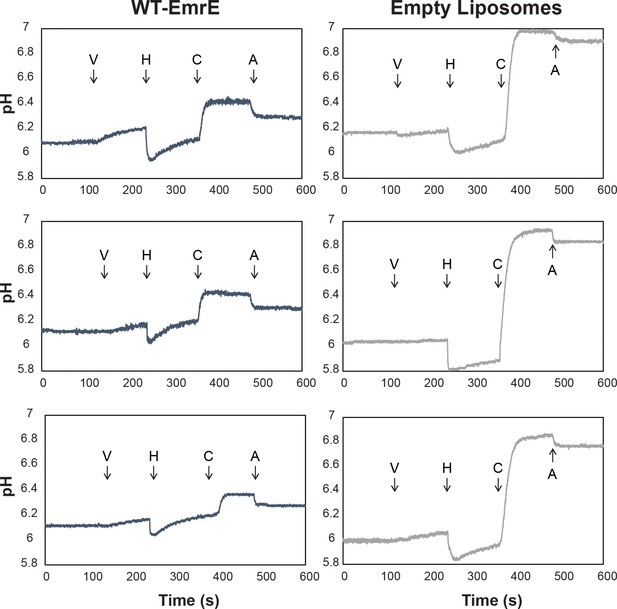
pH detected liposomal leak assay shows harmane dissipates ΔpH in an EmrE-dependent manner.
Proton-tight proteoliposomes and empty liposomes with a 3:1 ratio of POPC:POPG were prepared in a pH 7 internal buffer (50 mM MOPS, 100 mM KCl) and buffer exchanged into a pH 6 external buffer (50 μM MES, 1 mM KCl, and 99 mM NaCl) such that a ΔpH and Δ are present. The basal pH-dependent leak of WT-EmrE leads to a difference of ~0.1 pH units for WT-EmrE containing proteoliposomes relative to empty liposomes in the time it takes to buffer exchange and commence the measurement. However, the flat line prior to ionophore addition reveals this leak is slow on the timescale of the assay. Addition of valinomycin (V) allows a small number of protons to be exchanged across the membrane for both empty liposomes and proteoliposomes. Addition of harmane (H) lowers the pH of the weakly buffered solution external to the liposomes, then induces an EmrE-dependent, logarithmic leak in proteoliposomes that is distinct from the slow, linear leak present in empty liposomes. This leak dissipates the ΔpH across the proteoliposomes to a much greater extent than for the empty liposomes, such that addition of the ionophore CCCP (C) has a greatly reduced effect. Finally, a set amount of hydrochloric acid (A) is added for scale.
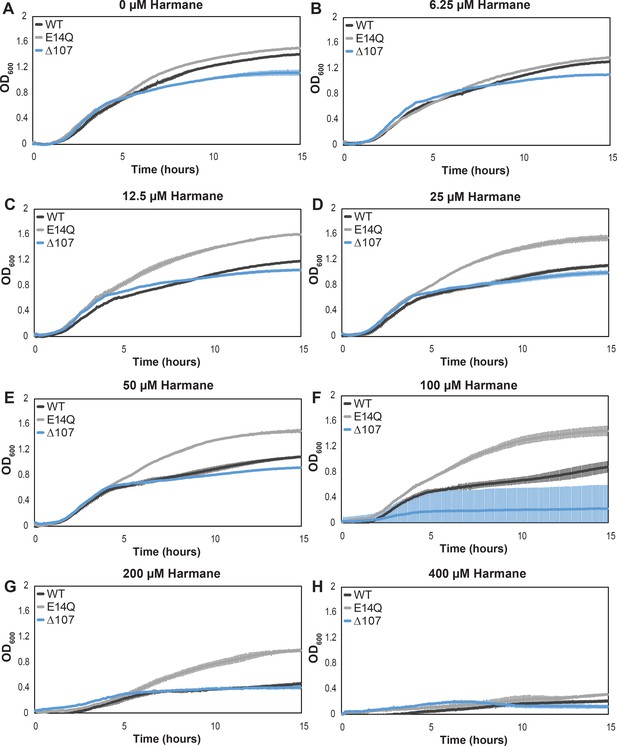
Growth assays show a differential impact of harmane on the growth of E. coli expressing WT-, E14Q-, or Δ107-EmrE.
Graphs represent the average growth in the presence of varying harmane concentrations as measured by OD600. Each curve is representative of an average of six technical replicates stemming from two biological replicates, and error bars are the standard deviation from the mean. Again, the growth defect in Δ107-EmrE expressing cells is evident in (A), and while the E14Q-EmrE expressing cells do not appear to have grown as well as is typical in (B), overall, the convergence of WT and Δ107-EmrE expressing cells as harmane concentration increases can be seen (C–E) before all cells begin to die due to a non-specific toxicity (F–H).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Escherichia coli) | EmrE | GenBank | Z11877 | |
| Strain, strain background (Escherichia coli) | BL21 Gold(DE3) | Agilent Technologies | 230312 | Competent cells |
| Strain, strain background (Escherichia coli) | MG1655-∆emre | Creative Biogen | Deletion of emre from K12 E. coli strain MG1655 | |
| Recombinant DNA reagent | pWB-EmrE (plasmid) | J. Spreacker, et al., Activating alternative transport modes in a multidrug resistance efflux pump to confer chemical susceptibility. Nat Commun 13, 7655 (2022). | Insertion of gene for EmrE or EmrE mutants (E14Q, ∆107) | |
| Recombinant DNA reagent | pET15b-EmrE (plasmid) | Novagen vector pET15b | Insertion of gene for EmrE or EmrE mutants (E14Q, ∆107) | |
| Peptide, recombinant protein | Thrombin (human) | Millipore Sigma | Cat #T7572 | |
| Other | 8-Hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (pyranine) | Millipore Sigma | CAS 6358-69-6 | pH-sensitive dye |
| Chemical compound, drug | Harmane | Millipore Sigma | CAS 486-84-0 | |
| Other | n-Decyl-b-D-maltopyranoside (decylmaltoside, DM) | Anatrace | Cat #D322 | Detergent |
| Other | 1-Palmitoyl-2-oleoyl-glycero-3- phosphocholine (POPC) | Avanti Polar Lipids | Cat #850457 | Lipid |
| Other | 1-Palmitoyl-2-oleoyl-glycero-3- phosphoglycerol (POPG) | Avanti Polar Lipids | Cat #840457 | Lipid |
| Other | 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) | Avanti Polar Lipids | Cat #850345 | Lipid |
| Software, algorithm | RAPTOR | http://github.com/uchicago-voth/raptor | commit f17fcc7 |





