Heat stress induces phage tolerance in Enterobacteriaceae
Figures
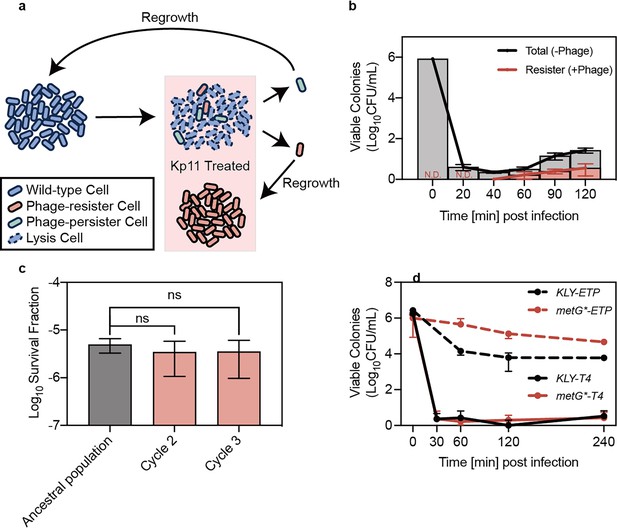
Bacterial persistence against phage infection.
(a) Schematic illustration of the experiments demonstrating the existence of phage persisters. (b) Viable colonies of K. pneumoniae ATCC 43816 at indicated time points post-infection with phage Kp11 (multiplicity of infection, MOI ~100), grown on both phage-containing (+phage) and phage-free (-phage) plates. Curves represent the mean ± standard deviation (s.d.) of three biological replicates. (c) Survival fraction after 40 min of phage Kp11 treatment for the ancestral strain ATCC 43816 and descendants of persisters. Data are presented as mean ± s.d. of three biological replicates; ns indicates not significant (p=0.6131 and 0.6415). (d) Viable colonies of KLY and its high-persistence derivative strain metG* that survived treatment with the antibiotic ertapenem (5 µg/mL, 20×MIC) and phage T4 infection (MOI ~100). Curves show the mean ± s.d. of three biological replicates.
-
Figure 1—source data 1
Data used for graphs presented in Figure 1b–d.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig1-data1-v1.xlsx
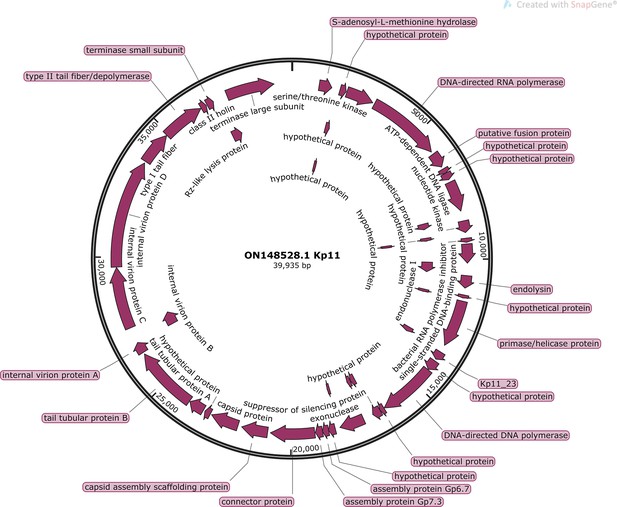
Complete genome of lytic phage Kp11 against Klebsiella Pneumoniae (GenBank: ON148528.1).
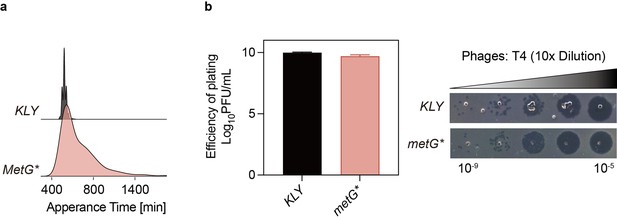
Susceptibility to phage T4 is similar for KLY and longer lag-time strain metG*.
(a) The distribution of appearance times of colonies for E. coli strain KLY and metG* was measured using ScanLag (refer to Materials and methods). The y-axis represents the normalized proportion of colony-forming units (CFUs) detected at each time point. (b) The relative minimum phage titer of KLY and metG* for T4 test and small drop assay phenotypes. Data are presented as the mean ± s.d. from three independent biological replicates. This data corresponds to the results discussed in Figure 1 of the main text, showing increased lag time does not confer protection against phage infection.
-
Figure 1—figure supplement 2—source data 1
Data used for graphs presented in Figure 1—figure supplement 2a and b.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig1-figsupp2-data1-v1.xlsx
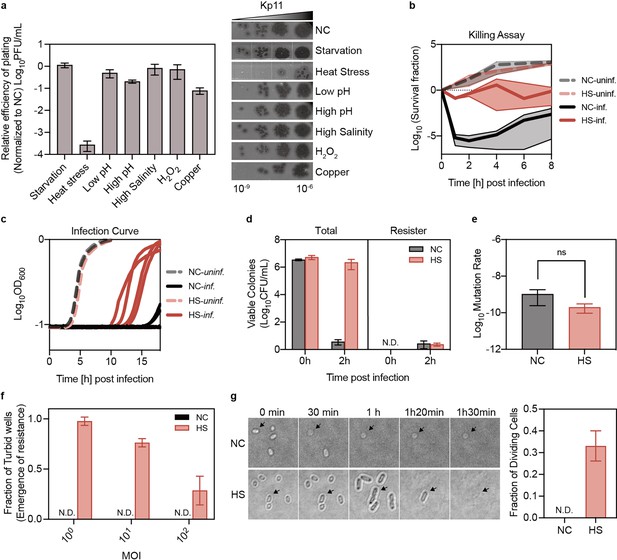
Phage tolerance induced by heat stress in Klebsiella pneumoniae.
(a) Plaque-forming units of the infection drop assay in K. pneumoniae ATCC 43816 following various pre-treatments. The morphotypes of bacteria corresponding to each stress pre-treatment are indicated: starvation for 36 hr in 0.9% NaCl; heat stress for 1.5 hr at 50 °C; and treatment for 1 hr with low pH (pH 3.2), high pH (pH 10), high salinity (5% NaCl), oxidative damage (10 mM H₂O₂), and copper (5 mM CuSO₄). Data are presented as mean ±s.d. of three biological replicates. NC: Negative Control, bacteria without high-temperature treatment prior to phage infection. (b) Survival of K. pneumoniae ATCC 43816 pre-treated with heat and subsequently infected with phage Kp11 (MOI ~100). Curves represent the mean ±s.d. of five biological replicates, with the solid line indicating the mean and the shaded area representing the s.d. of the mean. HS: Heat Stress, bacteria subjected to high-temperature treatment at 50 °C for 90 min prior to phage infection. (c) Growth dynamics of liquid cultures of K. pneumoniae ATCC 43816, with and without heat treatment, infected with phage Kp11 (MOI ~100) at 37 °C. Curves show the mean of four independent replicates. (d) Bacteria from the killing assay (panel b, time 0 and 2 hr) were collected and plated on plates with and without phage Kp11 (108 PFU). No phage-resistant colonies were detected at time 0 (N.D.: Non-detectable), and similar numbers of phage-resistant colonies were observed at time 2 hr in both populations. Data are presented as mean ±s.d. of three independent replicates. (e) Mutation rates of K. pneumoniae ATCC 43816 with and without heat treatment are shown. Data are presented as mean ±s.d. from five independent replicates and 14 plates for each replicate; ns indicates non-significant (p=0.3194). (f) Bacteria (~103 CFU) with and without heat treatment were cultured with phage Kp11 at various MOIs of 1, 10, and 100 for 24 hr, with the fraction of turbid wells shown indicating resistance emergence. Data are presented as mean ±s.d. of three independent replicates and 14 wells for each replicate, and no turbid wells were detected in negative control groups which were without high temperature treatment. (g) Microscopic analysis of K. pneumoniae ATCC 43816 in response to phage Kp11 infection: (Upper Panel) Untreated bacteria were lysed by phages before cell division could occur, whereas (Lower Panel) heat-treated bacteria were able to undergo cell division despite exposure to the lytic phage. Phage Kp11 was added at time 0; scale bar is 2 µm; Black arrows indicate example cells undergoing lysis or division. Quantitative analysis further demonstrated a significantly higher fraction of dividing cells in the heat-treated group under phage pressure compared to untreated bacteria.
-
Figure 2—source data 1
Data used for graphs presented in Figure 2a–f.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig2-data1-v1.xlsx
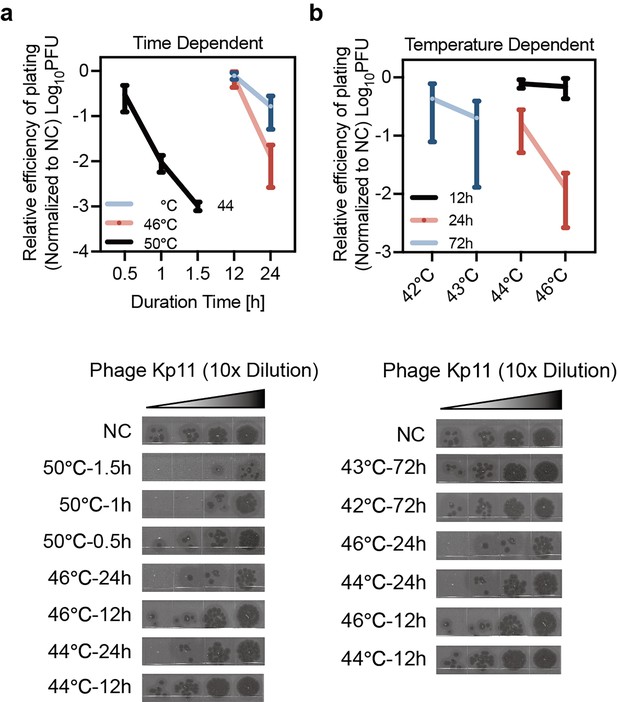
Heat-induced phage tolerance was impacted by treatment time and temperature.
Phage tolerance in K. pneumoniae induced by heat stress as a function of treatment duration (a) and treatment temperature (b). Data are presented as mean ±s.d. from three independent biological replicates. This data corresponds to the results discussed in Figure 2 of the main text, showing effects of heat-treated phage tolerance being both time- and temperature-dependent.
-
Figure 2—figure supplement 1—source data 1
Data used for graphs presented in Figure 2—figure supplement 1a and b.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig2-figsupp1-data1-v1.xlsx
Untreated bacteria were lysed by phages before cell division could occur.
Shown is the rapid lysis of untreated K. pneumoniae ATCC43816 infected with phage Kp11 at an MOI of 100. The bacteria are lysed before they can undergo cell division, demonstrating the efficiency of phage-induced lysis at this infection level. Watch as bacteria are quickly disrupted following phage exposure.
Heat-treated bacteria were able to undergo cell division despite exposure to the lytic phage.
Shown is the infection of heat-treated K. pneumoniae ATCC43816 by phage Kp11 at an MOI of 100. Unlike the untreated bacteria, these bacterial cells can be observed undergoing division before lysis occurs. The delayed lysis highlights the increased tolerance of heat-treated bacteria to phage infection under similar conditions. This increased frequency for bacterial division facilitates the evolution of phage resistance, highlighting the impact of heat treatment on bacterial survival under phage infection.
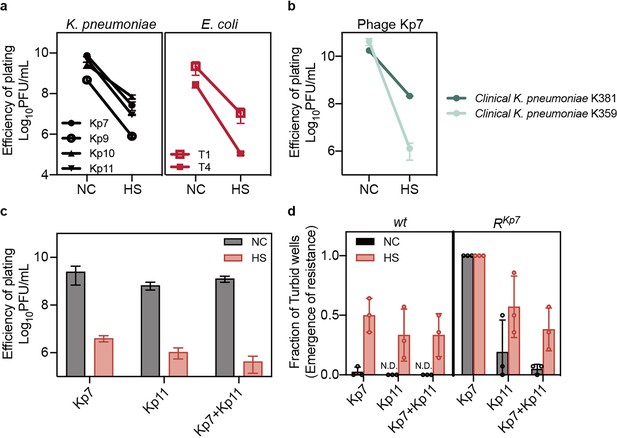
Heat-induced phage tolerance challenges phage therapy.
(a, b) Small drop plaque assays were conducted using 10-fold serial dilutions of phages Kp7, Kp9, Kp10, or Kp11 against K. pneumoniae ATCC 43816, as well as phage Kp7 against clinical K. pneumoniae strains K357 and K381, and phages T1 or T4 against E. coli KLY, both with and without heat treatment. Data are presented as mean ±s.d. of three independent replicates. (c) Efficiency of plating assays was performed for individual phages or phage combination on K. pneumoniae ATCC 43816, with and without heat treatment. Data are presented as mean ±s.d. of three independent replicates. (d) Bacteria (~103 CFU) with and without heat treatment were cultured with either a single phage or phage combination at an MOI of 100 for 24 hr. The fraction of turbid wells indicates resistance emergence. Data are presented as mean ±s.d. of three independent replicates and 14 wells for each replicate.
-
Figure 3—source data 1
Data used for graphs presented in Figure 3a–d.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig3-data1-v1.xlsx
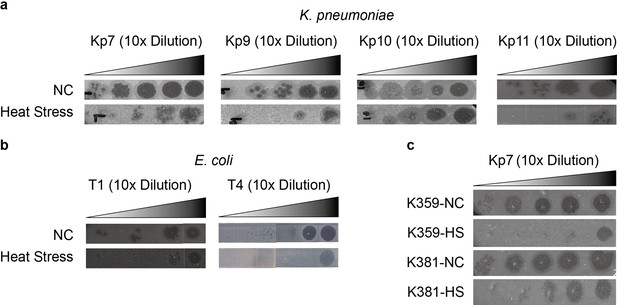
Phage tolerance induced by heat stress is a consistent response observed across various bacterial strains.
Morphological comparison of K. pneumoniae ATCC 43816 infected with phages Kp7, Kp9, Kp10, and Kp11 (a), E. coli KLY infected with phages T1 and T4 (b), and clinical K. pneumoniae strains K359 and K381 infected with phage Kp7 (c), before and after heat treatment. This data corresponds to the results discussed in Figure 3 of the main text.
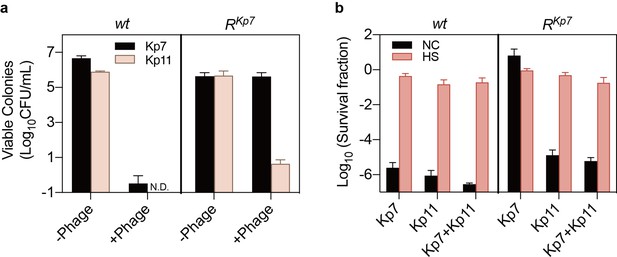
Heat stress–induced phage tolerance reduces the efficacy of phage combinations.
(a) The Kp7-resistant strain (RKp7) remained sensitive to phage Kp11. Colonies of RKp7 were detectable only on the Kp7 phage plates but not on the Kp11 phage plates, while the wild-type (wt) strain was sensitive to both phages, showing no viable colonies on either plate. Data are presented as the mean ± s.d. independent biological replicates. (b) Killing assay of wt and RKp7 strains with phage Kp7, Kp11, and their combination, before and after heat treatment. Heat stress increased bacterial survival under all conditions, including exposure to Kp7 alone, Kp11 alone, and the combination of Kp7 and Kp11. Data are presented as the mean ± s.d. from three independent biological replicates. This data corresponds to the results discussed in Figure 3 of the main text.
-
Figure 3—figure supplement 2—source data 1
Data used for graphs presented in Figure 3—figure supplement 2a and b.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig3-figsupp2-data1-v1.xlsx
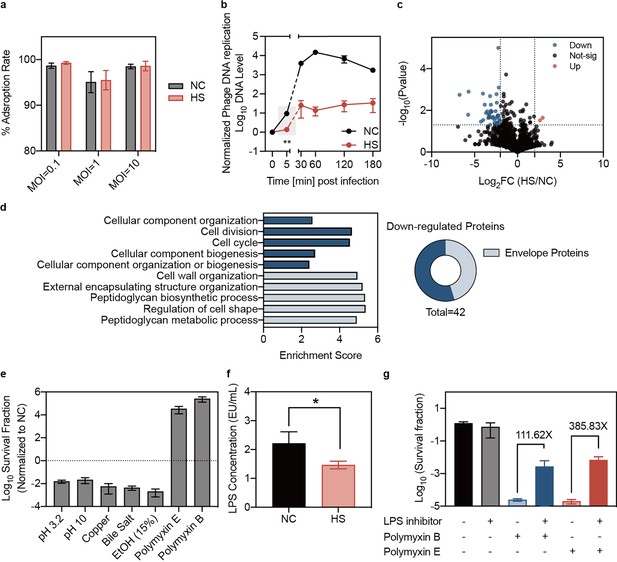
Reduced phage DNA entry and altered envelope stress responses in heat-treated bacteria.
(a) Adsorption assays of phage Kp11 to K. pneumoniae ATCC43816 were performed with and without heat treatment at various MOIs of 0.1, 1, and 10 for 3 min. Data are presented as mean ±s.d. of three independent replicates. (b) The phage DNA levels in bacteria were measured at different time points. Phage Kp11 (MOI ~100) was added at time 0. Data are presented as mean ±s.d. of three biological replicates. Statistical significance was determined using significance levels set at **p<0.01 (p=0.0046). (c) Comparison of the protein profiling results for K. pneumoniae ATCC43816 with and without heat treatment. The color of each dot represents a p value calculated based on the log2 ratio of protein abundance in the two populations (log2(HS/NC)) (see Materials and methods for the statistical test used). The best p values from all technical replicates are shown. The intensity is the normalized mean mass spectrometry peak intensity of each protein associated with the best p value among the three replicates. (d) Gene ontology (GO) analysis of the downregulated proteins in biological process. (e) Normalized survival fraction of heat-treated K. pneumoniae ATCC43816 under various stresses, including pH changes (pH 3.2 and pH 10 for 1 hr), copper (5 mM CuSO4 for 1 hr), ethanol (15% ethanol for 1 hr), bile salt (10 mg/mL for 3 hr) and polymyxin (Polymyxin B, E 25 µg/mL for 1 hr). Data are presented as mean ±s.d. of three independent replicates. (f) The production of LPS in bacteria was quantified using the Chromogenic LAL Endotoxin Assay. Bars show mean ± s.d. of three biological replicates. Statistical significance was determined using significance levels set at *p<0.05 (p=0.0377). (g) Survival fraction of K. pneumoniae treated with polymyxin for 1 hr, with or without the LPS inhibitor CHIR-090. Data are presented as mean ±s.d. of three independent replicates.
-
Figure 4—source data 1
Data used for graphs presented in Figure 4a, b and Figure 4d–g.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig4-data1-v1.xlsx
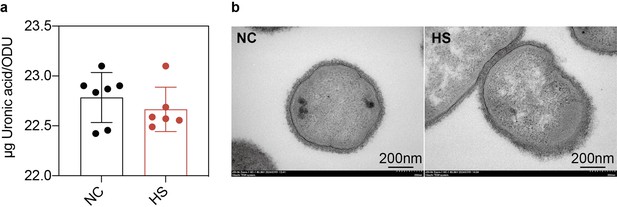
Production and structure of the bacterial capsule in heat-treated and untreated bacteria.
(a) Quantification of capsular production of untreated bacteria and heat-treated bacteria. Capsular production was measured using the uronic acid method and expressed as the amount of uronic acid per OD600. Data are presented as the mean ± s.d. from six independent biological replicates. (b) Detection of bacterial capsular structure by transmission electron microscopy (TEM) before and after heat treatment. This data corresponds to the results discussed in Figure 4 of the main text, showing the production and structure of the bacterial capsule remained unchanged.
-
Figure 4—figure supplement 1—source data 1
Data used for graphs presented in Figure 4—figure supplement 1a.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig4-figsupp1-data1-v1.xlsx
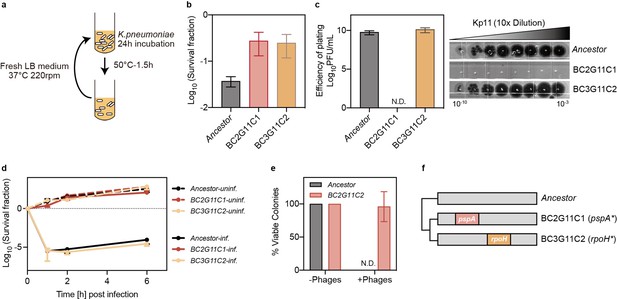
Mutation in pspA confers dual insensitivity to thermal and phage stress.
(a) Experimental design for cyclic exposure to heat treatment, where cultures of ATCC 43816 were subjected to a 50 °C bath for 90 min. Following heat treatment, cultures were resuspended in fresh medium and incubated for 24 hr. (b) Survival fraction of ancestral and evolved strains at 50 °C for 90 min. Data are presented as mean ± s.d. of three independent replicates. BC: Batch Culture, G: Evolution Cycle Number, C: Colony. BC2G11C1 refers to the first colony from batch culture 2 after 11 rounds of heat treatment. (c) Efficiency of plating assay for phage Kp11 to ancestral and evolved strains. Data are presented as mean ± s.d. of three independent replicates. (d) Survival fraction of ancestral and evolved strains from phage Kp11 infection (MOI ~100). Curves show the mean ± s.d. of three independent replicates. (e) Viable colonies of ancestral and evolved strains grown on both phage plates (108 PFU) and phage-free plates. Data are presented as the mean ± s.d. of three independent replicates. (f) Nonsynonymous mutations identified in the strains isolated from the evolved culture.
-
Figure 5—source data 1
Data used for graphs presented in Figure 5b–e.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig5-data1-v1.xlsx
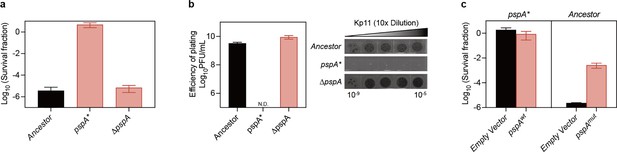
Impact of pspA mutations on bacterial survival and phage resistance.
(a, b) The pspA knockout strain did not display the phenotype associated with the pspA mutation in survival (a) and efficiency of plating (b) when exposed to phage Kp11. (c) Reintroduction of wild-type PspA into the mutant strain did not restore the original phenotype; however, overexpression of the mutant PspA enhanced survival during phage infection. Data are presented as the mean ± s.d. from three biological replicates. This data corresponds to the results discussed in Figure 5 of the main text, showing the mutant in gene pspA was a gain-of-function mutation.
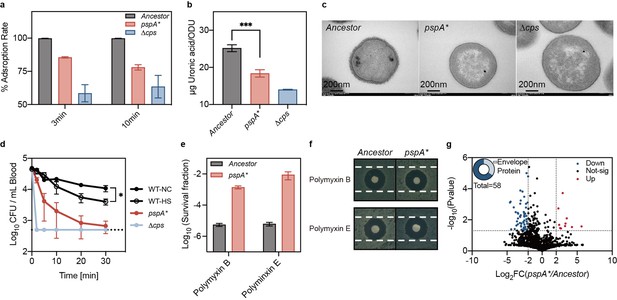
pspA mutation impaired phage adsorption and altered envelope stress responses.
(a) Adsorption assay of phage Kp11 to the ancestral strain K. pneumoniae ATCC 43816, pspA* and the non-capsular strain Δcps at an MOI of 0.1. (b) Quantification of capsular production of the ancestral strain, pspA* and Δcps. The capsular polysaccharide (CPS) was quantified using the uronic acid method, presented as the amount of uronic acid per OD600. Data are presented as the mean ± s.d. of three independent replicates; statistical significance was assessed using an unpaired two-tailed t-test, with statistical significance levels set at ***p<0.005 (p=0.001). (c) Detection of bacterial capsular structure using transmission electron microscopy (TEM). (d) Virulence traits were assessed by intravenously (i.v.) infection of ICR mice with ~106 of each bacterial strain (ancestral with and without heat treatment, pspA*, and Δcps) to determine bacteremia levels. Curves show the mean of three independent replicates, and error bars are s.d. of the mean. Statistical significance was assessed using an unpaired two-tailed t-test, with significance levels set at *p<0.05 (p=0.011). (e) Survival fraction of bacteria (ancestral and evolved strain pspA*) after polymyxin treatment (polymyxin B and E, 25 µg/mL for 1 hr). Data are presented as the mean ± s.d. of three independent replicates. (f) Disk fusion assay (polymyxin B and E, 10 µg) of the ancestral and evolved strain pspA*. (g) Comparison for the protein profiling results for the ancestor and pspA*. The color of each dot represents a p value calculated based on the log2 ratio of protein abundance in the two populations (log2(pspA*/Ancestor)).
-
Figure 6—source data 1
Data used for graphs presented in Figure 6a, b and Figure 6d, e.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig6-data1-v1.xlsx
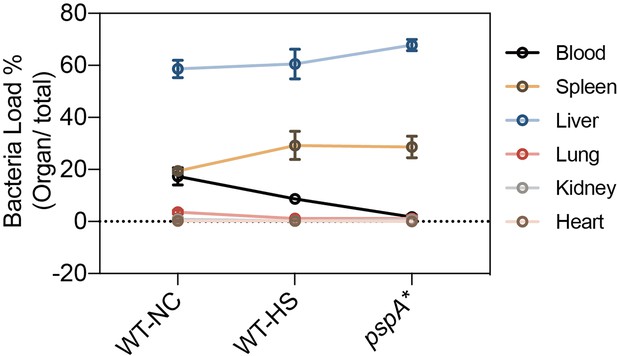
Superior protection of untreated ancestral strain ATCC 43816 against immune clearance of septic infection.
Female ICR mice were intravenously infected with approximately 106 CFU of the ancestral strain ATCC 43816, with and without heat treatment, and the pspA* mutant. CFU counts in the blood and major organs were determined at 30 min post-infection. Data are presented as the mean ±s.d. from three independent biological replicates. This data corresponds to the results discussed in Figure 6 of the main text, showing a significant reduction in immune evasion capacity both in pspA* and heat-treated ancestral strain.
-
Figure 6—figure supplement 1—source data 1
Data used for graphs presented in Figure 6—figure supplement 1a–c.
- https://cdn.elifesciences.org/articles/105703/elife-105703-fig6-figsupp1-data1-v1.xlsx
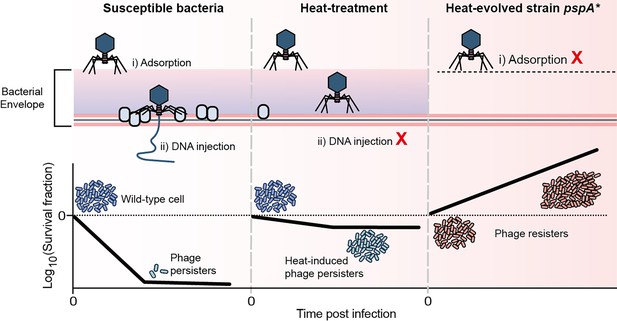
Schematical model of this work.
A small proportion of phage-sensitive bacteria can survive during lytic phage infections, exhibiting persistence against phage. Heat treatment systematically reduces envelope-associated proteins in bacteria, inhibiting the entry of phage DNA and thereby inducing phage tolerance. Mutation in the gene pspA resulted in the loss of capsule, conferring receptor-deficient phage resistance.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/105703/elife-105703-mdarchecklist1-v1.docx
-
Supplementary file 1
List of bacterial strains and phages used in this study.
- https://cdn.elifesciences.org/articles/105703/elife-105703-supp1-v1.docx
-
Supplementary file 2
List of plasmids and primers used in this study.
- https://cdn.elifesciences.org/articles/105703/elife-105703-supp2-v1.docx





