IK1 channels do not contribute to the slow afterhyperpolarization in pyramidal neurons
Figures
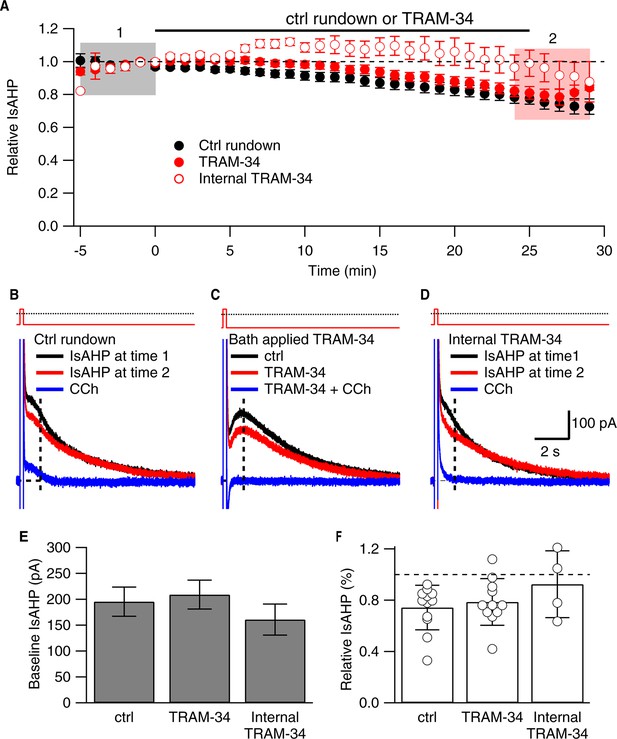
TRAM-34 (1 µM; 22-24˚C) does not affect the IsAHP.
(A) Time course of the normalized amplitude of the IsAHP from control rundown (ctrl, closed black symbols), or TRAM-34 treated cells either bath applied (closed red symbols) or internally delivered (open red symbols) in CA1 pyramidal neurons. (B, C, D) Representative CA1 pyramidal neuron tail currents elicited by the voltage protocol shown above the traces for control rundown (B), bath applied TRAM-34 (C) and internally delivered TRAM-34 (D) at −5 to 0 min (black), 25-30 min (red) and 10– 15 min after CCh application (blue). Vertical dash line at 1 sec after the pulse indicates time point for IsAHP measurement in time course plot of (A). (E) Bar plot of the amplitudes of the IsAHP during a 5 min baseline period for control rundown (Ctrl), bath applied TRAM-34 and internal TRAM-34. (F) Bar plot of the IsAHP measured at 25–30’ (red shaded area panel A) relative to 5 min baseline (black shaded area panel A) for control rundown (Ctrl; n = 10) and bath applied TRAM-34 (n = 11) and internal TRAM-34 (n = 4). Error bars are ± SEM.
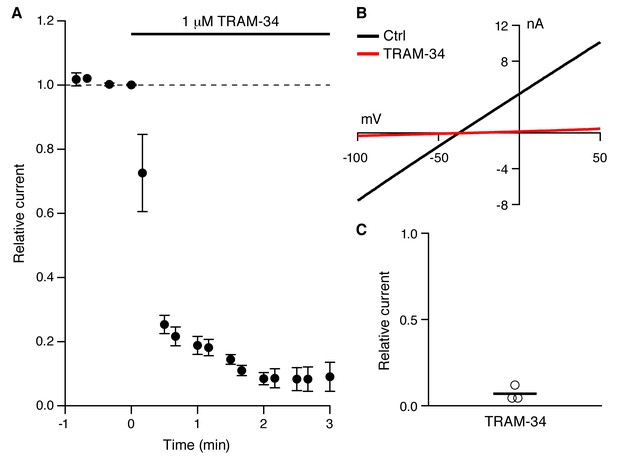
TRAM-34 blocks cloned IK1 channels.
(A) Time course of TRAM-34 block of IK1 channels expressed in HEK293 cells (n = 3). (B) Representative whole-cell recordings with 10 µM Ca2+ in the patch pipette. Currents were evoked from HEK293 cells expressing IK1 by voltage ramp commands (0.16 mV/ms) in control bath solution (black) and after TRAM-34 application (red) (1 µM; 22– 24 ˚C). (C) Scatter plot of TRAM-34 block of IK1 current (n = 3).
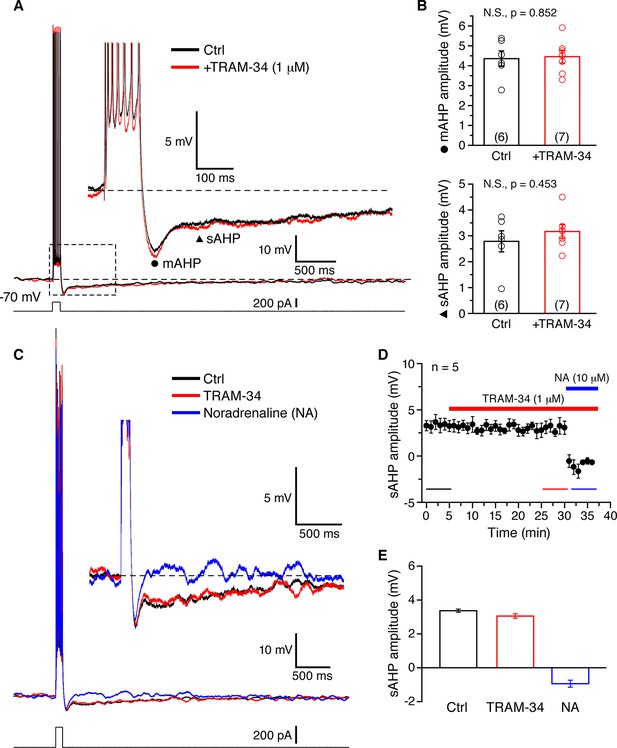
TRAM-34 has no significant effects on the medium afterhyperpolarization (mAHP) or slow afterhyperpolarization (sAHP) in CA1 pyramidal cells.
(A) Brief spike trains (7 spikes/100 ms) were evoked by depolarizing current pulses from a holding potential of -70 mV. Black and red traces represent recordings from two pyramidal cells, in control medium and after incubation with TRAM-34 (1µM) for 30 minutes. Inset, showing mAHP(⚫) and sAHP (▲) at an enlarged scale. (B) mAHP and sAHP amplitudes were not significantly different between control and TRAM-34 treated groups. Data are given as, mean ± SEM. (C) Representative traces of the effect of TRAM-34 (1 µM, red) and noradrenaline (NA, 10 µM, blue) on the mAHP and sAHP in CA1 pyramidal neurons. (D) Time course of the sAHP amplitude measured in panel A. Bath application of TRAM-34 (1 µM) for 25 min did not significantly reduce the sAHP (n = 5). However, subsequent application of noradrenaline (NA, blue line) rapidly eliminated the sAHP. (E) Summary bar plots showing the sAHP amplitude averaged over a period of five minutes during control (black bar), TRAM-34 (red bar) and NA (blue bar) application. The averaged periods are represented by color bottom lines in panel D.
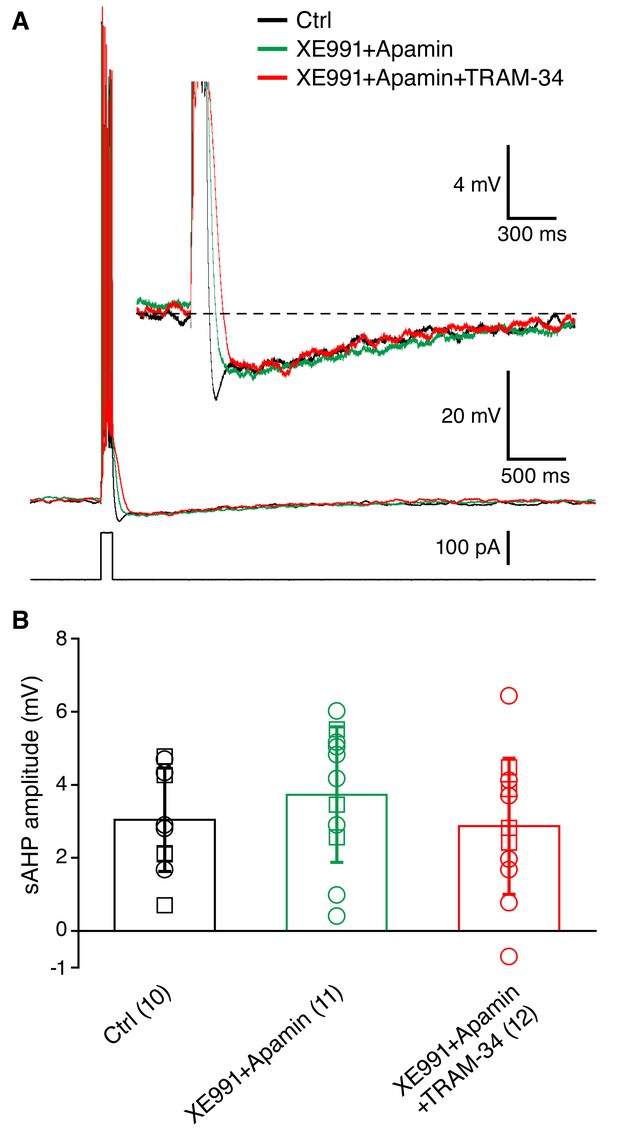
Incubation of acute hippocampal slices and organotypic slice cultures with TRAM-34 (1 μM) did not reduce the sAHP in CA1 pyramidal neurons.
(A) Example traces showing the mAHP and sAHP in three different conditions tested. Cells in the control group (black trace) were recorded in normal ACSF. Cells in the second group (XE991+Apamin, green trace) were recorded after incubation for at least 30 minutes in ACSF with XE991 (10 μM) and apamin (100 nM). Cells in the third group (XE991+Apamin+TRAM-34, red trace) were recorded after incubation for at least 30 minutes in ACSF with XE991 (10 μM), apamin (100 nM) and TRAM-34 (1 μM). (B) Summary of the results from slices (open circles) and organotypic cultures (open squares). The sAHP amplitude did not differ between the three groups of cells: (1) control (n = 10), (2) XE991+Apamin (n = 11), and (3) XE991+Apamin+TRAM-34 (n = 12).
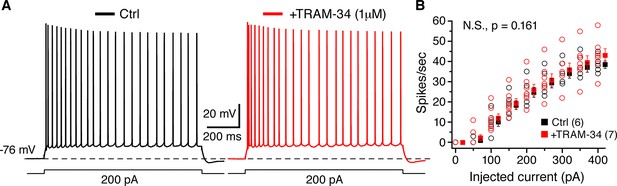
TRAM-34 (1 µM) had no significant effect on the excitability of CA1 pyramidal cells.
(A) Representative spike trains evoked by 1s long depolarizing current (200 pA) injections from -76 mV, recorded from pyramidal cells in control medium (left, black trace) and after incubation of TRAM-34 (right, red trace). (B) Comparison of spike rates (spikes/s) between control (black) and TRAM-34 (red) treated groups evoked by depolarizing, 1 s long current pulses (0–400 pA). Mono-exponential fits were used to compare spike rates in control medium and TRAM-34 treated groups, by using the function: f(X) = A[exp(-x/τ)]+ y0. No significant differences were found between control and TRAM-34 treated groups [control, τ: 263 (58) pA; TRAM-34, τ: 371 (65) pA, N.S, p = 0.161, t-test after Box-Cox transformation (Minitab 17)].
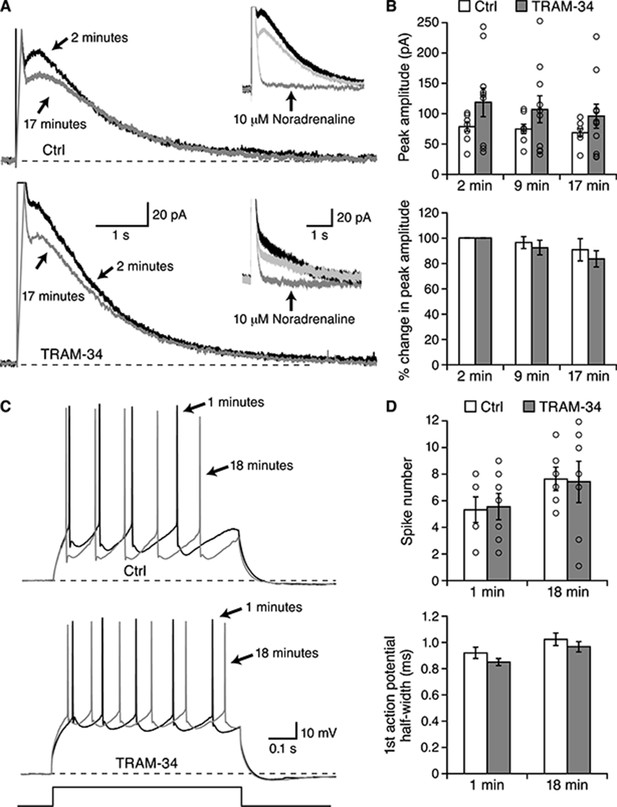
TRAM-34 did not block the IsAHP in BLA pyramidal neurons.
Whole-cell voltage clamp recording from BLA pyramidal neurons. (A) Representative current traces for IsAHP current evoked from a holding potential of −50 mV under control conditions (upper traces) and with 1 µM TRAM-34 added to the pipette solution. Insets show the effects of 10 µM noradrenaline. (B) The peak IsAHP current, measured 500 ms after the voltage step is plotted under the two conditions in control and TRAM-34 loaded neurons at the indicated times after onset of the whole-cell recording configuration (break-in). The lower panel shows the same data normalized to the amplitude 2 minutes after break-in. (C) Current clamp recordings from the neurons shown in (A), discharge evoked by a 600 ms current injection. (D) Plotted are the action potential half width and number of evoked action potentials evoked by the current injection at the indicated times in control and TRAM-34.
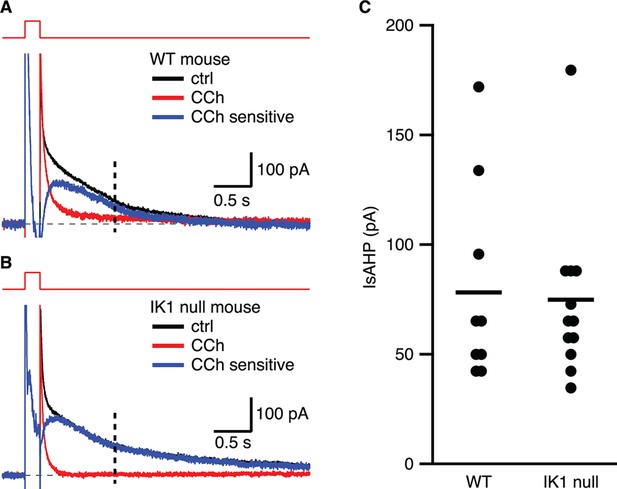
The IsAHP in IK1 null mice is not different from wild type.
(A,B) Tail currents elicited by the voltage protocol from −63 to 7 mV shown above the traces for wild type (A) and IK1 null (B). Black traces are control, red traces are after CCh application, and blue traces are the subtracted CCh sensitive currents. (C) Scatter plot of the IsAHP amplitude for wild type and IK1 null mice. Mean indicated by horizontal line.





