Lateral orbitofrontal neurons acquire responses to upshifted, downshifted, or blocked cues during unblocking
Figures
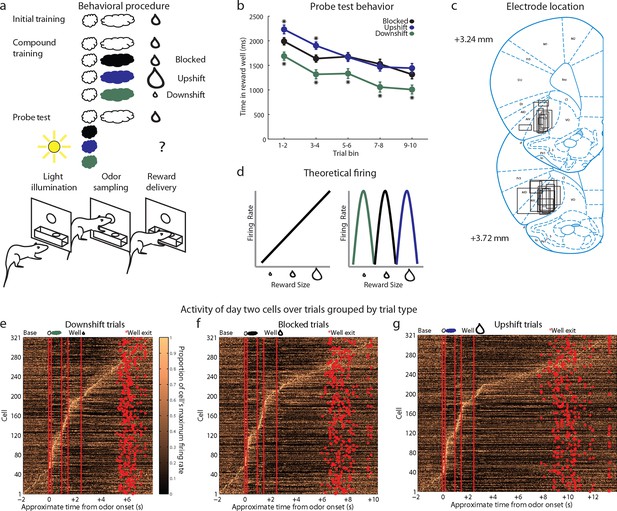
Experimental outline, behavior summary, recording sites, and recorded cells.
(a) Thirsty rats were initially trained to enter an odor port after a house light lit up, then to go to the reward well below to receive a drop of chocolate milk. There were 4 trial types in the unblocking session. The first was a reminder of initial training. On the other three trial types, the originally trained odor was briefly presented, followed by one of three novel odors. The reward following the novel odors was either unchanged (black; blocked trials), larger in size (blue; upshift trials), or smaller in size (green; downshift trials). In the probe test stage, we assessed learning by presenting the novel odors without a subsequent reward. (b) Time in the reward well on the probe test trials. ANOVA for time spent in the reward well with odor (blocked, upshift, downshift), and trial (1–10) as factors found a significant effect of odor (ANOVA, F2,1770 =37.8, p<0.001) and trial (ANOVA, F9,1770 =15.7, p<0.001). Planned comparisons confirmed that in the first four-trial block, rats spent significantly more time in the reward well following the upshift odor (p<0.01) relative to the blocked odor. Rats also spent less time in the reward well following the downshift odor on all trials relative to the blocked odor (p<0.01). *p<0.01. Error bars indicate SEM. (c) Single unit activity was recorded from the lateral orbital and agranular insular cortices. Locations are shown at 3.24 and 3.72 mm anterior to bregma. AIV, AID = agranular insular area, LO = lateral orbital cortex. (d) Theoretical firing pattern for cells that signal value monotonically (left) or are tuned to individual reward sizes (right). (e–g) Activity of day two cells with a baseline firing rate under 10 Hz sorted by when each cell reaches its’ maximal firing rate over the course of a trial for e) Downshift, f) Blocked, g) and Upshift trials. Cells are sorted independently on each trial type and thus the cell numbers do not correspond between trial types.
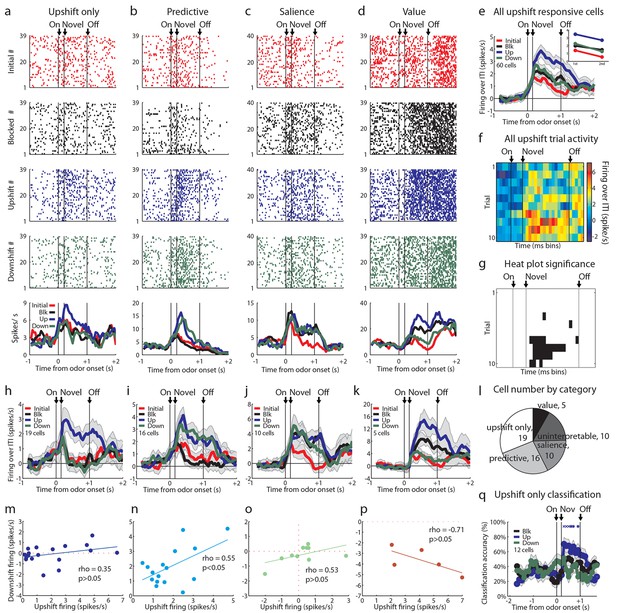
Single unit and population firing of upshift-responsive neurons.
(a–d) Raster plots for firing of single units on initial (red), blocked (black), upshift (blue), and downshift (green) trials. All raster plots are from the first day of unblocking. Odor onset (On) is indicated by the first vertical line, novel odor onset (Novel) by the second vertical line, and odor offset (Off) by the third. Each tick represents a spike. Average activity across all trials is plotted by odor (bottom) showing a) Selective firing to the upshift odor, b) Putative predictive firing to the upshift and downshift odors, c) Putative salience firing to all three novel odors but not the initially trained odor, d) Putative value firing, exhibiting a monotonic firing pattern. (e) Mean neural activity (novel odor epoch - ITI) for the upshift-responsive population (n=60) is plotted. Line colors as in raster plots; shaded areas indicate standard error of mean. ANOVA with bin and odor as factors found significant effects of bin, odor, and the bin x odor interaction (F59,14160=19.81, F3, 14160=52.47, F177, 14160=1.46, ps<0.001). ANOVA restricted to the novel odor period with odor and time (1st 500 ms vs. 2nd 500 ms, shown in upper right inset) as factors found a main effect of odor and time (F3,472=13.93, F1,472 = 10.89, ps<0.01). At both times there was no difference between blocked and downshift firing rates. (f) Baseline-normalized firing to the upshift cue for the upshift-responsive population on the first 10 trials of the first unblocking day (n = 33). Firing was calculated in a 150 ms sliding window for each 50-ms bin moving away from novel odor onset. The firing rate over ITI was then plotted, with dark red bins indicating maximal firing to the upshift cue and blue indicating minimal firing. Heat plot values are shown on right of heat plot. (g) The significance of the increased firing to the upshift cue was determined by performing a one-tailed t-test, comparing increases in firing to 0, using a significance of p<0.001 and a sliding window as in Figure 2f. Black bins indicate significant elevations in firing to the upshift cue. (h–k) Mean neural activity (novel odor epoch - ITI) for different upshift-responsive populations is plotted as in Figure 2d for h) Cells that respond only to the upshift cue over baseline, i) Cells that respond to the upshift and downshift cues over baseline but do not respond to the blocked cue. j) Cells that respond to the novel odor cues but not the initially trained cue over baseline. k) Cells whose signal increases monotonically with increasing reward amount. (l) Pie chart of the proportion of upshift-responsive neurons in each category. (m–p) Scatter plots of blocked-cue and baseline-normalized upshift and downshift firing rate for m) Upshift only n) Predictive o) Salience p) Value populations. (q) Classification accuracy for all cells that respond only to the upshift cue on day 1. Chance is indicated with a dashed red line. Classification accuracy significance above chance is indicated above time bins in a color matching the trial type. *p<0.05, xp<0.01. Error bars indicate SEM.
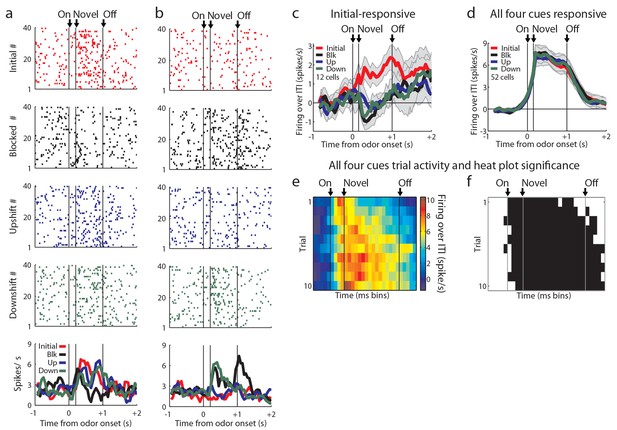
Odor-responsive units not analyzed in the main text.
Of the 82 units not analyzed in the main text, neurons showed responses to different combinations of odors. (a) Neuron fired maximally to initial, upshift, and downshift odors, possibly signaling information and thus not responsive to the blocked cue. (b) Neuron fired maximally to blocked and downshift odors, possibly signaling cues that were not predictive of reward. However, these patterns were rare and were not reflective of the odor-responsive population. (c) 12 cells fired over baseline only to the initial odor. (d) 52/174 (30%) of the excitatory odor-responsive cells showed a significant phasic response to each of the four odor cues. Neurons were classified as putative sensory neurons only if they were not value-coding. These neurons fired to all of the odors, even the blocked odor, thus their firing cannot be easily explained as signaling information about the predicted outcomes. However they might be signaling information about the cues themselves, such as their shared sensory features or intrinsic salience. When we examined the firing of neurons in this population on the first 10 trials of unblocking, (e-f) Heat plots and significance plots as in Figure 2f-g on unblocking day 1 (n = 21) for all four cues show elevated firing over baseline from odor onset, p<0.01. Error bars indicate SEM.
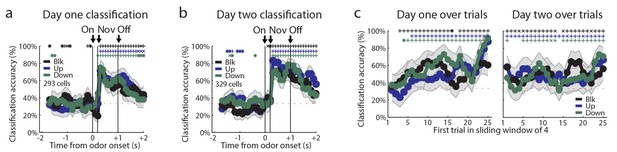
Recorded units not restricted to odor-responsive units.
(a-b) Classification accuracy as in Figure 2q for a) Day one cells b) Day two cells. (c) Classification accuracy over a sliding window of 4 trials for day one cells as in Figure 3n for day one (left) and day two (right) cells. Classification accuracy significance above chance is indicated above time bins in a color matching the trial type. *p<0.05, xp<0.01., +p<0.001. Error bars indicate SEM.
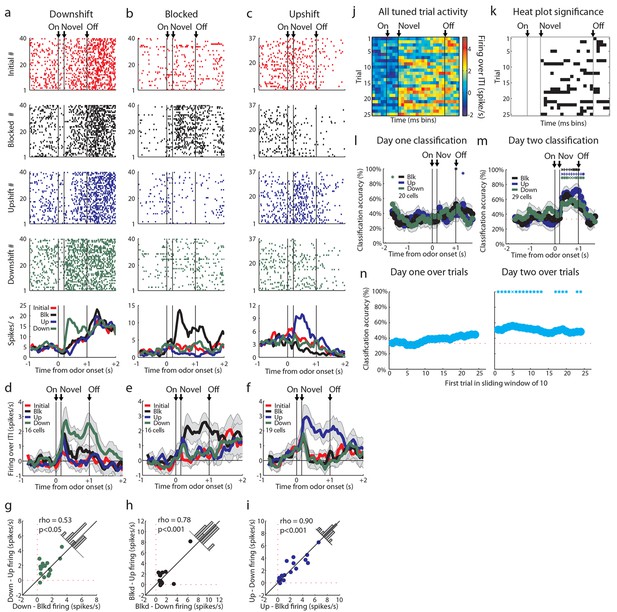
Tuned cells and population activity.
Raster plots as in Figure 2a–d for firing of single units that are selective for one cue on unblocking day 2 are shown for units responsive to only the (a) Downshift cue (b) Blocked cue (c) Upshift cue. (d-f) Mean neural activity (novel odor epoch - ITI) as in Figure 2e for the d) Downshift, e) Blocked, f) Upshift responsive populations. (g-i) Scatter plots in which firing for each non-preferred cue is subtracted from the preferred cue for tuned populations. Bar graphs show the distribution of difference between the two indices for each neuron. To the extent that cells do not differentiate between the two non-preferred cues, scatter points should congregate around the diagonal, the histogram bars should peak in the center, and a t-test should indicate that the distribution of the mean is not significantly different from 0. g) Scatter plots and histograms are shown for the downshift population. A t-test of the diagonal distribution data found that the distribution of the mean is not significantly different from 0, p=0.07. h) Scatter plots and histograms are shown for the blocked population. A t-test of the diagonal distribution data found that the distribution of the mean is not significantly different from 0, p=0.80. i) Scatter plots and histograms are shown for the upshift population. A t-test of the diagonal distribution data found that the distribution of the mean is not significantly different from 0, p=0.63. (j-k) Heat plots and p-value plots as in Figure 2 f-g, p<0.01, for all tuned cells on unblocking days 1 and 2 (n = 51) normalized to the initially trained cue. (l-m) Classification accuracy over time from odor onset as in Figure 2q for l) 20 tuned cells on unblocking day 1 and m) 29 tuned cells on unblocking day 2. Classification accuracy significance above chance is indicated above time bins in a color matching the trial type. *p<0.05, xp<0.01., +p<0.001. (n) Classification accuracy for tuned cells over trials on day one (n=20) and day two (n=29) over a sliding window of 10 trials averaged by trial type. Classification accuracy significance above chance is indicated above time bins. *p<0.05, xp<0.01. On day one, accuracy does not rise above chance, but there is a correlation between trial number and classification accuracy, rho = 0.85, p<0.001. Error bars indicate SEM.
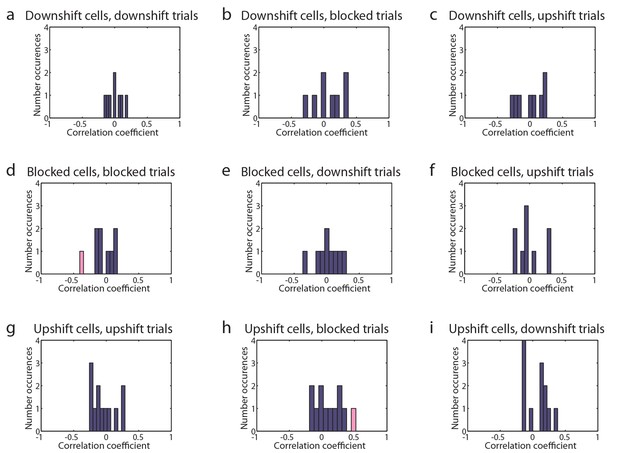
Tuned cells’ trial firing and latency to reward well.
(a-i) Histograms showing the correlation coefficient between trial firing and latency to reward well on day two with significant correlation colored pink for (a-c) Downshift-tuned cells (n=8) on a) Downshift, b) Blocked, and c) Upshift trials, for (d-f) Blocked-tuned cells (n=9) on d) Blocked, e) Downshift, and f) Upshift trials, for (g-i) Upshift-tuned cells (n=12) on g) Upshift, h) Blocked, and i) Downshift trials.
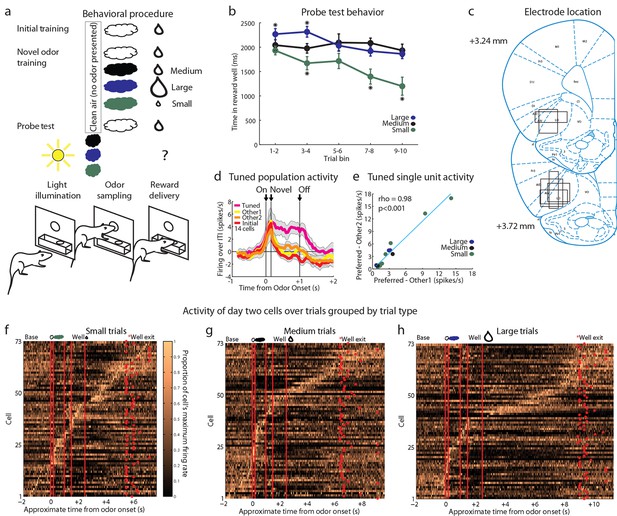
Control experimental outline, behavior summary, recording sites, and neural results.
(a) Procedure is nearly identical to that in Figure 1a except a control procedure was used rather than a blocking procedure. The reward following the novel odors was either medium (black), large (blue), or small (green). (b) Time in the reward well on the probe test trials. ANOVA for time spent in the reward well with odor (small, medium, large), and trial (1–10) as factors found a significant effect of odor (ANOVA, F2,450 =22.61, p<0.001) and trial (ANOVA, F9,450 =2.7, p<0.01). Planned comparisons confirmed that in the first four-trial block, rats spent significantly more time in the reward well following the large odor (p<0.05) relative to the medium odor. Rats also spent less time in the reward well following the small odor on all trials relative to the medium odor (p<0.001). *p<0.05 (c) Single unit activity was recorded from the lateral orbital and agranular insular cortices. Locations are shown at 3.24 and 3.72 mm anterior to bregma. AIV, AID = agranular insular area, LO = lateral orbital cortex. (d) Mean neural activity (novel odor epoch - ITI) is plotted for all of the tuned cells (n=14) combined, with the tuned cue in pink and the initially trained cue in red. Other 1 = medium for small-responsive and large-responsive and small for medium-responsive. Other 2 = large for small-responsive and medium-responsive and small for large-responsive. Error bars indicate SEM. (e) Scatter plot as in Figure 3g-I for all tuned cells. Other 1 = medium for small-responsive and large-responsive and small for medium-responsive. Other 2 = large for small-responsive and medium-responsive and small for large-responsive. (f-h) Activity of day two cells as in Figure 1e-g for f) Small, g) Medium, h) Large trials.





