Neto auxiliary proteins control both the trafficking and biophysical properties of the kainate receptor GluK1
Figures
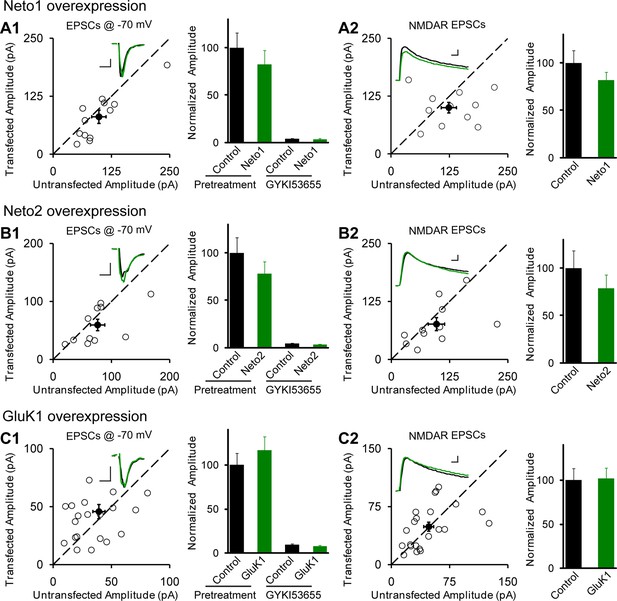
Individual overexpression of Neto1, Neto2 or GluK1 has no effect on synaptic transmission.
Rat hippocampal slice cultures were biolistically transfected with Neto1 (A, n=12), Neto2 (B, n=11) or GluK1 (C, n=22). Simultaneous dual whole-cell recordings from a transfected CA1 pyramidal neuron (green trace) and a neighboring wild type one (black trace) were performed. The evoked EPSCs (eEPSCs) were measured at −70 mV and +40 mV (the current amplitudes were measured 100 ms after stimulation). Open and filled circles represent amplitudes for single pairs and mean ± SEM, respectively. Insets show sample current traces from control (black) and experimental (green) cells. The scale bars for representative eEPSC trace were 25 pA and 25 ms. Bar graphs show normalized eEPSC amplitudes (mean ± SEM) of −70 mV (A1, 82.24 ± 14.64% control, p > 0.05; B1, 77.9 ± 12.9% control, p > 0.05 and C1, 116.58 ± 15.53% control, p > 0.05) and +40 mV (A2, 81.74 ± 8.42% control, p > 0.05; B2, 78.53 ± 14.35% control, p > 0.05 and C2, 101.8 ± 12.06% control, p > 0.05) presented in scatter plots. All the statistical analyses are compared to respective control neurons with two-tailed Wilcoxon signed-rank sum test. The eEPSC amplitudes measured at −70 mV after GYKI53655 (100 μM) wash-in in A1, B1 and C1 were also normalized according to respective pretreated control neurons.
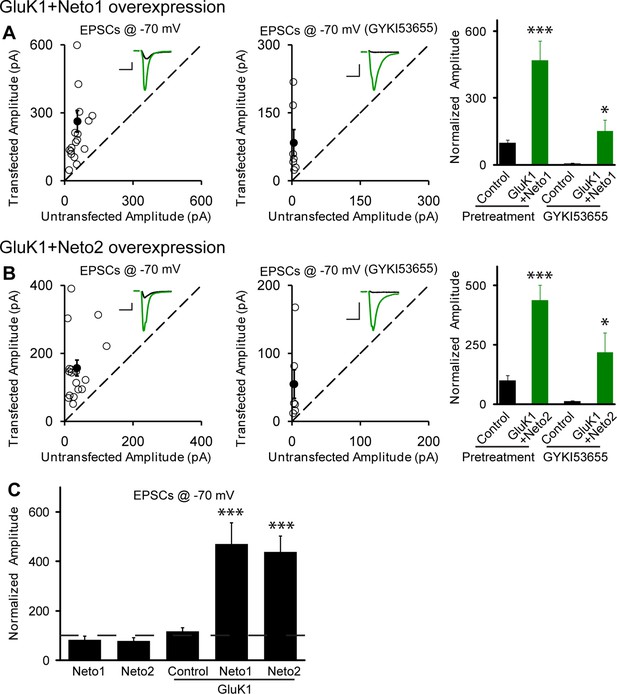
Neto1 and Neto2 promote GluK1 receptor synaptic targeting.
(A) Scatter plots show eEPSC amplitudes of control and GluK1/Neto1-cotransfected CA1 neurons in rat hippocampal slice cultures measured at −70 mV in the absence or presence of GYKI53655. Filled circles show mean ± SEM. Insets show sample current traces from control (black) and GluK1/Neto1-expressing (green) cells. The scale bars for representative eEPSC trace were 50 pA and 25 ms. Bar graph show normalized eEPSC amplitudes (mean ± SEM) of pretreated (n=19, 470.65 ± 85.6% control, *** p < 0.0005) and GYKI53655 treated (n=7, 150.72 ± 51.8% control pretreatment, * p < 0.05) cells. (B) Scatter plots show eEPSC amplitudes of control and GluK1/Neto2-cotransfected CA1 neurons in rat hippocampal slice cultures measured at −70 mV in the absence or presence of GYKI53655. Filled circles show mean ± SEM. Insets show sample current traces from control (black) and GluK1/Neto1-expressing (green) cells. The scale bars for representative eEPSC trace were 50 pA and 25 ms. Bar graph show normalized eEPSC amplitudes (mean ± SEM) of pretreated (n=17, 689.52 ± 195.16% control, *** p < 0.0005) and GYKI53655 treated (n=7, 317.63 ± 83.12% control pretreatment, * p < 0.05) cells. (C) Summary of the normalized evoked EPSC amplitudes at −70 mV as percent of respective control ± SEM for each indicated transfection. All the statistical analyses are compared to respective control neurons with two-tailed Wilcoxon signed-rank sum test.
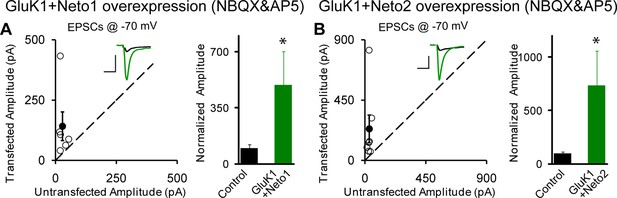
Neto1 and Neto2 regulation of GluK1 synaptic expression is independent of synaptic activity.
Scatter plots show eEPSC amplitudes of control and GluK1/Neto1 (A) or GluK1/Neto2 (B) transfected neurons in rat hippocampal slice. Slices were treated with 25 μM NBQX and 100 μM AP5 during the incubation culture and measured at −70 mV. Filled circles show mean ± SEM. Insets show sample current traces from control (black) and experimental (green) cells. The scale bars for representative eEPSC trace were 50 pA and 25 ms. Bar graph show normalized eEPSC amplitudes (mean ± SEM) (A: GluK1/Neto1, n=6, 493.41 ± 206.95% control, * p < 0.05; B: GluK1/Neto2, n=7, 734.43 ± 321.83% control, * p < 0.05) presented in scatter plots. All the statistical analyses are compared to respective control neurons with two-tailed Wilcoxon signed-rank sum test.
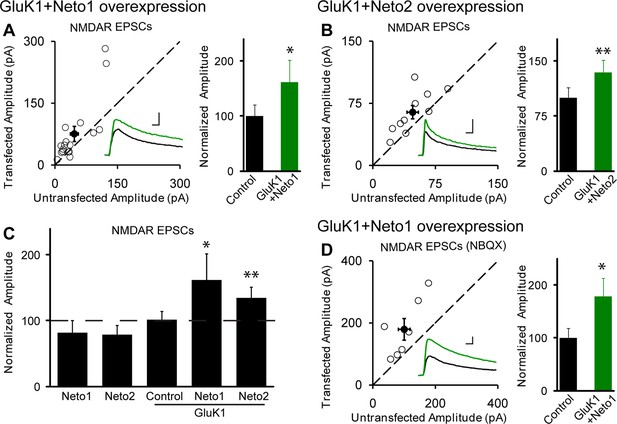
NMDAR EPSCs are increased in GluK1/Neto1 and GluK1/Neto2 expressing neurons.
Scatter plots show eEPSC amplitudes of control and GluK1/Neto1 or GluK1/Neto2 transfected neurons in rat hippocampal slice cultures measured at +40 mV (100 ms after stimulation). Filled circles show mean ± SEM. Insets show sample current traces from control (black) and experimental (green) cells. The scale bars for representative eEPSC trace were 50 pA and 25 ms. Bar graphs show normalized eEPSC amplitudes (mean ± SEM) (A: GluK1/Neto1, n=7, 162.15 ± 39.44% control, * p < 0.05; B: GluK1/Neto2, n=10, 134.28 ± 16.85% control, ** p < 0.01) presented in scatter plots. (C) Summary of the normalized evoked NMDAR EPSC amplitudes at +40 mV as percent of respective control ± SEM for each transfection. (D) Scatter plots show peak eEPSC amplitudes of control and GluK1/Neto1 transfected neurons in rat hippocampal slice cultures measured at +40 mV in the presence of NBQX (25 μM). Bar graphs show normalized eEPSC amplitudes (mean ± SEM) (n=7, 178.73 ± 34.72% control, * p < 0.05) presented in scatter plots. All the statistical analyses are compared to respective control neurons two-tailed Wilcoxon signed-rank sum test.
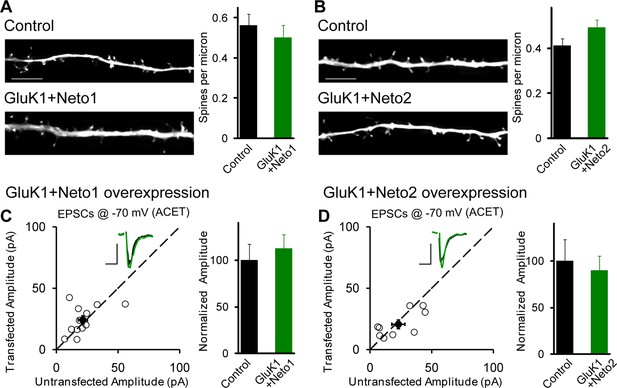
GluK1 synaptic expression has no effect on spinogenesis and does not replace endogenous synaptic AMPA receptors.
(A) Sample images of primary apical dendrites from control (upper) and GluK1/Neto1 overexpressed (lower) neurons imaged using super-resolution structured illumination microscopy (SIM). Bar graph in right shows average spine density (control, n = 8, 0.56 ± 0.06/μm; GluK1/Neto1, n = 9, 0.5 ± 0.06/μm; p > 0.05). Scale bar: 5 μm. (B) Sample images of primary apical dendrites from control (upper) and GluK1/Neto2 overexpressed (lower) neurons imaged using SIM. Bar graph in right shows average spine density (control, n = 8, 0.41 ± 0.03/μm; GluK1/Neto2, n = 7, 0.49 ± 0.03/μm; p > 0.05). Scale bar: 5 μm. All the statistical analyses are compared to respective control neurons with unpaired two-tailed t test. (C and D) Scatter plots show eEPSC amplitudes of control and GluK1/Neto1 (C) or GluK1/Neto2 (D) cotransfected neurons in rat hippocampal slice cultures measured at −70 mV in the presence of the GluK1 antagonist ACET (1 μM). Filled circles show mean ± SEM. Insets show sample current traces from control (black) and experimental (green) cells. The scale bars for representative eEPSC trace were 25 pA and 25 ms. Bar graph show normalized eEPSC amplitudes (mean ± SEM) (A: n=12, 112.33 ± 15.36% control, p > 0.05; B: n=9, 89.7 ± 15.38% control, p > 0.05) presented in scatter plots. All the statistical analyses are compared to respective control neurons with two-tailed Wilcoxon signed-rank sum test.
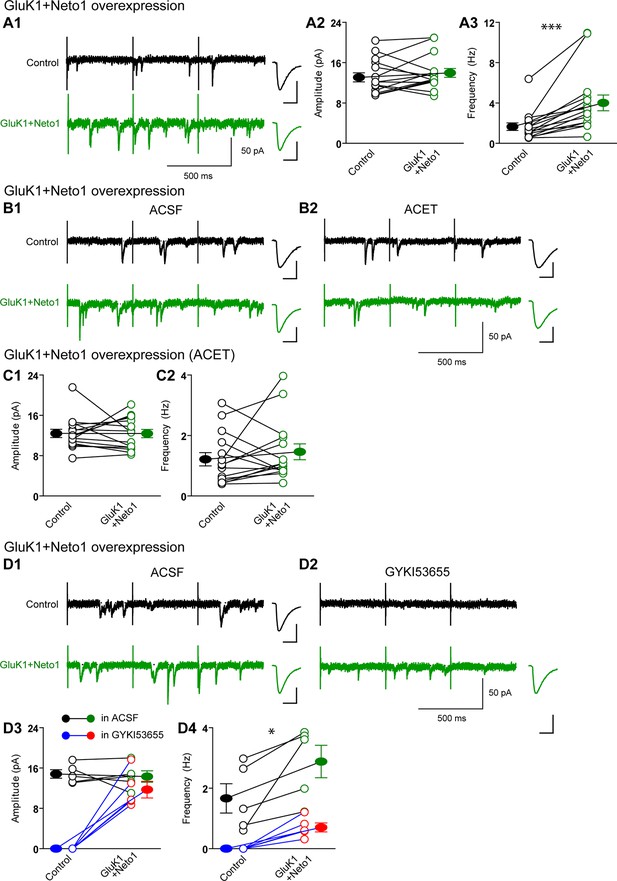
Neto1 specifically targets GluK1 receptors to silent synapse.
(A1) Representative sample traces of asynchronous EPSCs (aEPSCs) simultaneously recorded in the presence of Sr2 + from control (black) and GluK1/Neto1-coexpressed (green) neurons. The first 50 ms following stimulation was excluded from analysis. The scale bars for single representative aEPSC traces were 10 pA and 10 ms. (A2) aEPSC amplitude is not significantly changed in GluK1/Neto1-expressing neurons (n=15, control: 13.08 ± 0.89 pA, GluK1/Neto1: 13.96 ± 0.89 pA, p > 0.05). Plot shows single pairs (open circles) and mean ± SEM (filled circles). (A3) aEPSC frequency is significantly increased in neurons expressing GluK1 and Neto1 (n=15, control: 1.65 ± 0.38 Hz, GluK1/Neto1: 4.01 ± 0.79 Hz, *** p < 0.0001). Plot shows single pairs (open circles) and mean ± SEM (filled circles). (B1 and B2) Representative sample traces of aEPSCs recorded in the presence of Sr2+ from control (black) and GluK1/Neto1-coexpressed (green) neurons before (B1, left) and after (B2, right) ACET treatment. The first 50 ms following stimulation was excluded from analysis. The scale bars for single representative aEPSC trace were 10 pA and 10 ms. (C1) Plot shows single pairs (open circles) and mean ± SEM (filled circles) of aEPSC amplitude from control and GluK1/Neto1 transfected neurons (n=15, control: 12.41 ± 0.82 pA, GluK1/Neto1: 12.38 ± 0.83 pA, p > 0.05) recorded in the presence of ACET. (C2) The aEPSC frequency in neurons expressing GluK1 and Neto1 is not significantly different from control ones in the presence of ACET (n=15, control: 1.21 ± 0.22 Hz, GluK1/Neto1: 1.46 ± 0.26 Hz, p > 0.05). Plot shows single pairs (open circles) and mean ± SEM (filled circles). (D1 and D2) Representative sample traces of aEPSCs recorded in the presence of Sr2 from control (black) and GluK1/Neto1-coexpressed (green) neurons before (D1, left) and after (D2, right) GYKI53655 (30 μM) treatment. The first 50 ms following stimulation was excluded from analysis. The scale bars for single representative aEPSC trace were 10 pA and 10 ms. (D3-D4) Plots show single paired (open circles) and mean ± SEM (filled circles) of aEPSC amplitude (D3) and frequency (D4) from control and GluK1/Neto1-cotransfected neurons before (black and green, n=5; amplitude: control: 14.81 ± 0.85 pA, GluK1/Neto1: 14.3 ± 1.13 pA, p > 0.05; frequency: control: 1.66 ± 0.19 Hz, GluK1/Neto1: 2.88 ± 0.54 Hz, p < 0.05) and after 30 μM GYKI53655 treatment (blue and red, amplitude: GluK1/Neto1: 11.71 ± 1.66 pA; frequency: GluK1/Neto1: 0.7 ± 0.15 Hz). All the statistical analyses are compared to respective control neurons with two-tailed Wilcoxon signed-rank sum test.
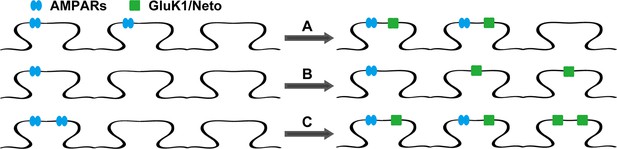
The models of Neto proteins-regulated synaptic GluK1 receptors localization.
Endogenous AMPARs are blue and GluK1 receptors are green. (A) AMPARs and GluK1 receptors are localized at the same synapses. (B) AMPARs and GluK1 receptors are localized at different synapses. (C) Endogenous AMPARs are redistributed by synaptic GluK1 receptors and some of the synapses are populated with both AMPARs and GluK1 receptors.
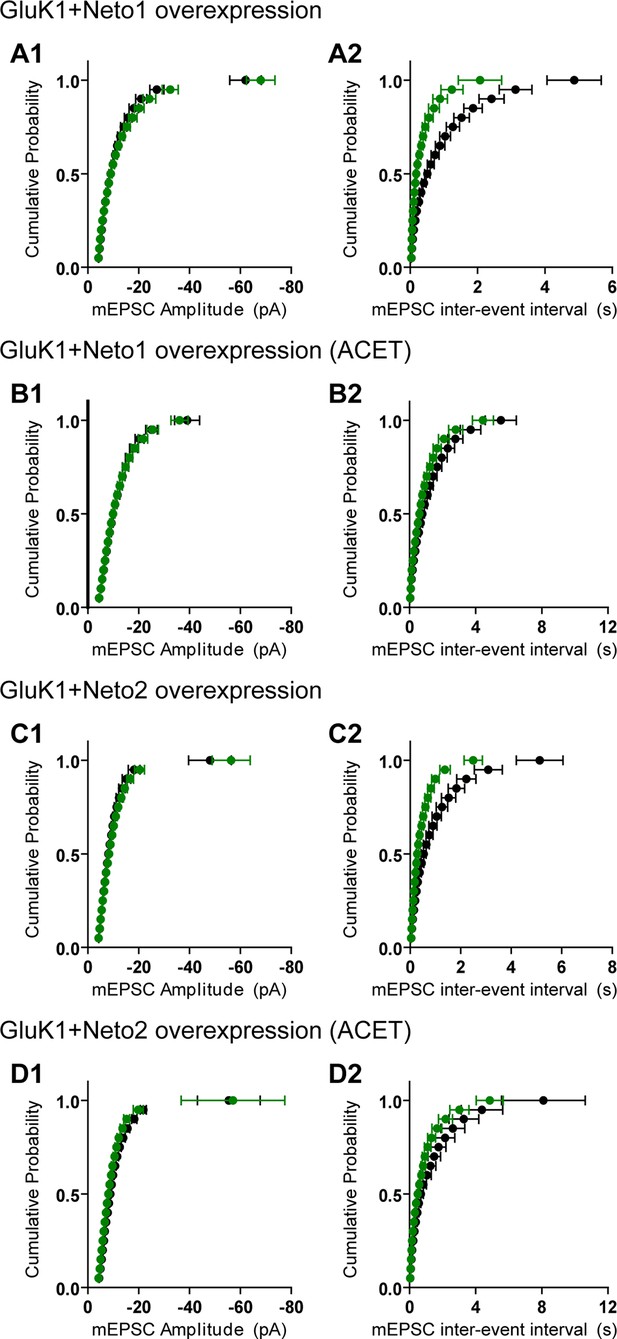
GluK1 receptors specifically traffic to silent synapses in the presence of Neto1 or Neto2.
(A1 and A2) Cumulative distribution plots of aEPSC amplitude and frequency from control (black) and GluK1/Neto1-coexpressed (green) neurons. Cumulative distribution functions show no irregularities. (B1 and B2) Cumulative distribution plots of aEPSC amplitude and frequency from control (black) and GluK1/Neto1-coexpressed (green) neurons in the presence of ACET. Cumulative distribution functions show no irregularities. (C1 and C2) Cumulative distribution plots of aEPSC amplitude and frequency from control (black) and GluK1/Neto2-coexpressed (green) neurons. Cumulative distribution functions show no irregularities. (D1 and D2) Cumulative distribution plots of aEPSC amplitude and frequency from control (black) and GluK1/Neto2-coexpressed (green) neurons in the presence of ACET. Cumulative distribution functions show no irregularities.
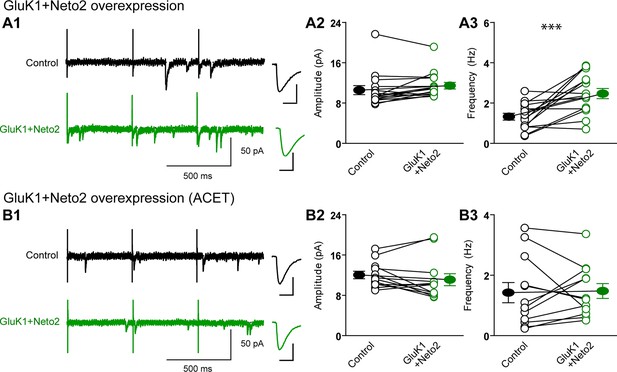
Neto2 specifically targets GluK1 receptors to silent synapse.
(A1) Representative sample traces of aEPSCsfrom control (black) and GluK1/Neto2-coexpressed (green) neurons. The first 50 ms following stimulation was excluded from analysis. The scale bars for single representative aEPSC trace were 10 pA and 10 ms. (A2) aEPSC amplitude is not significantly changed in GluK1/Neto2 expressed neurons (n=15, control: 10.56 ± 0.89 pA, GluK1/Neto2: 11.44 ± 0.67 pA, p > 0.05). Plot shows single pairs (open circles) and mean ± SEM (filled circles). (A3) aEPSC frequency is significantly increased in neurons expressing GluK1 and Neto2 (n=15, control: 1.32 ± 0.17 Hz, GluK1/Neto2: 2.45 ± 0.26 Hz, *** p < 0.0005). Plot shows single pairs (open circles) and mean ± SEM (filled circles). (B1) Representative sample traces of aEPSCs simultaneously recorded in the presence of Sr2+ and ACET from control (black) and GluK1/Neto2-coexpressed (green) neurons. The first 50 ms following stimulation was excluded from analysis. The scale bars for single representative aEPSC trace were 10 pA and 10 ms. (B2) Plot shows single pairs (open circles) and mean ± SEM (filled circles) of aEPSC amplitude from control and GluK1/Neto2 transfected neurons (n=12, control: 12.03 ± 0.74 pA, GluK1/Neto2: 11.1 ± 1.19 pA, p > 0.05). (B3) The aEPSC frequency in neurons expressing GluK1 and Neto2 is not significantly different from control ones in the presence of ACET (n=12, control: 1.42 ± 0.33 Hz, GluK1/Neto1: 1.48 ± 0.25 Hz, p > 0.05). Plot shows single pairs (open circles) and mean ± SEM (filled circles). All the statistical analyses are compared to respective control neurons with two-tailed Wilcoxon signed-rank sum test.
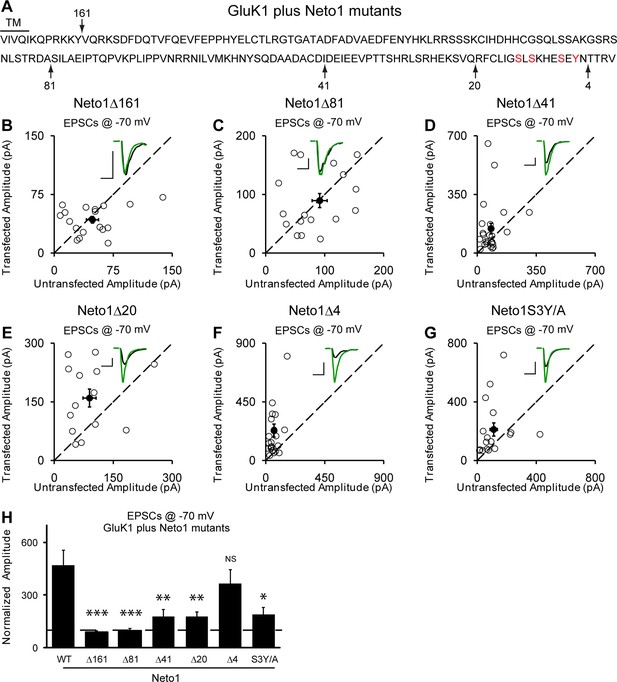
Neto1-mediated GluK1 synaptic trafficking is dependent on the critical serine and tyrosine residues in the intracellular region.
(A) Amino acid sequence of the Neto1 C-tail. The truncation mutants generated are indicated by arrows. The Neto1S3Y/A is a mutant in which the highlighted three serines and one tyrosine residues within the last 20 amino acids are mutated to alanines. (B–G) Scatter plots of eEPSCs at −70 mV for GluK1 co-expressed with various Neto1 mutants. Open circles are individual pairs and filled are mean ± SEM. Insets show sample current traces from control (black) and experimental (green) cells. The scale bars for representative eEPSC trace were 50 pA and 25 ms. (H) Normalized evoked EPSC amplitudes at −70 mV as percent of respective control ± SEM for each transfection (GluK1/Neto1: n=19, 470.65 ± 85.59% control; GluK1/Neto1Δ161: n=18, 87.7 ± 8.67% control, *** p < 0.0001; GluK1/Neto1Δ81: n=18, 97.8 ± 13.1% control, *** p < 0.0001; GluK1/Neto1Δ41: n=24, 179.2 ± 39.0% control, ** p < 0.005; GluK1/Neto1Δ20: n=14, 178.1 ± 25.5% control, ** p < 0.005; GluK1/Neto1Δ4: n=23, 365.62 ± 80.22% control, p > 0.05; GluK1/Neto1S3Y/A: n=17, 189.48 ± 40.26% control, * p < 0.05). All the statistical analyses are tested with the group co-overexpressing GluK1 and wildtype Neto1 using Mann-Whitney U-test.
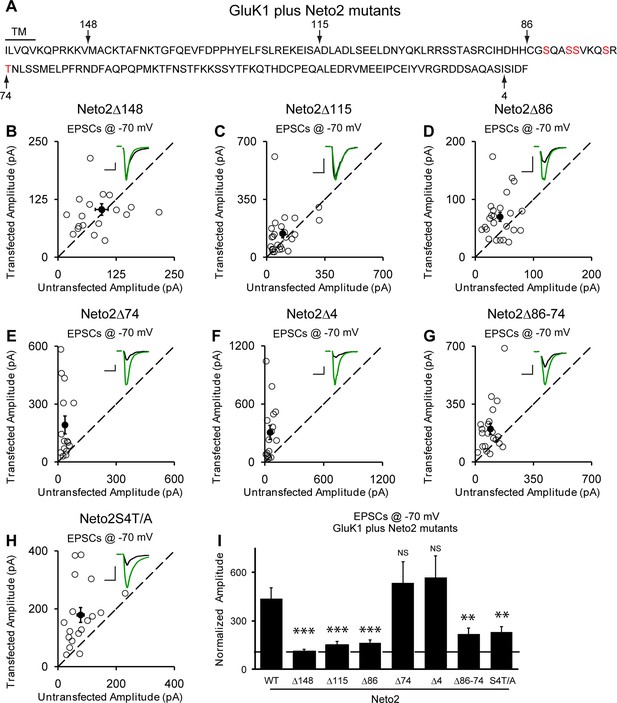
Neto2-mediated GluK1 synaptic trafficking is dependent on the critical serine and threonine residues in the intracellular region.
(A) Amino acid sequence of the Neto2 C-tail. The truncation mutants generated are indicated by arrows. The Neto2S4T/A is a mutant in which the highlighted four serines within region 86-74 and one threonine just next to this region are mutated to alanines. (B–H) Scatter plots of eEPSCs at −70 mV for GluK1 co-expressed with various Neto2 mutants. Open circles are individual pairs and filled are mean ± SEM. Insets show sample current traces from control (black) and experimental (green) cells. The scale bars for representative eEPSC trace were 50 pA and 25 ms. (I) Normalized evoked EPSC amplitudes at −70 mV as percent of respective control ± SEM for each transfection (GluK1/Neto2: n=17, 473.69 ± 65.08% control; GluK1/Neto2Δ148: n=19, 109.96 ± 13.27% control, *** p < 0.0001; GluK1/Neto2Δ115: n=25, 148.51 ± 25.59% control, *** p < 0.0005; GluK1/Neto2Δ86: n=24, 163.72 ± 18.08% control, *** p < 0.0005; GluK1/Neto2Δ74: n=15, 535.85 ± 129.35% control, p > 0.05; GluK1/Neto2Δ4: n=16, 567.38 ± 134.55% control, p > 0.05; GluK1/Neto2Δ86–74: n=21, 219.09 ± 35.39% control, ** p < 0.01; GluK1/Neto2S4T/A: n=18, 229.98 ± 32.99% control, ** p < 0.01). All the statistical analyses are tested with the group co-overexpressing GluK1 and wildtype Neto1 using Mann-Whitney U-test.
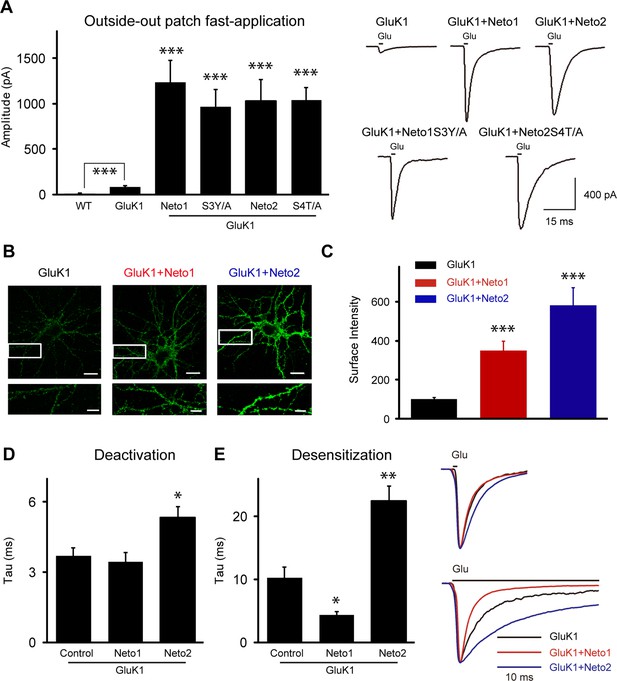
Neto1 and Neto2 increase GluK1 receptor surface trafficking and biophysical properties.
(A) Bar graphs show the amplitude of GluK1 currents (mean ± SEM) from outside-out patches pulled from wild type and transfected CA1 neurons with indicated plasmids and exposed to 1 or 100 ms applications of 10 mM glutamate and 100 μM GYKI53655 (WT, n=7, 8.57 ± 2.51 pA, *** p < 0.0005; GluK1: n=10, 81.65 ± 11.26 pA; GluK1/Neto1: n=9, 1231.94 ± 242.92 pA, *** p < 0.0001; GluK1/Neto1S3Y/A: n=7, 967.14 ± 138.30 pA, *** p < 0.0005; GluK1/Neto2: n=10, 1022.84 ± 241.81 pA, *** p < 0.0005; GluK1/Neto2S4T/A: n=9, 1035.22 ± 115.00 pA, *** p < 0.0001). All the statistical analyses are compared to GluK1 single overexpression using Mann-Whitney U-test. Sample traces and scale bar are shown to the right. (B) DIV 10 neurons were transfected with HA-GluK1 and Neto1 or Neto2, as indicated. At DIV 13, cells were stained for surface GluK1 and the intensity of surface GluK1 was quantitated (3 dendrites per neuron) using Metamorph analysis software. Scale bar, 20 μm. Images at the bottom of each panel are higher magnification from the enclosed region. Scale bar, 5 μm. (C) Bar graph shows the surface expression of GluK1 (mean ± SEM) from three independent experiments (GluK1: n=34; GluK1/Neto1: n=33; GluK1/Neto2: n=34). An unpaired two-tailed t-test was used to determine the significance of the data: *** p < 0.0001. (D and E) Bar graphs show mean ± SEM GluK1 deactivation (d, GluK1: n=10, 3.7 ± 0.35 ms; GluK1/Neto1: n=7, 3.43 ± 0.42 ms, p > 0.05; GluK1/Neto2: n=10, 5.33 ± 0.46 ms, * p < 0.05) and desensitization (e, GluK1: n=6, 12.42 ± 2.26 ms; GluK1/Neto1: n=8, 4.93 ± 0.59 ms, * p < 0.05; GluK1/Neto2: n=7, 27.49 ± 3.26 ms, ** p < 0.005) from outside-out patches pulled from indicated transfection CA1 neurons and exposed to 1 or 100 ms applications of 10 mM glutamate and 100 μM GYKI53655, respectively. All the statistical analyses are compared to GluK1 single overexpression using Mann-Whitney U-test. Sample traces are shown to the right and are peak-normalized.
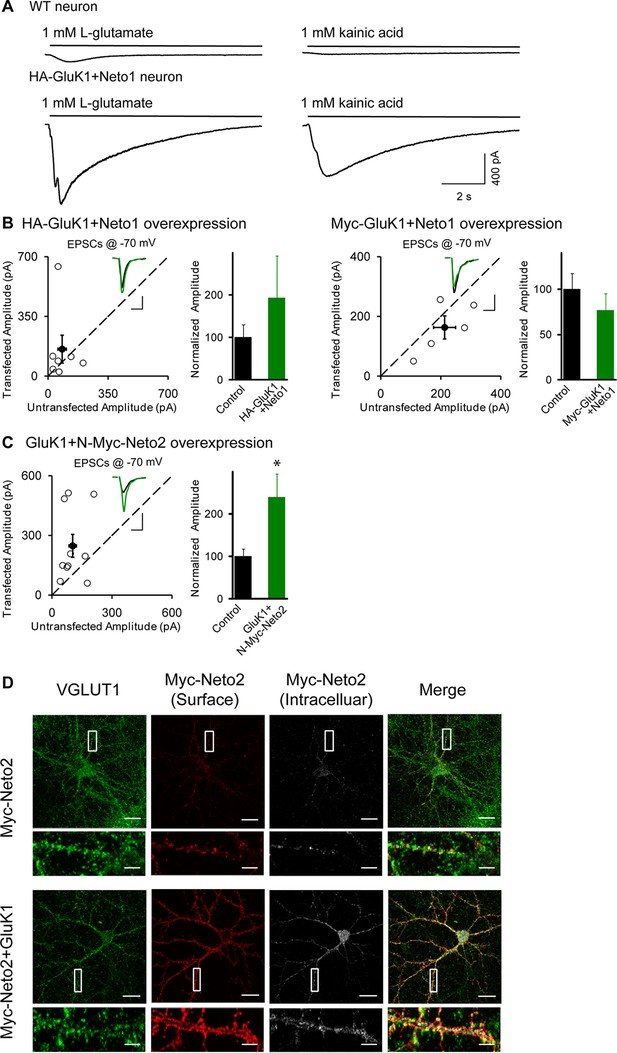
GluK1 receptor is localized at synapse.
(A) Electrophysiogical recordings of whole-cell puffing with 1 mM Glutamate (left) or 1 mM kainic acid (right) from wildtype (upper) or HA-GluK1/Neto1 coexpressed (lower) CA1 pyramidal neurons. (B) Scatter plots show eEPSC amplitudes measured at −70 mV of control and HA-GluK1/Neto1 or Myc-GluK1/Neto1 transfected neurons in rat hippocampal slice. Filled circles show mean ± SEM. Insets show sample current traces from control (black) and experimental (green) cells. The scale bars for representative eEPSC trace were 50 pA and 25 ms. Bar graph show normalized eEPSC amplitudes (mean ± SEM) (HA-GluK1/Neto1, n=7, 192.41 ± 99.67% control, p > 0.05; Myc-GluK1/Neto1, n=8, 78.89 ± 14.04% control, p > 0.05) presented in scatter plots. (C) Scatter plots show eEPSC amplitudes measured at –70 mV of control and GluK1/Myc-Neto2 transfected neurons in rat hippocampal slice. Filled circles show mean ± SEM. Insets show sample current traces from control (black) and experimental (green) cells. The scale bars for representative eEPSC trace were 100 pA and 25 ms. Bar graph show normalized eEPSC amplitudes (mean ± SEM) (n=10, 238.94 ± 55.43% control, * p < 0.05) presented in scatter plots. (D) DIV 10 neurons were transfected with Myc-Neto2 alone or together with GluK1 as indicated. At DIV 13, cells were stained for surface and intracellular Neto2 as well as presynaptic marker VGLUT1 using Metamorph analysis software. Scale bar, 20 μm. Images at the bottom of each panel are higher magnification from the enclosed region. Scale bar, 5 μm.





