Glycan modification of antigen alters its intracellular routing in dendritic cells, promoting priming of T cells
Figures
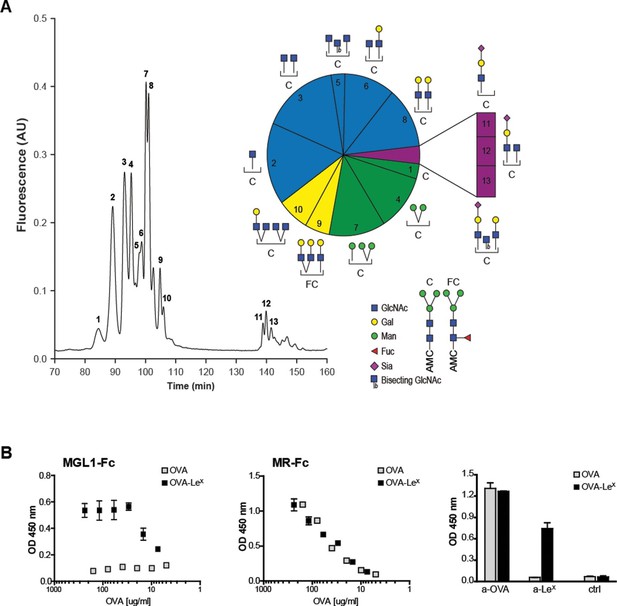
Generation of OVA-neo-glycoconjugates with LeX that confers binding of OVA to MGL1.
(A) A glycan profile of OVA was generated using a multidimensional normal phase nano-HPLC coupled with an electrospray ionization interface mass spectrometer with an intercalated nanofluorescence detector. The different glycan species, indicated by numbers, are shown on the right; their relative proportion is represented in a pie chart. (B) ELISA showing functional modification of OVA with LeX glycans, as detected with anti-LeX antibodies and resulting in binding of MGL1-Fc. Unconjugated OVA does not carry any ligands for MGL1. Modification of OVA with LeX did not alter the ability to bind to MR as illustrated by equal binding kinetics of MR-Fc to native OVA and OVA-LeX. OVA and OVA-LeX preparations contain similar amounts of OVA as detected with anti-OVA antibodies.
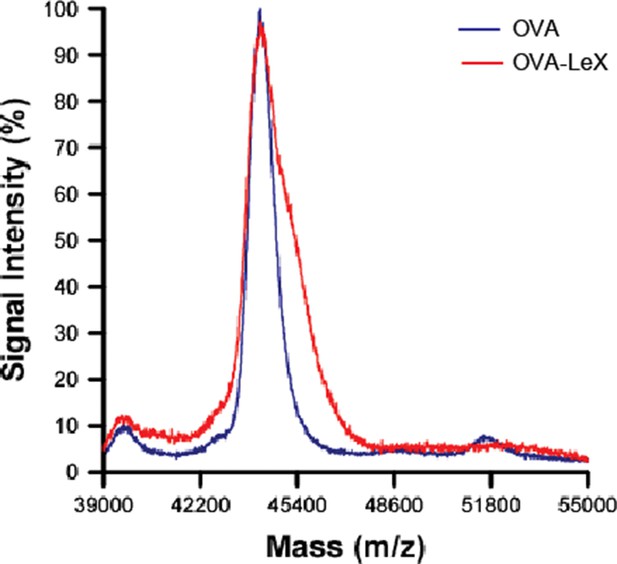
The MALDI-TOF/TOF mass spectrum of OVA-LeX (Red) shows an increase of 1,2 KDa compared to unconjugated OVA (Blue), corresponding to addition of two LeX molecules per OVA molecule.
https://doi.org/10.7554/eLife.11765.004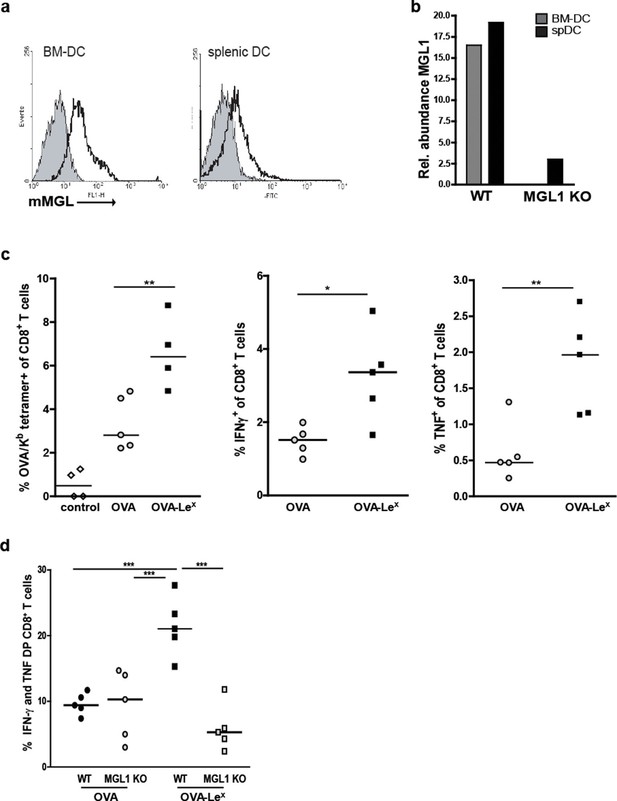
Immunization with OVA-LeX induces increased CD8+ T cell responses in vivo.
(A) Expression of murine MGL on BM-DCs and CD11c+ spDCs was analyzed by flow cytometry. (B) MGL1 mRNA expression by BM-DC and splenic DC from WT and MGL1 KO mice was determined using qRT-PCR. GAPDH was used as a reference gene and the results are representative of three independent experiments. C57BL/6 mice were immunized s.c. with either OVA-LeX or native OVA mixed with anti-CD40 using a prime-boost protocol. Spleens were analyzed by flow cytometry to determine the frequency of (C) H2-Kb/SIINFEKL-tetramer-binding CD8+ T cells and IFN-γ or TNF production by activated CD8+ T cells was determined by intracellular staining after OVA-specific re-stimulation ex vivo. Dots represent individual mice (n=4–5 mice/group; **p<0.01). Bars indicate median of each group. Graphs shown are representative of two independent experiments. (D) C57BL/6 and MGL1 KO mice were prime-boosted with either OVA-LeX or native OVA mixed with anti-CD40. Frequencies of IFN-γ and TNF-double-producing CD8+ T cells were determined by intracellular staining after OVA-specific re-stimulation of splenocytes ex vivo. Dots represent individual mice (n=4–5 mice/group; *p<0.05 ***p<0.001). Bars indicate median of each group. Data are representative of 2 independent experiments.
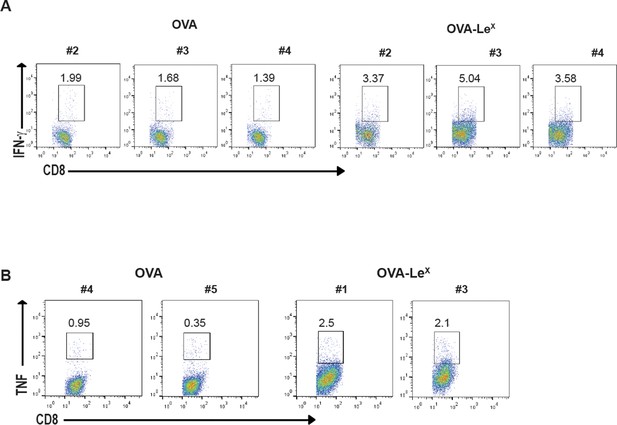
Representative flow cytometry plots of (A) IFN-γ and (B) TNF- producing CD8+ T cells in spleens of C57BL/6 mice that were immunized with either OVA-LeX or native OVA mixed with anti-CD40 using a prime-boost protocol; numbers above the gates designate the percentage of IFN-γ+ or TNF+ CD8+ T cells.
https://doi.org/10.7554/eLife.11765.006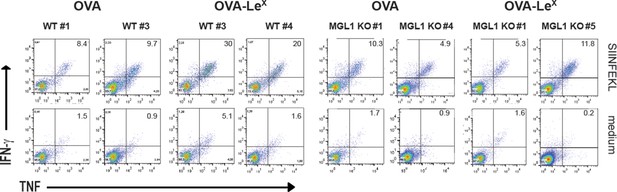
C57BL/6 and MGL1 KO mice were prime-boosted with either OVA-LeX or native OVA mixed with anti-CD40.
Frequencies of IFN-γ and TNF-double-producing CD8+ T cells were determined by intracellular staining after re-stimulation of splenocytes ex vivo. Representative facs plots of indicated mice are shown; numbers designate the percentage of IFN-γ and TNF-double positive CD8+ T cells.
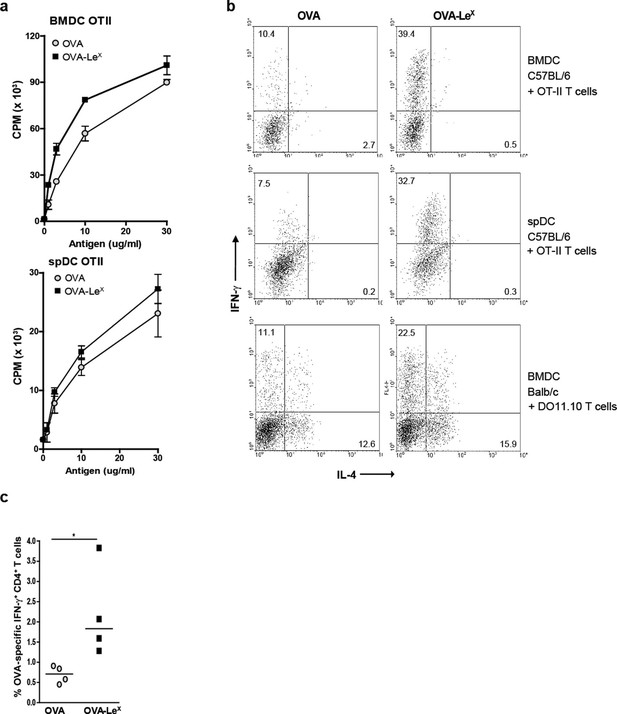
Modification of OVA with LeX structures skews naive CD4+ T cells towards the Th1-effector lineage.
(A) Pulsing of CD11c+ spDCs or BM-DCs with OVA-LeX results in equal OT-II proliferation as native OVA. Expansion of OVA-specific T cells was determined using 3H-thymidine incorporation. Data are shown as mean ± SD of triplicate cultures, representative of three independent experiments. (B) Flow cytometric analysis of OT-II or DO11.10 T cells differentiated by OVA-LeX or OVA-loaded spDCs or BM-DCs. Cells were gated on CD4+ T cells. Numbers in dot plots indicate the percentage of IFN-γ+ or IL-4+ of CD4+ T cells. Dot plots are representative of five independent experiments. (C) C57BL/6 mice were immunized s.c. with either OVA-LeX or native OVA mixed with anti-CD40 using a prime-boost protocol and the frequency of IFN-γ-producing activated CD4+ T cells in spleen was determined by intracellular staining after OVA-specific re-stimulation ex vivo. Dots represent individual mice, bars indicate median of each group (n=5 mice/group, **p<0.01). Graphs shown are representative of two independent experiments.
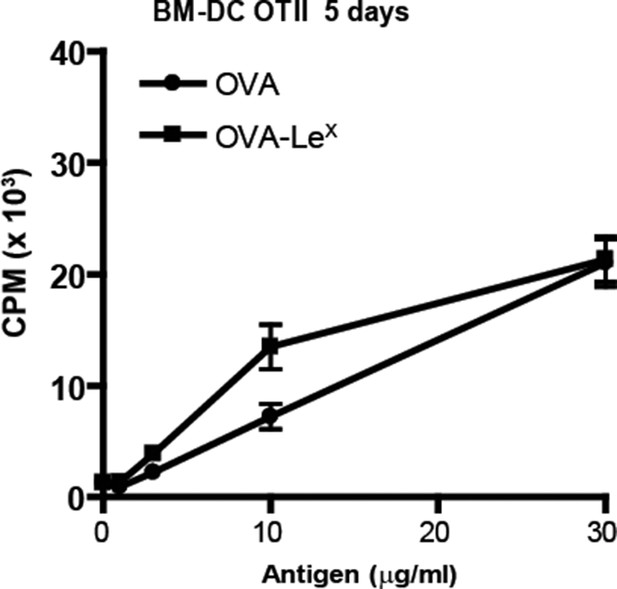
No enhanced expansion of OT-II T cells when co-cultured for six days with OVA-LeX pulse-loaded DCs.
WT BM-DC were loaded with OVA-LeX or native OVA for 4h and subsequently co-cultured with OT-II T cells for six days. Proliferation of OT-II T cells was determined by [3H]-thymidine uptake and presented as mean ± SD of triplicate cultures. Data shown are representative of two independent experiments.
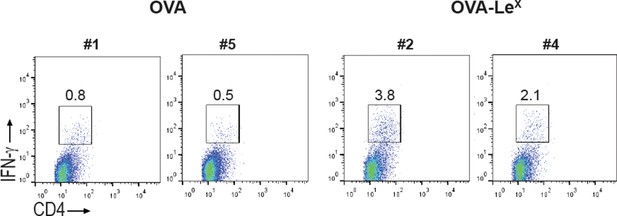
C57BL/6 mice were immunized s.c. with either OVA-LeX or native OVA mixed with anti-CD40 using a prime-boost protocol and the frequency of IFN-γ-producing activated CD4+ T cells in spleen was determined by intracellular staining after OVA-specific re-stimulation ex vivo.
Representative facs plots of indicated mice are shown; numbers above the gates designate the percentage of IFN-γ+ CD4+ T cells.
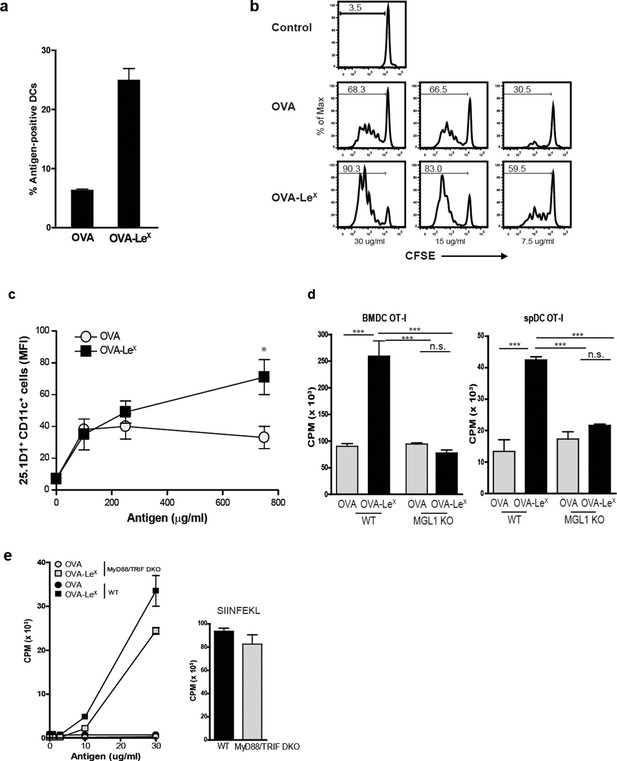
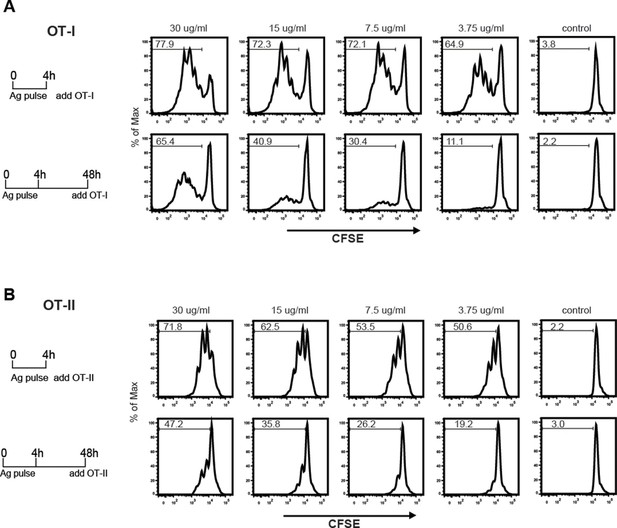
MGL1 mediates cross-presentation of OVA-LeX independently of TLR signaling.
(A) Uptake of fluorescent-labeled OVA-LeX or native OVA (30 µg/ml) by WT BM-DCs was analyzed by flow cytometry after 90 min. Graphs indicate the mean ± SD of triplicates and are representative of three independent experiments. (B) CFSE-labeled OT-I T cells were incubated with BM-DCs pulse-loaded with indicated concentrations of OVA-LeX or OVA for 4h. Un-loaded DC served as controls. Proliferation of OT-I T cells was analyzed after 3 days by flow cytometry. Percentages of divided OT-I cells are indicated. (C) OVA-LeX induces more OVA257-264/H2-Kb I complexes at the cell-surface of DCs than native OVA, as shown by 25.1D1 staining 18h after pulse loading of BM-DCs with OVA-LeX or native OVA. *p<0.05. (D) WT or MGL1 KO BM-DCs or CD11c+ spDCs are pulsed with OVA-LeX (black bars) or native OVA and OT-I proliferation was determined on day 3 by [3H]-thymidine uptake. Data are presented as mean ± SD of triplicates, representative of three independent experiments. ***p<0.001, ns not significant. (E) Cross-presentation of OVA-LeX is independent of MyD88 and/or TRIF signaling. BM-DC from WT or MyD88/TRIF DKO mice were pulsed with indicated concentrations of antigen and co-cultured with OT-I T cells. DCs pulsed with the nominal epitope SIINFEKL served as controls (right panel). Proliferation was determined by [3H]-thymidine uptake. Data are representative of two experiments and indicated as mean ± SD of triplicates.
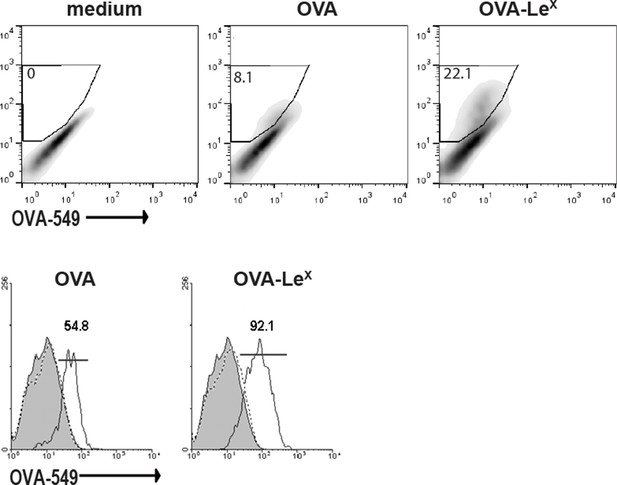
Uptake of fluorescent-labeled OVA-LeX or native OVA (30 µg/ml) by WT BM-DCs was analyzed by flow cytometry after 90 min (upper panel).
Control cells were incubated with medium. The percentage of gated antigen-positive DCs are indicated. Lower panel: histograms indicating the mean uptake of OVA (left, black line) or OVA-LeX (right, black line) versus medium (grey filled histograms) and EGTA (dashed lines) controls. Numbers indicate the MFI of OVA-positive DCs.
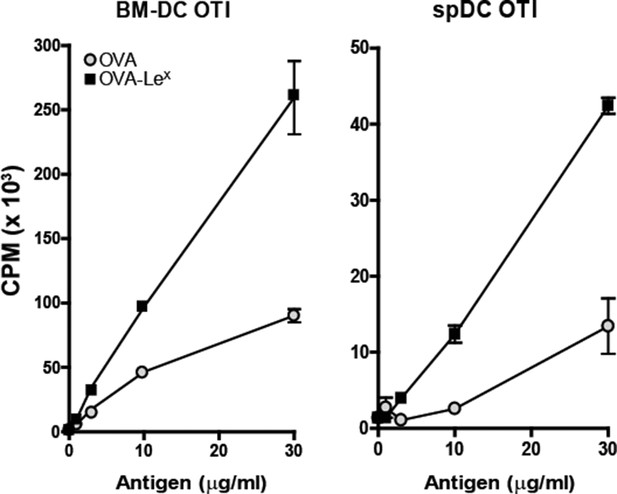
Enhanced cross-presentation of OVA-LeX by DCs as measured by 3H-Thymidine incorporation.
BM-DCs and CD11c+ spDCs loaded with OVA-LeX enhanced OT-I proliferation compared to native OVA loaded DCs. Proliferation was determined on day 3 by [3H]-Thymidine uptake and presented as mean ± SD of triplicate cultures. Data are representative of four independent experiments.
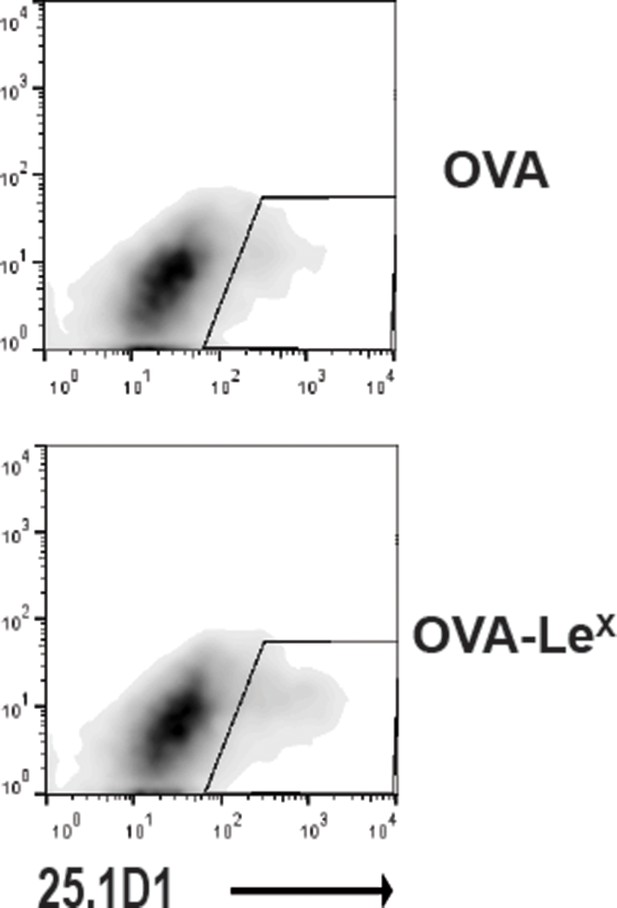
Representative flow cytometry plots of 25.1D1 staining of BM-DCs 18h after pulse loading with OVA-LeX or native OVA (750 ug/ml).
https://doi.org/10.7554/eLife.11765.014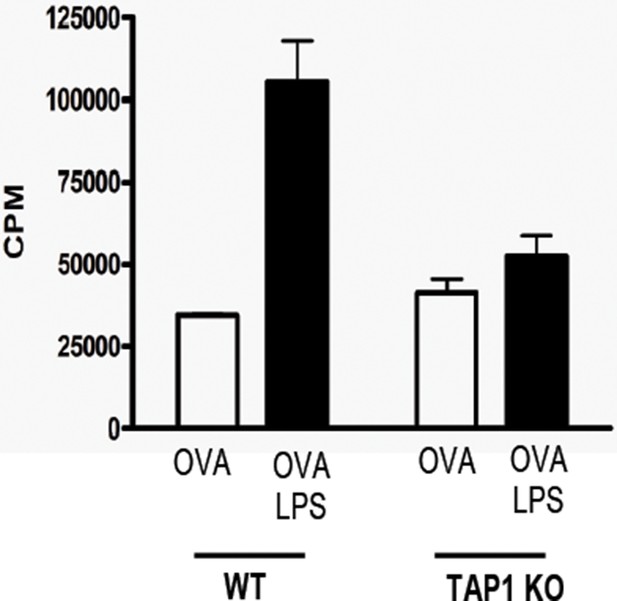
Cross-presentation of OVA requires TLR4 triggering and is TAP-dependent.
WT or TAP1-deficient BM-DC were loaded with OVA (0.5 mg/ml) in the presence or absence of 100 ng/ml LPS and subsequently co-cultured with purified OT-I T cells for 3 days. [3H]-Thymidine is incorporated during the last 18h and is presented as mean ± SD of triplicate cultures. Data are representative of two independent experiments.
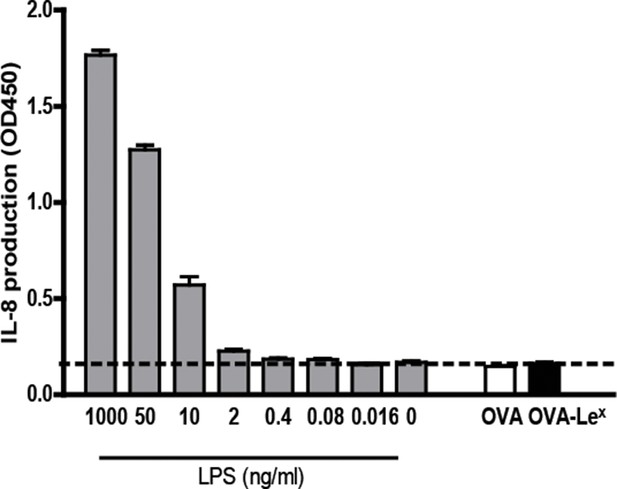
OVA-LeX formulations are free of endotoxins.
Both OVA and OVA-LeX were tested for endotoxin levels. Human embryonic kidney (HEK)293-TLR4/MyD88 transfectants (kind gift of Dr. D. Golenbock) were cultured in the presence of either antigen preparation (30 µg/ml) or indicated amounts of E. coli-derived LPS (Sigma Aldrich). The HEK transfectants respond to LPS by secreting IL-8. In both preparations, LPS was below detection limits (dashed line). Results are representative of two independent experiments.
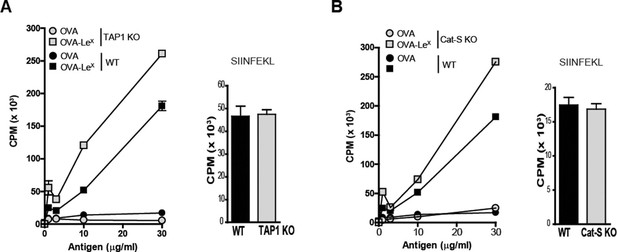
LeX-modified antigen is cross-presented in a TAP- and Cathepsin-S-independent fashion.
To examine whether cross-presentation of OVA-LeX involves TAP or Cathepsin-S (A) TAP1 KO and (B) Cat-S KO BM-DCs and WT BM-DCs were pulsed with OVA-LeX or native OVA and co-cultured with OT-I T cells for 3 days. DCs exogenously loaded with SIINFEKL for 3h served as control. Proliferation was determined by [3H]-Thymidine uptake and data are presented as mean ± SD of triplicates (representative of three experiments).
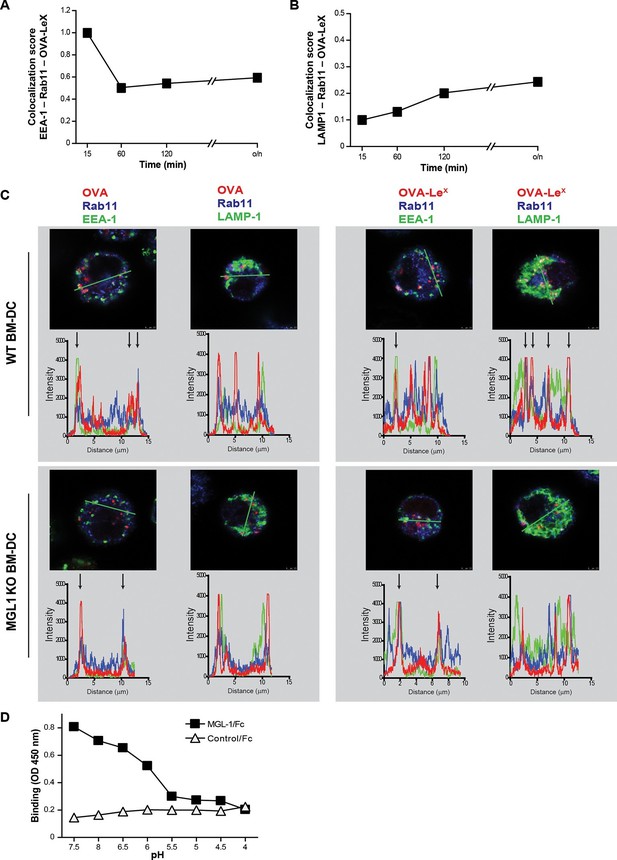
OVA-LeX is routed to Rab11+LAMP1+ compartments where it is stored for presentation in MHC class I.
(A, B) WT BM-DCs were pulsed with AlexaFluor 674-OVA-LeX (30 µg/ml) and chased at the indicated time-points to assess triple co-localization scores of OVA-LeX with (A) EEA-1 and Rab11 or (B) LAMP1 and Rab11 using imaging flow cytometry. (C) WT (upper panels) and MGL1 KO (lower panels) BM-DCs were incubated with Dylight-633-OVA-LeX or native OVA (30 µg/ml) and 2h later co-localization of OVA antigen (Red) with early endosomal (EEA-1, Green) or endosomal/lysosomal (LAMP1, Green) and recycling endosomal (Rab11, Blue) compartments was analyzed using CSLM. From a z-stack, histograms were created for a selected area (indicated by a line, upper part of each panel) using the Leica confocal software. Histograms were created from each fluorochrome and overlays were made by the program. Arrows indicate co-localization of antigen (Red) with EEA1&Rab11 or LAMP1&Rab11. (D) MGL1-Fc binding to LeX-PAA was determined at indicated pH by ELISA.
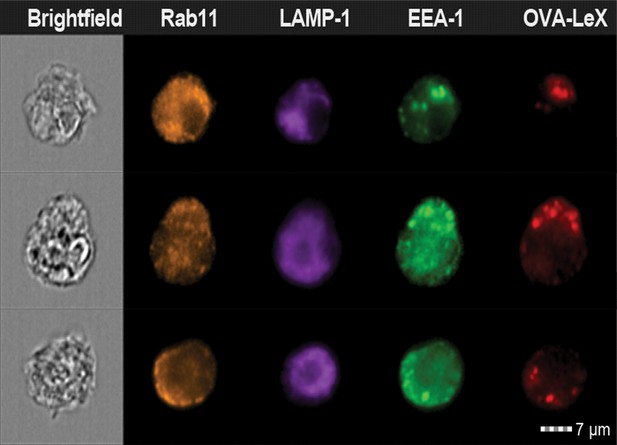
Examples of WT BM-DCs pulsed with AlexaFluor 674-OVA-LeX displaying high co-localization of OVA-LeX with EEA-1 and Rab11 as measured by imaging flow cytometry.
https://doi.org/10.7554/eLife.11765.019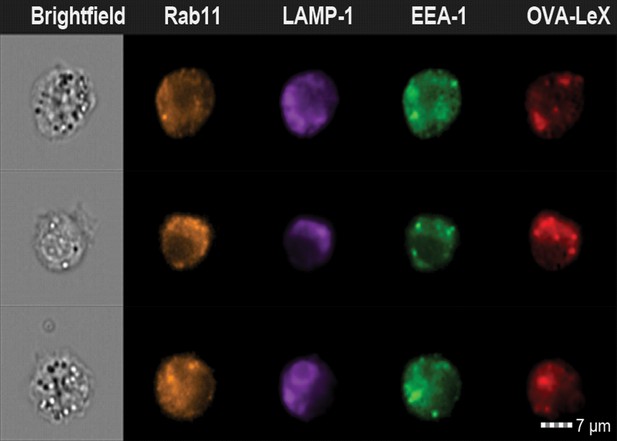
Examples of WT BM-DCs pulsed with AlexaFluor 674-OVA-LeX displaying high co-localization of OVA-LeX with LAMP1 and Rab11 as measured by imaging flow cytometry.
https://doi.org/10.7554/eLife.11765.020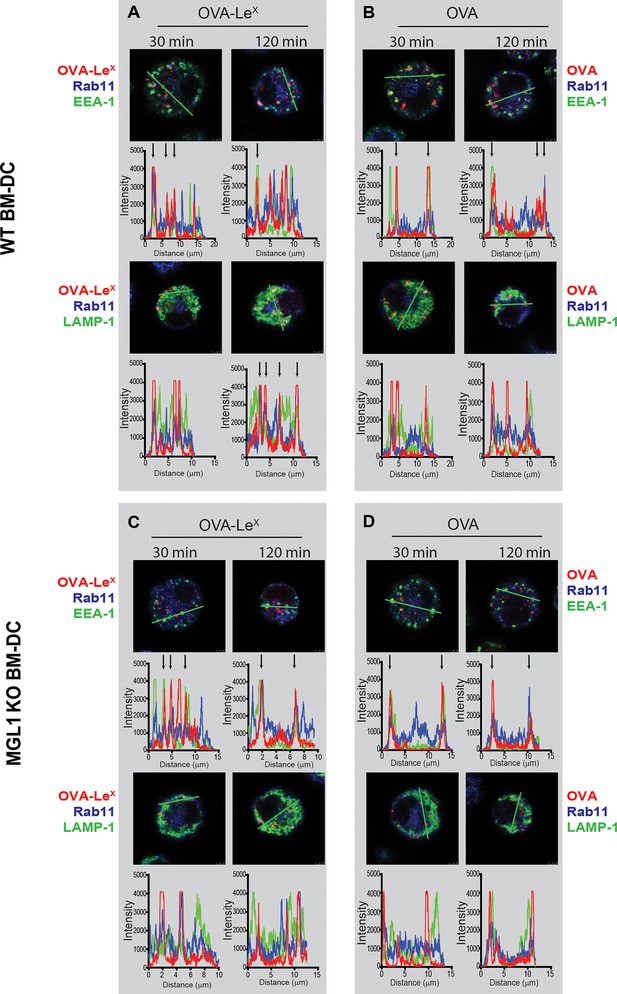
WT (upper panels) and MGL1 KO (lower panels) BM-DCs were incubated with Dylight-633-OVA-LeX or native OVA (30 µg/ml) and 0.5 h and 2 h later co-localization of OVA antigen (Red) with early endosomal (EEA-1, Green) or endosomal/lysosomal (LAMP1, Green) and recycling endosomal (Rab11, Blue) compartments was analyzed using CSLM.
From a z-stack, histograms were created for a selected area (indicated by a line) using the Leica confocal software. Histograms were created from each fluorochrome and overlays were made by the program. Arrows indicate co-localization of antigen (Red) with EEA1&Rab11 or LAMP1&Rab11.
MGL1 targeting with OVA-LeX shows sustained antigen presentation in MHC-I.
WT BM-DCs were pulsed for 4 h with titrated amounts of OVA-LeX and washed with culture medium. DCs were then chased for 48 h in antigen-free medium. (A) BM-DCs pulsed for 4h with OVA-LeX induced MHC-I antigen presentation as measured by CFSE-labeled OVA-specific OT-I cells (upper panel). Sustained presentation is shown after 48 h (lower panel). (B) MHC-II antigen presentation 4h and 48h after pulse-loading with OVA-LeX, analyzed by OT-II proliferation. Data are presented as percentage of proliferated T cells and representative of three independent experiments.
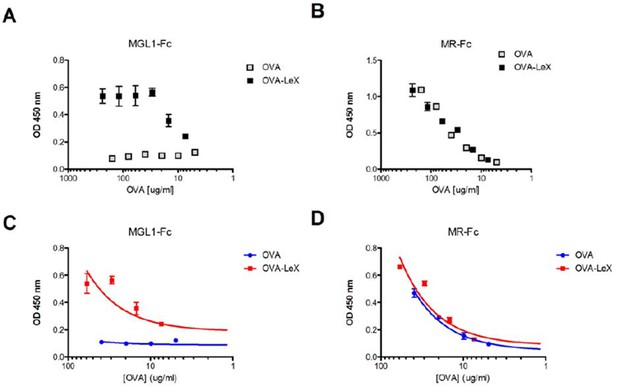
Generation of OVA-neo-glycoconjugates with LeX that confers binding of OVA to MGL1.
(A) ELISA showing functional modification of OVA with LeX glycans, resulting in binding of MGL1-Fc. Unconjugated OVA does not carry any ligands for MGL1. Modification of OVA with LeX did not alter the ability to bind to MR as illustrated by equal binding kinetics of MR-Fc to OVA and OVA-LeX (B). (C+D) Linear regression analysis of MGL1-Fc and MR-Fc binding to OVA and OVA-LeX at the concentrations used in the study, clearly shows differential binding of OVA-LeX to MGL1 but not to MR due to the modification with LeX.





