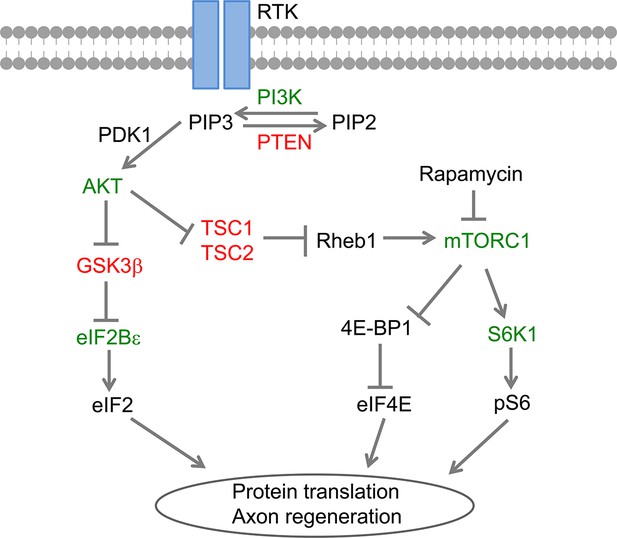
GSK3β regulates AKT-induced central nervous system axon regeneration via an eIF2Bε-dependent, mTORC1-independent pathway
Figures
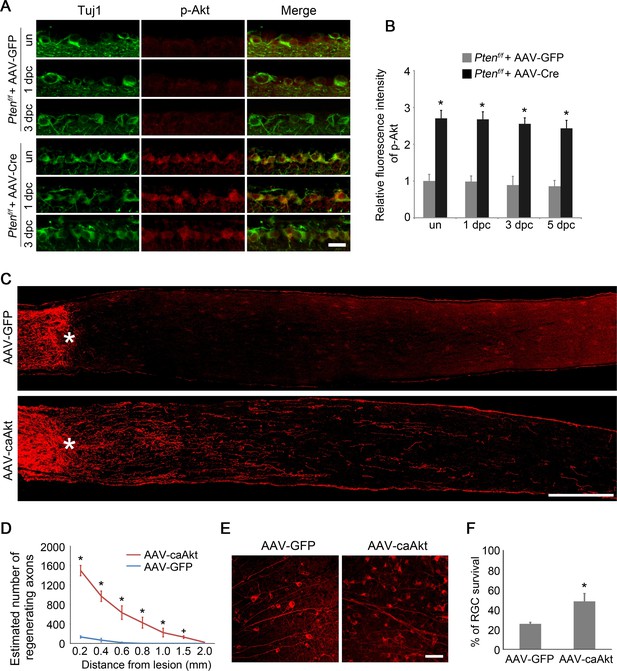
AKT activation promotes axon regeneration and RGC survival.
(A) Detection of AKT Phosphorylation at Ser473 in Pten-deleted RGCs labeled by anti-Tuj1 immunohistochemistry, with or without (un) optic nerve injury. Scale bar, 20 µm. (B) Quantification of phospho-AKT immunofluorescence intensity following Pten deletion. Data are presented as mean ± s.d., n=4 per group. *p<0.001, Two-way ANOVA with Bonferroni post hoc test. (C) Confocal images of optic nerve sections showing regenerating axons labeled by anti-GAP43 immunohistochemistry at 2 weeks after optic nerve injury from AAV-GFP or AAV-caAkt injected eyes. *, crush site. Scale bar, 200 µm. (D) Quantification of regenerating axons at different distances distal to the lesion site. Data are presented as mean ± s.d., n=5 per group. *p<0.01, +p<0.05, Two-way ANOVA with Bonferroni post hoc test. (E) Confocal images of retinal whole-mounts showing surviving Tuj1+ RGCs at 2 weeks after optic nerve injury. Scale bar, 50 µm. (F) Quantification of RGC survival at 2 weeks after injury, expressed as a percentage of total Tuj1+ RGCs in the uninjured retina. Data are presented as mean ± s.d., n=5 per group. *p<0.01, Student’s t test.
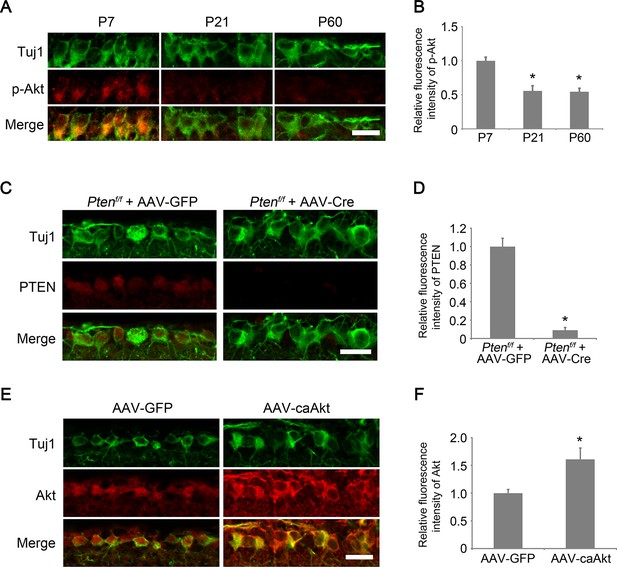
Developmental analysis of AKT phosphorylation in RGCs.
(A) Confocal images of retinal sections showing phospho-AKT immunoreactivity in Tuj1+ RGCs at postnatal day 7, 21, and 60. Scale bar, 20 µm. (B) Quantification of phospho-AKT immunofluorescence intensity relative to postnatal day 7. Data are presented as mean ± s.d., n=5 per group. *p<0.001, One-way ANOVA with Dunnett's test. (C) Confocal images of retinal sections showing PTEN immunoreactivity in Tuj1+ RGCs from AAV-GFP or AAV-Cre injected Ptenf/f eyes, Scale bar, 20 µm. (D) Quantification of PTEN immunofluorescence intensity. Data are presented as mean ± s.d., n=3 per group. *p<0.001, Student’s t test. (E) Confocal images of retinal sections showing double immunolabeling for Tuj1+ RGCs and AKT from AAV-GFP or AAV-caAkt injected eyes, Scale bar, 20 µm. (F) Quantification of AKT immunofluorescence intensity. Data are presented as mean ± s.d., n=3 per group. *p<0.01, Student’s t test.
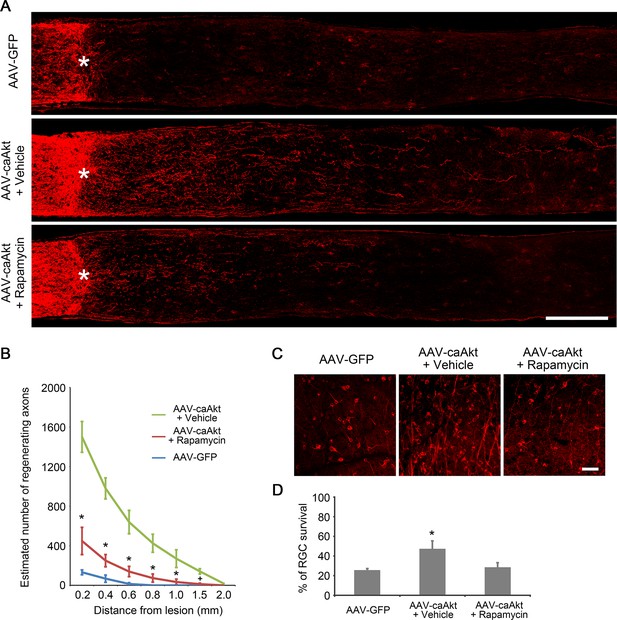
Inhibition of mTORC1 partially reduces AKT-induced axon regeneration.
(A) Confocal images of optic nerve sections showing regenerating axons labeled by anti-GAP43 immunohistochemistry at 2 weeks after injury from AAV-GFP or AAV-caAkt injected eyes treated with vehicle or rapamycin. *, crush site. Scale bar, 200 µm. (B) Quantification of regenerating axons at different distances distal to the lesion site. Data are presented as mean ± s.d., n=6 per group. *p<0.01, +p<0.05, Two-way ANOVA with Bonferroni post hoc test. (C) Confocal images of retinal whole-mounts showing surviving Tuj1+ RGCs at 2 weeks after optic nerve injury. Scale bar, 50 µm. (D) Quantification of RGC survival at 2 weeks after injury, expressed as a percentage of total Tuj1+ RGCs in the uninjured retina. Data are presented as mean ± s.d., n=5 per group. *p<0.001, One-way ANOVA with Dunnett's test. Data from AAV-GFP was used as comparison control.
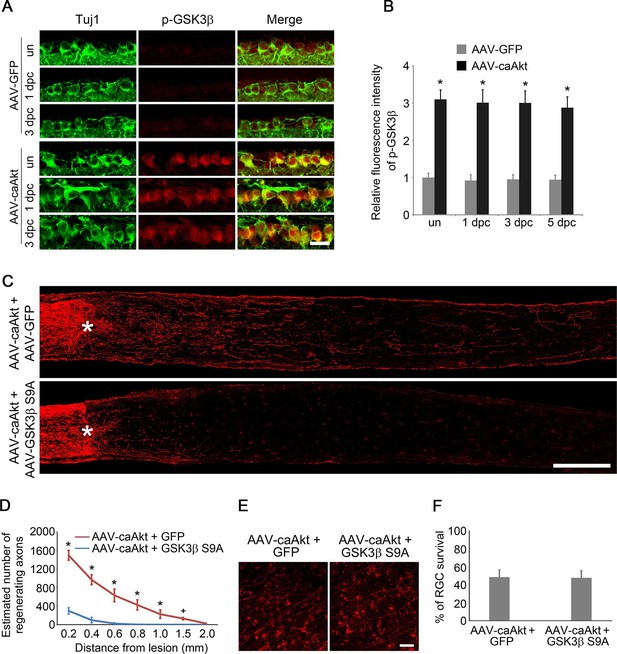
GSK3β is an essential downstream effector to mediate AKT-induced axon regeneration.
(A) Immunohistochemical detection of GSK3β phosphorylation at Ser9 in retinal sections from AAV-GFP or AAV-caAkt-injected eyes, either with or without optic nerve injury. Scale bar, 20 µm. (B) Quantification of phospho-GSK3β immunofluorescence intensity. Data are presented as mean ± s.d., n=4 per group. *p<0.001, Two-way ANOVA with Bonferroni post hoc test. (C) Confocal images of optic nerve sections showing regenerating axons labeled by anti-GAP43 immunohistochemistry at 2 weeks after optic nerve injury from AAV-caAKT + AAV-GFP or AAV-caAkt + AAV-GSK3β S9A injected eyes. *, crush site. Scale bar, 200 µm. (D) Quantification of regenerating axons at different distances distal to the lesion site. Data are presented as mean ± s.d., n=5 per group. *p<0.01, +p<0.05, Two-way ANOVA with Bonferroni post hoc test. (E) Confocal images of retinal whole-mounts showing surviving Tuj1+ RGCs at 2 weeks after optic nerve injury. Scale bar, 50 µm. (F) Quantification of RGC survival at 2 weeks after injury, expressed as a percentage of total Tuj1+ RGCs in the uninjured retina. Data are presented as mean ± s.d., n=5 per group.
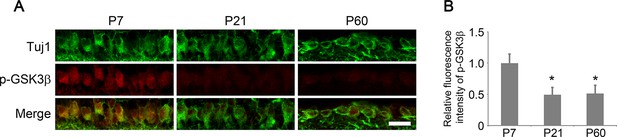
Developmental analysis of GSK3β phosphorylation in RGCs.
(A) Confocal images of retinal sections showing double immunolabeling for Tuj1+ RGCs and phospho-GSK3β at postnatal day 7, 21, and 60. Scale bar, 20 µm. (B) Quantification of phospho-GSK3β immunofluorescence intensity relative to postnatal day 7. Data are presented as mean ± s.d., n=5 per group. *p<0.001, One-way ANOVA with Dunnett's test.
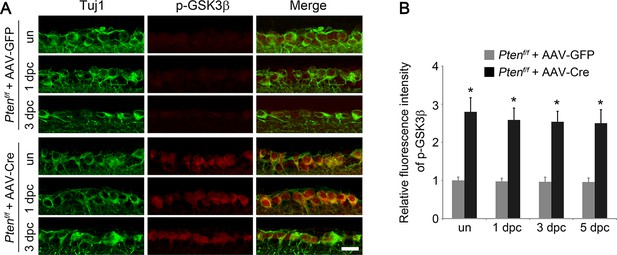
Pten deletion results in GSK3β phosphorylation in RGCs.
(A) Detection of GSK3β phosphorylation in RGCs by double immunolabeling for phospho-GSK3β (Ser9) and Tuj1 in retinal sections from Ptenf/f mice injected with AAV-GFP or AAV-Cre, with or without optic nerve crush. Scale bar, 20 µm. (B) Quantification of phospho-GSK3β immunofluorescence intensity. Data are presented as mean ± s.d., n=4 per group. *p<0.001, Two-way ANOVA with Bonferroni post hoc test.
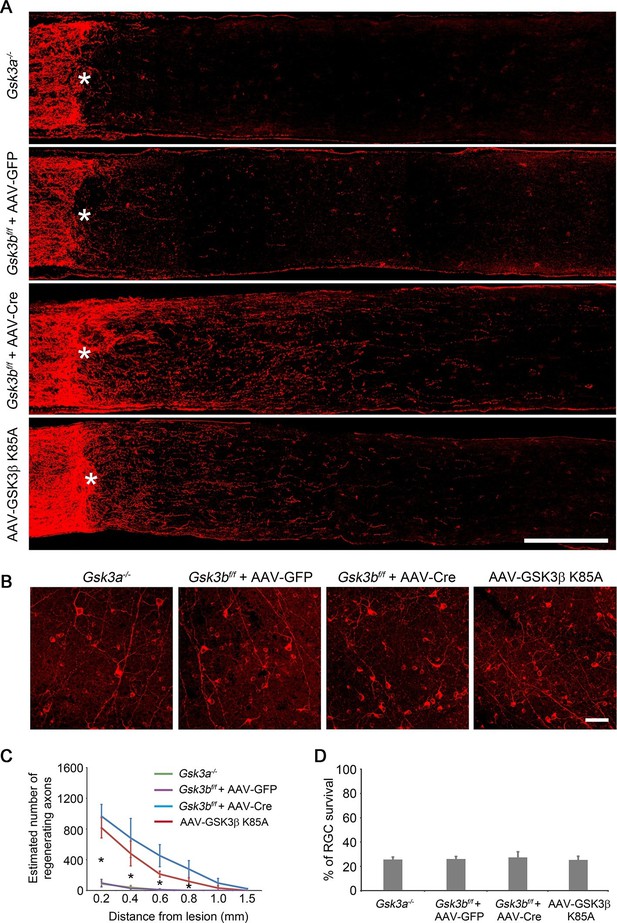
Deletion or inactivation of GSK3β promotes axon regeneration.
(A) Confocal images of optic nerve sections showing regenerating axons labeled by anti-GAP43 immunohistochemistry at 2 weeks after optic nerve crush from Gsk3a−/− mice, Gsk3bf/f mice injected with AAV-GFP or AAV-Cre, or AAV-GSK3β K85A injected wild-type mice. *, crush site. Scale bar, 200 µm. (B) Confocal images of retinal whole-mounts showing surviving Tuj1+ RGCs at 2 weeks after optic nerve injury. Scale bar, 50 µm. (C) Quantification of regenerating axons at different distances distal to the lesion site. Data are presented as mean ± s.d., n=5 per group. *p<0.01, Two-way ANOVA with Bonferroni post hoc test. (D) Quantification of RGC survival at 2 weeks after injury, expressed as a percentage of total Tuj1+ RGCs in the uninjured retina. Data are presented as mean ± s.d., n=5 per group.
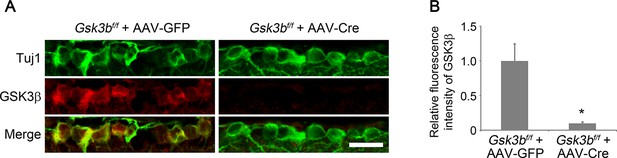
Cre-mediated Gsk3b deletion in RGCs.
(A) Confocal images of retinal sections showing double immunolabeling for Tuj1+ RGCs and GSK3β from AAV-GFP or AAV-Cre-injected Gsk3bf/f eyes, Scale bar, 20 µm. (B) Quantification of GSK3β immunofluorescence intensity. Data are presented as mean ± s.d., n=3 per group. *p<0.01, Student’s t test.
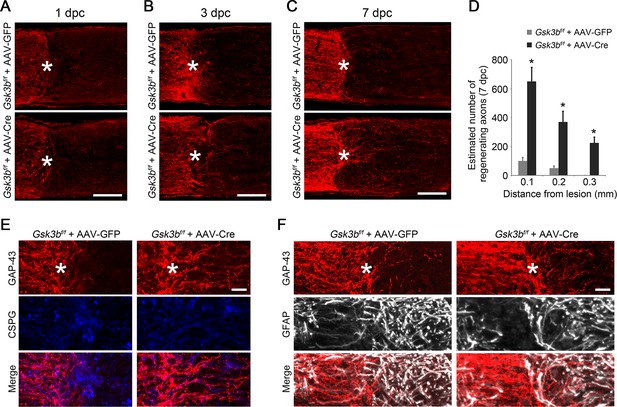
A time course study of axon regeneration in Gsk3b-deleted RGCs.
(A-C) Confocal images of optic nerve sections showing regenerating axons labeled by anti-GAP43 immunohistochemistry at 1, 3, or 7 days from Gsk3bf/f mice injected with AAV-GFP or AAV-Cre. *, crush site. Scale bar, 100 µm. (D) Quantification of regenerating axons at 7 days after injury from Gsk3bf/f mice injected with AAV-GFP or AAV-Cre at different distances distal to the lesion site. Data are presented as mean ± s.d., n=4 per group. *p<0.01, Two-way ANOVA with Bonferroni post hoc test. (E) Confocal images of optic nerve sections showing double immunolabeling for GAP43+ regenerating axons and chondroitin sulfate proteoglycan (CSPG). Scale bar, 20 µm. (F) Confocal images of optic nerve sections showing double immunolabeling for GAP43+ regenerating axons and glial fibrillary acidic protein (GFAP). Scale bar, 20 µm.
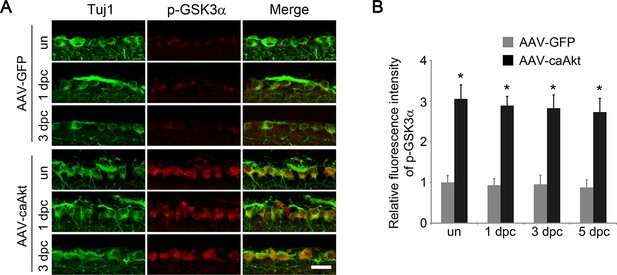
AKT activation increases GSK3α phosphorylation.
(A) Immunohistochemical detection of GSK3α phosphorylation at Ser21 in retinal sections from AAV-GFP or AAV-caAkt injected eyes, with or without optic nerve injury. Scale bar, 20 µm. (B) Quantification of RGC phospho-GSK3α immunofluorescence intensity. Data are presented as mean ± s.d., n=4 per group. *p<0.001, Two-way ANOVA with Bonferroni post hoc test.
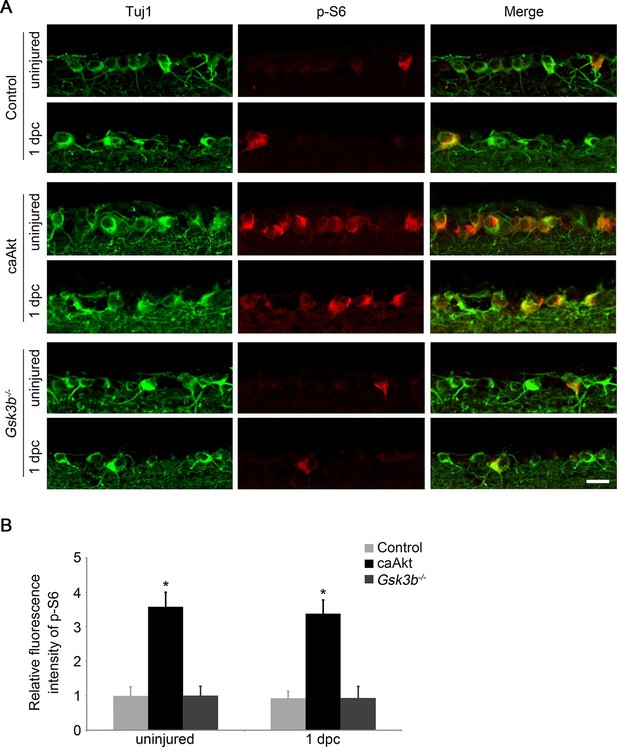
Gsk3b deletion does not alter the mTORC1 pathway.
(A) Confocal images of retinal sections showing double immunolabeling for Tuj1+ RGCs and phospho-S6 from AAV-GFP, AAV-caAkt, or AAV-Cre (Gsk3bf/f)-injected eyes, with or without optic nerve injury. Scale bar, 20 µm. (B) Quantification of phospho-S6 immunofluorescence intensity. Data are presented as mean ± s.d., n=4 per group. *p<0.001, Two-way ANOVA with Bonferroni post hoc test.
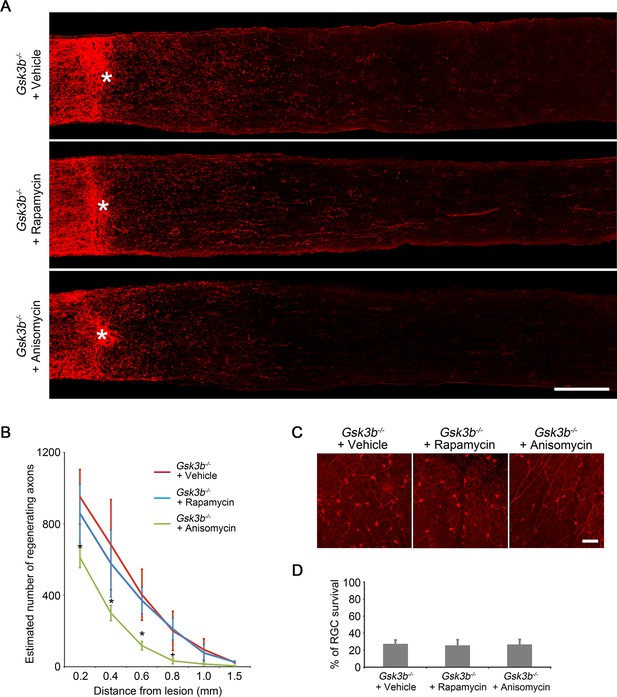
Gsk3b deletion-induced axon regeneration is sensitive to protein synthesis inhibition but not to mTORC1 inhibition.
(A) Confocal images of optic nerve sections showing regenerating axons labeled by anti-GAP43 immunohistochemistry at 2 weeks after injury in Gsk3bf/fmice injected with AAV-Cre with vehicle, rapamycin, or anisomycin treatment. *, crush site. Scale bar, 200 µm. (B) Quantification of regenerating axons at different distances distal to the lesion site. Data are presented as mean ± s.d., n=6 per group. *p<0.01, +p<0.05, Two-way ANOVA with Bonferroni post hoc test. (C) Confocal images of retinal whole-mounts showing surviving Tuj1+ RGCs at 2 weeks after optic nerve injury. Scale bar, 50 µm. (D) Quantification of RGC survival at 2 weeks after injury, expressed as a percentage of total Tuj1+ RGCs in the uninjured retina. Data are presented as mean ± s.d., n=5 per group.
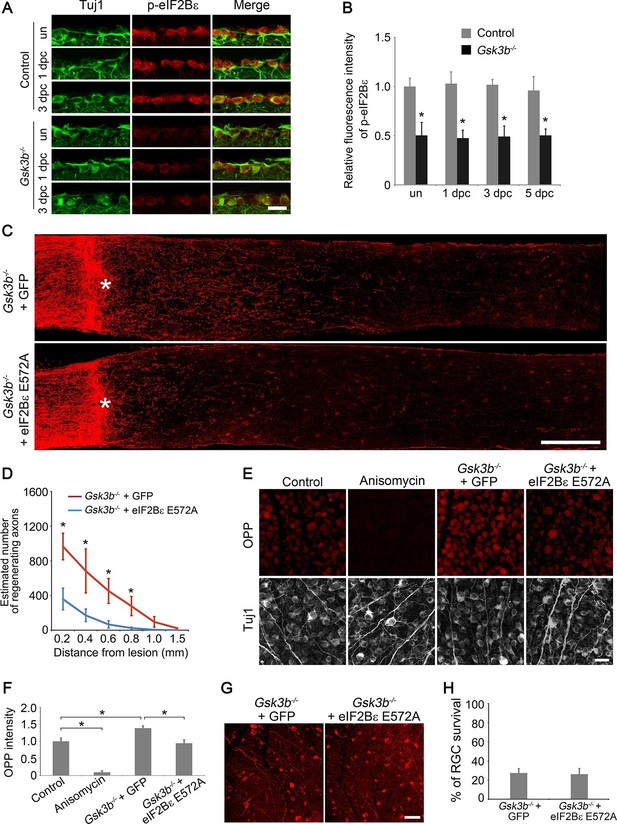
eIF2Bε is required for Gsk3b deletion-induced axon regeneration.
(A) Confocal images of retinal sections showing double immunolabeling for Tuj1+ RGCs and phospho-eIF2Bε from Gsk3bf/fmice injected with AAV-GFP (Control) or AAV-Cre (Gsk3b-/-), with or without optic nerve injury. Scale bar, 20 µm. (B) Quantification of phospho-eIF2Bε immunofluorescence intensity. Data are presented as mean ± s.d., n=5 per group. *p<0.001, Two-way ANOVA with Bonferroni post hoc test. (C) Confocal images of optic nerve sections showing regenerating axons labeled by anti-GAP43 immunohistochemistry at 2 weeks after optic nerve crush from Gsk3bf/fmice injected with AAV-Cre + AAV-GFP or AAV-Cre + AAV-eIF2Bε E572A. *, crush site. Scale bar, 200 µm. (D) Quantification of regenerating axons from Gsk3bf/f mice injected with AAV-Cre + AAV-GFP or AAV-Cre + AAV-eIF2Bε E572A at different distances distal to the lesion site. Data are presented as mean ± s.d., n=9 per group. *p<0.01, Two-way ANOVA with Bonferroni post hoc test. (E) OPP Alexa Fluor 594 protein synthesis assay in retinal whole-mounts from Gsk3bf/f mice injected with AAV-GFP (Control), AAV-Cre (Gsk3b-/-) + AAV-GFP, or AAV-Cre + AAV-eIF2Bε E572A or treated with anisomycin. Scale bar, 25 µm. (F) Quantification of OPP fluorescence intensity. Data are presented as mean ± s.d., n=5 per group. *p<0.01, One-way ANOVA with Tukey’s test. (G) Confocal images of retinal whole-mounts showing surviving Tuj1+ RGCs at 2 weeks after optic nerve injury. Scale bar, 50 µm. (H) Quantification of RGC survival at 2 weeks after injury, expressed as a percentage of total Tuj1+ RGCs in the uninjured retina. Data are presented as mean ± s.d., n=5 per group.
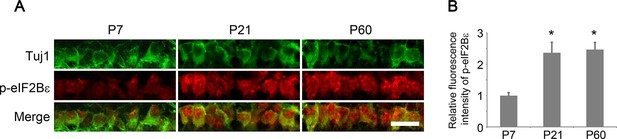
Developmental analysis of eIF2Bε phosphorylation in RGCs.
(A) Confocal images of retinal sections showing double immunolabeling for Tuj1+ RGCs and phospho-eIF2Bε at postnatal day 7, 21, and 60. Scale bar, 20 µm. (B) Quantification of phospho-eIF2Bε immunofluorescence intensity relative to postnatal day 7. Data are presented as mean ± s.d., n=5 per group. *p<0.001, One-way ANOVA with Dunnett's test.
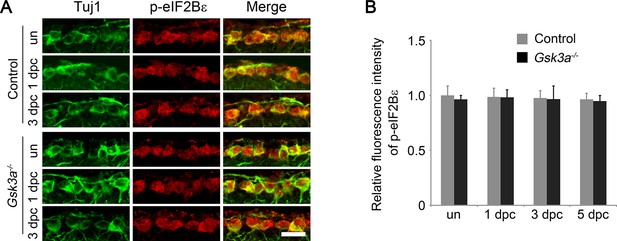
Analysis of eIF2Bε phosphorylation in Gsk3a knockout mice.
(A) Confocal images of retinal sections showing double immunolabeling for Tuj1+ RGCs and phospho-eIF2Bε in Gsk3a-/- mice or their wild type litter mates as a control, with or without optic nerve injury. Scale bar, 20 µm. (B) Quantification of phospho-eIF2Bε immunofluorescence intensity. Data are presented as mean ± s.d., n= 5 per group.
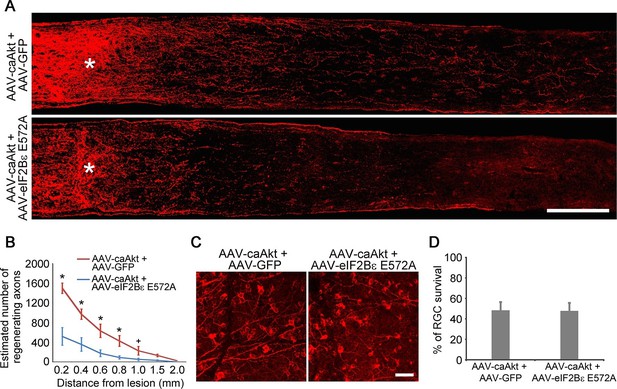
eIF2Bε is required for AKT-induced axon regeneration.
(A) Confocal images of optic nerve sections showing regenerating axons labeled by anti-GAP43 immunohistochemistry at 2 weeks after injury from AAV-caAKT + AAV-GFP or AAV-caAkt + AAV-eIF2Bε E572A injected eyes. *, crush site. Scale bar, 200 µm. (B) Quantification of regenerating axons from AAV-caAKT + AAV-GFP or AAV-caAkt + AAV-eIF2Bε E572A injected eyes at different distances distal to the lesion site. Data are presented as mean ± s.d., n=5 per group. *p<0.05, +p<0.05, Two-way ANOVA with Bonferroni post hoc test. (C) Confocal images of retinal whole-mounts showing surviving Tuj1+ RGCs at 2 weeks after optic nerve injury. Scale bar, 50 µm. (D) Quantification of RGC survival at 2 weeks after injury, expressed as a percentage of total Tuj1+ RGCs in the uninjured retina. Data are presented as mean ± s.d., n=5 per group.
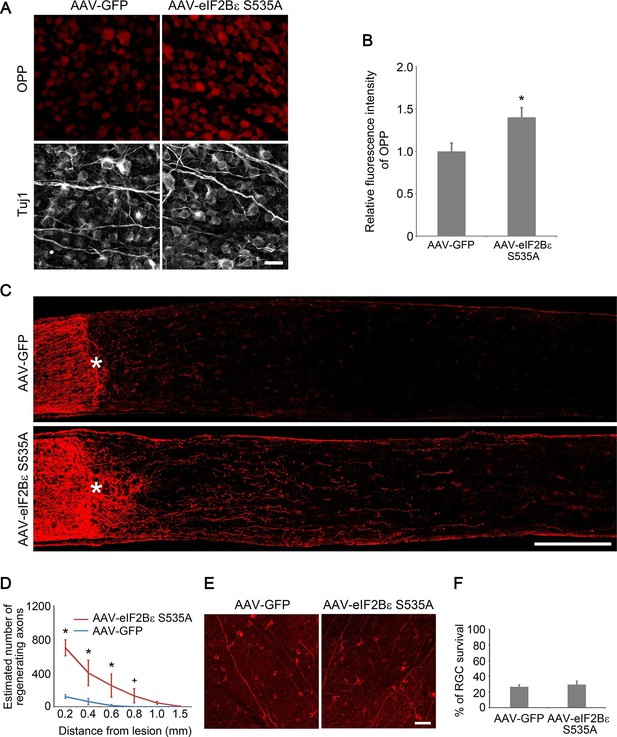
eIF2Bε activation promotes axon regeneration.
(A) OPP Alexa Fluor 594 protein synthesis assay in retinal whole-mounts from AAV-GFP or AAV-eIF2Bε S535A injected eyes. Scale bar, 25 µm. (B) Quantification of OPP fluorescence intensity. Data are presented as mean ± s.d., n=5 per group. *p<0.01, Student’s t test. (C) Confocal images of optic nerve sections showing regenerating axons labeled by anti-GAP43 immunohistochemistry at 2 weeks after injury from wild-type mice injected with AAV-GFP or AAV-eIF2Bε S535A. *, crush site. Scale bar, 200 µm. (D) Quantification of regenerating axons from retinas injected with AAV-GFP or AAV-eIF2Bε S535A at different distances distal to the lesion site. Data are presented as mean ± s.d., n=5 per group. *p<0.01, +p<0.05, Two-way ANOVA with Bonferroni post hoc test. (E) Confocal images of retinal whole-mounts showing surviving Tuj1+ RGCs at 2 weeks after optic nerve injury. Scale bar, 50 µm. (F) Quantification of RGC survival at 2 weeks after injury, expressed as a percentage of total Tuj1+ RGCs in the uninjured retina. Data are presented as mean ± s.d., n=5 per group.
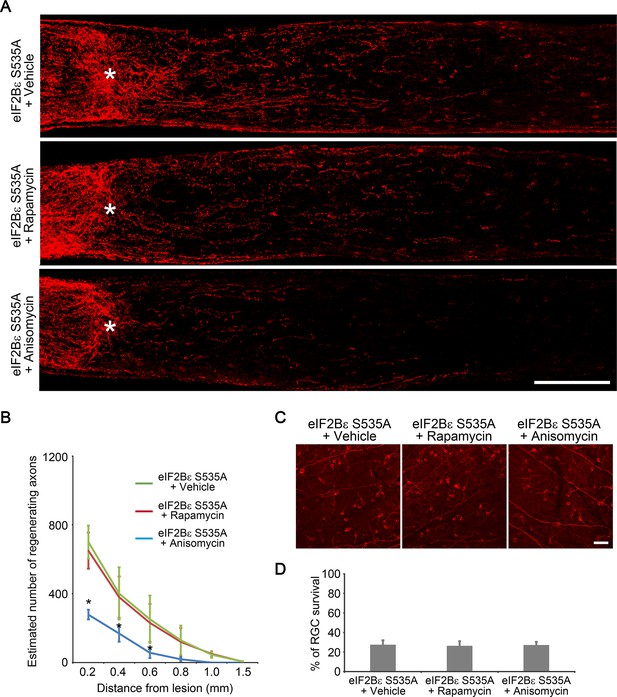
eIF2Bε-induced axon regeneration is sensitive to protein synthesis inhibition but not to mTORC1 inhibition.
(A) Confocal images of optic nerve sections showing regenerating axons labeled by anti-GAP43 immunohistochemistry at 2 weeks after injury from AAV-eIF2Bε S535A injected eyes with vehicle, rapamycin, or anisomycin treatment. *, crush site. Scale bar, 200 µm. (B) Quantification of regenerating axons at different distances distal to the lesion site. Data are presented as mean ± s.d., n=5 per group. *p<0.01, Two-way ANOVA with Bonferroni post hoc test. (C) Confocal images of retinal whole-mounts showing surviving Tuj1+ RGCs at 2 weeks after optic nerve injury. Scale bar, 50 µm. (D) Quantification of RGC survival at 2 weeks after injury, expressed as a percentage of total Tuj1+ RGCs in the uninjured retina. Data are presented as mean ± s.d., n=5 per group.