Translational control of nicotine-evoked synaptic potentiation in mice and neuronal responses in human smokers by eIF2α
Figures
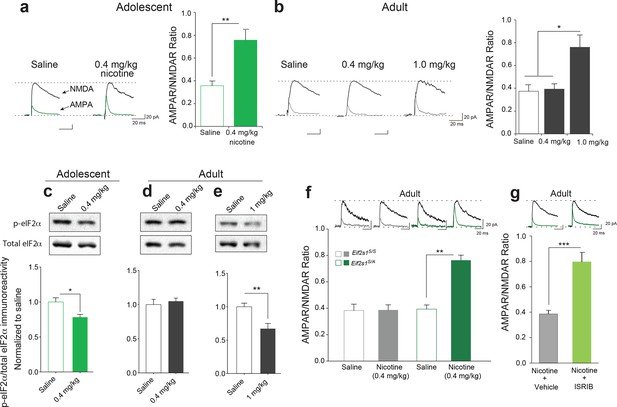
Reduced p-eIF2α-mediated translational control increases the susceptibility to nicotine-induced LTP.
(a-b) Left, Representative traces of AMPAR and NMDAR EPSCs recorded from VTA DA neurons 24 hr after i.p. injection of saline or the indicated dose of nicotine. A relatively low dose of nicotine (0.4 mg/kg) induced LTP, shown by an increase in AMPAR/NMDAR ratio in VTA DA neurons (a, Right, P<0.01, n=6,6 saline/0.4 mg/kg nicotine, t10=4.026) from adolescent mice (5 weeks old), but not in those from adult mice (3–5 months old, b, Right, P=0.802, n=6/7/6 saline/0.4 mg/kg nicotine/1.0 mg/kg nicotine, F2,16=9.029). A higher dose of nicotine (1.0 mg/kg) was required to increase the AMPAR/NMDAR ratio in VTA DA neurons from adult mice (b, Right, P<0.05 vs. saline or 1.0 mg/kg nicotine, n=6/7/6 saline/0.4 mg/kg nicotine/1.0 mg/kg nicotine, F2,16=9.029). (c-d) A low dose of nicotine (0.4 mg/kg) reduced p-eIF2α in the VTA of adolescents (c, P<0.05, n=9/5 saline/0.4 mg/kg nicotine, t12=2.479), but not adult mice (d, P=0.5710, n=7/11 saline/0.4 mg/kg nicotine, t16=0.5784). (e) A higher dose of nicotine (1 mg/kg) was required to reduce p-eIF2α in VTA of adult mice (P<0.01, n=11/5 saline/1 mg/kg nicotine, t14=3.428). (f) A low dose of nicotine (0.4 mg/kg) failed to induce LTP in VTA DA neurons from adult WT (Eif2s1S/S) mice (Left, P=0.964, n=5 per group, t8=0.05), but elicited significant LTP in adult Eif2s1S/A mice (Right, P=0.003, n=5 per group, t8=6.73). (g) A low dose of nicotine (0.4 mg/kg) induced LTP in ISRIB-injected adult mice compared to vehicle-injected mice (P<0.001, n=7/7 nicotine+vehicle/nicotine+ISRIB, t12=5.222).
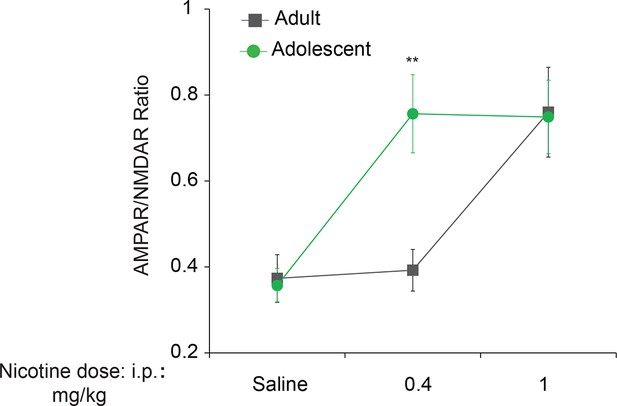
Adolescent mice are more susceptible than adult mice to nicotine-induced synaptic potentiation.
Adolescent (5 weeks old, n=6–7 per group) or adult mice (3–5 months old, n=6–7 per group) were i.p-injected with saline or nicotine at indicated doses and whole-cell recordings were performed in VTA DA neurons. An increase in the AMPAR/NMDAR ratio (an index of LTP) was induced with the 0.4 mg/kg dose of nicotine (F2,32=4.34, P<0.01 vs. saline) in adolescent mice, whereas 1.0 mg/kg was required for a significant increase in adults (F2,32=4.34, P<0.05 vs. saline or 0.4 mg/kg nicotine).
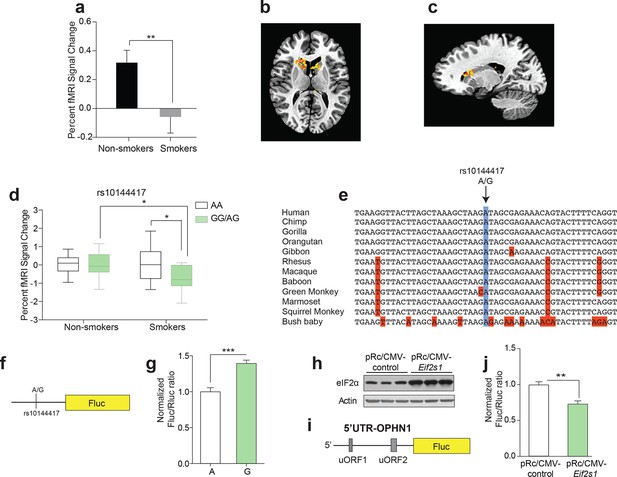
The effect of a single nucleotide polymorphism (SNP) in the promoter of the Eif2s1 gene on reward-dependent striatal activity in human tobacco smokers.
(a-c) Reward-related activity in caudate/putamen is lower in smokers than non-smokers (b, P<0.01, n=33/55, t86=2.678). (b-c) Transverse (b) and sagittal (c) views of significant fMRI BOLD signal in caudate/putamen of non-smokers compared to smokers in response to juice reward. (d) Interaction between smoking and rs10144417 genotype (P<0.05, F1,86=5.836). (e) Partial alignment of Eif2s1 promoter sequences in human and related animals. Note high level of nucleotide conservation: the rs10144417 SNP is indicated in blue and the non-conserved nucleotides in red. (f) Schematic of firefly luciferase reporter constructs, in which a 5-kb Eif2s1 promoter fragment containing either the A or G allele was cloned upstream of the firefly luciferase gene in the p15Amp reporter vector. A renilla luciferase reporter was co-transfected with reporters containing the A or G variant and firefly luciferase (Fluc) activity was normalized to renilla (Rluc) activity. (g) Effects of A and G variants of SNP rs10144417 on the transcriptional activity of the Eif2s1 promoter as assessed by a luciferase reporter assay in HEK-293 cells. The data are from three independent experiments (P<0.001, n=6 per group, t10=5.405). (h) Western blotting showing that overexpression of Eif2s1 (pRc/CMV-Eif2s1) increased eIF2α levels compared to control (vector alone pRc/CMV). (i) Diagram of the 5′ UTR-Ophn1-Fluc reporter, which consists of the 5’UTR of Ophn1 mRNA fused to the coding region of Firefly luciferase (Fluc). A renilla luciferase (Rluc) reporter vector was co-transfected into HEK293T as a transfection control. (j) Overexpression of Eif2s1 reduced expression of 5′ UTR-Ophn1-Fluc (P<0.01, n=6, t10=3.9425).
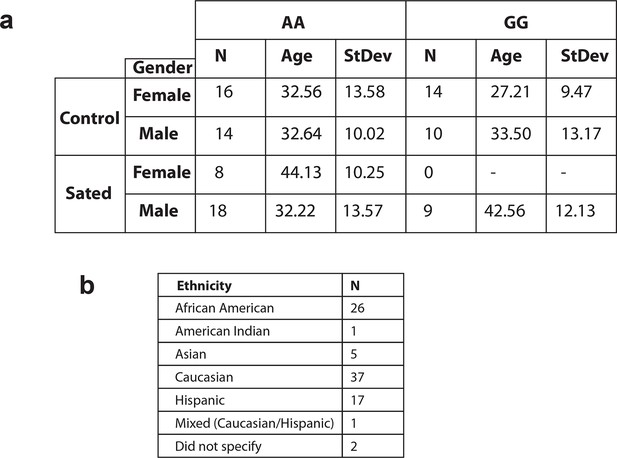
Demographic information of human participants involved in fMRI studies.
(a) Table showing the number of participants by gender, age, and smoking status carrying the A or the G variant in the Eif2s1 gene. (b) Table showing the number of participants by their self-reported ethnicities The participants have no history of any other drug dependance.
fMRI recording paradigm and reward-stimulus pairing in human smokers.
(a) The fMRI recording session consisted of four 5–7 min blocks of light-juice pairings. A self-paced break (“inter-run interval”) was included between runs to allow participants to ask any questions and to allow the investigators to provide feedback on participant motion within the scanner. (b) A total of fifty-five light-juice (1 mL) pairings were presented. The delay between light and juice was 7 s.
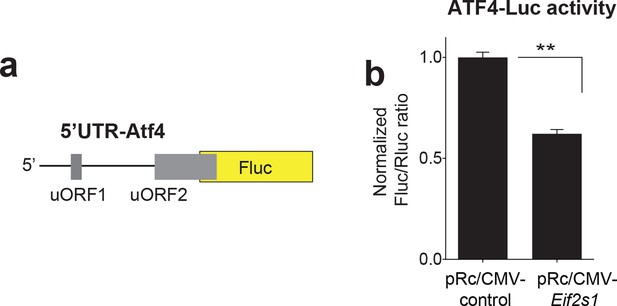
ATF4-Luciferase construct design and activity with Eif2s1.
(a) Diagram of the 5 UTR-ATF4 Fluc reporter, which consists of the 5’UTR of ATF4 mRNA fused to the coding region of Firefly luciferase (Fluc). A renilla luciferase (Rluc) reporter vector was co-transfected into HEK293T as a transfection control. (b) Overexpression of Eif2s1 reduced expression of 5 UTR-ATF4-Fluc (P<0.0001, n=4 per group, t6=11.33).





