Rhodopsin targeted transcriptional silencing by DNA-binding
Figures
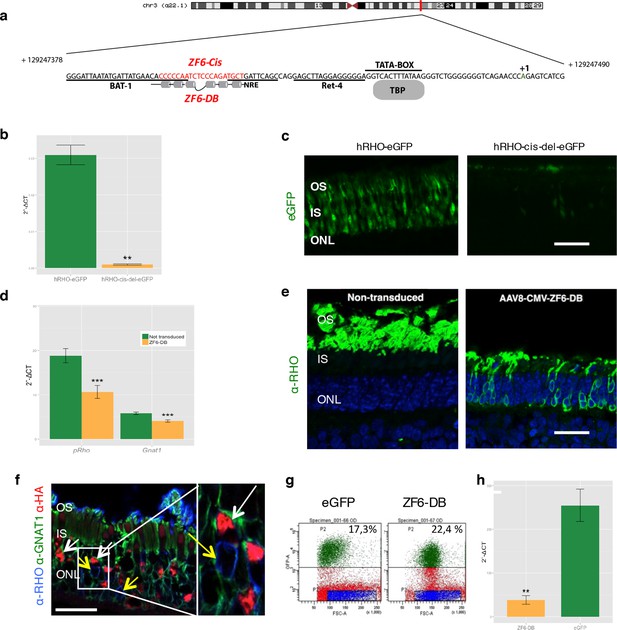
Delivery of ZF6-DB DNA-binding synthetic trans-acting factor targeted to a 20 bp of RHO cis-acting regulatory element (CRE) dramatically reduces Rho expression in photoreceptors.
(a) Schematic representation of the chromosomal location of the RHO locus and its proximal promoter elements indicating the transcription start site (in green, +1) and the location of ZF6-DB binding site (in red, ZF6-Cis) and ZF6-DB (based on Mitton et Al., 12); BAT1, Bovine A/T-rich sequence1; NRE, NRL response element; TBP, TATA box binding protein. (b) qReal Time PCR of mRNA levels (2^-ΔCT) on the adult porcine retina 15 days after vector delivery of either AAV8-hRHO-eGFP (n=2) or AAV8-hRHO-cis-del-eGFP (n=2) subretinally administered at a dose of 1x1010, showed that AAV8-hRHO-cis-del-eGFP resulted in decreased transduction (about fifty fold) compared with hRHO. (c) Histology confirmed the decrease of eGFP expression in hRHO-cis-del-eGFP injected retina compared with the retina injected with hRHO-eGFP. Scale bar, 50 µm. (d) qReal Time PCR of mRNA levels (2^-ΔCT) of adult porcine retina injected subretinally with AAV8-CMV-ZF6-DB (n=6) at a vector dose of 1x1010 genomes copies (gc) compared with non-transduced area (n=7) of the same eye 15 days after vector delivery, resulted in robust transcriptional repression of the Rho transcript. pRHO, porcine Rhodopsin; Gnat1, Guanine Nucleotide Binding Protein1. (e) Rho Immunofluorescence (green) histological confocal analysis of AAV8-CMV-ZF6-DB treated porcine retina compared with non-transduced area. Scale bar, 100 um. The treatment with ZF6-DB determined collapse of the outer-segment (OS) with apparent retention of nuclei (stained with DAPI) in the outer nuclear layer (ONL). (f) Immunofluorescence triple co-localization staining of porcine retina shown in (b) with Rho (blue), rod specific protein Gnat1 (green) and HA (ZF6-DB, red) antibodies. White arrows indicate co-localization of both HA-tag-ZF6-DB and Gnat1 rods depleted of Rho, whereas yellow arrows showed residual Rho and Gnat1 positive cells lacking ZF6-DB. A magnification of the triple staining (box) is highlighted. Scale bar, 100 µm. OS, outer segment; IS, inner segment; ONL, outer nuclear layer; INL, inner nuclear layer. (g) Representative fluorescence-activated cell sorting (FACS) of porcine retina 15 days after injections of either AAV8-GNAT1-eGFP (dose 1x1012 gc) or co-injection with both AAV8-GNAT1-eGFP and AAV8-CMV-ZF6-DB (dose of eGFP, 1x1012 gc; ZF6-DB dose 5x1010 gc). eGFP positive sorted cells (AAV8-GNAT1-eGFP) corresponded to 17,3% of the analysed population (left panel; P2 area, green dots), whereas, 22,4% of eGFP positive cells in the retina that received both vectors (AAV8-GNAT1-eGFP and AAV8-CMV-ZF6-DB; right panel; P2 area, green dots). (h) qReal Time PCR on sorted rods treated with AAV8-GNAT1-eGFP (n=3) and AAV8-CMV-ZF6-DB (n=3) showed a repression of about 85% of total rhodopsin when compared with rods treated with eGFP (mRNA levels: 2^-ΔCT). Error bars, means +/- s.e.m. n =; *p<0.05, **p<0.01, ***p<0.001; two-tailed Student’s t test.
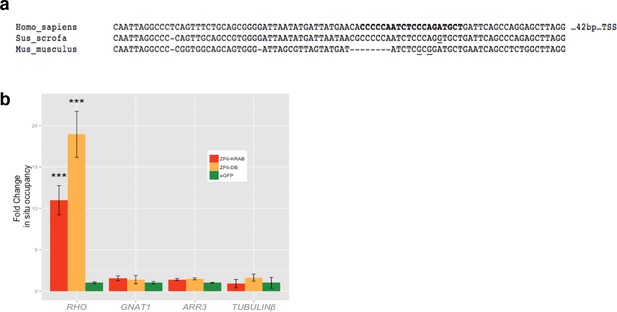
Chromatin Immunoprecipitation (ChIP) of ZF6-DB and ZF6-KRAB.
(a) Alignment of RHODOPSIN proximal promoter between human, mouse and pig DNA sequences; in bold ZF6-cis sequence, underlined the sequence differences present in ZF6-cis bound by ZF6-DB. (b) qPCR ChIP analysis of RHO TSS region including ZF6-cis site), GNAT1, ARR3 and tubulin beta proximal promoters controls in transfected HEK293 cells (n=3 indipendent expermients). RHO-specific enrichment is shown on RHO TSS region. ***p<0.001; two-tailed Student’s t test.
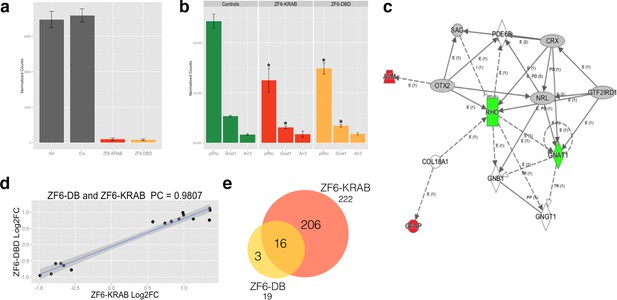
Photoreceptor delivery of ZF6-DB resulted in reduced genome-wide transcript perturbations.
(a) RNA-Seq expression levels (Mean Normalized Counts) comparison between 2 endogenous TFs (Crx and Nrl) and the expression levels resulting from transduction of AAV8-CMV-ZF6-DB and AAV8-CMV-ZF6-KRAB, 15 days after retinal delivery (AAV8-CMV-ZF6-DB n= 6; AAV8-CMV-ZF6-KRAB n= 4 and 7 controls, non-transduced area). (b) Rho and rod Gnat1 and Cone Arrestin 3 expression levels in treated and control retina. (c) Ingenuity Pathway Analysis of DEGs after ZF6-DB AAV delivery in porcine retina showed a network of 13 genes. The 2 phototransduction genes RHO and GNAT1 are shown in green (down-regulated) whereas the 2 genes associated with primary inflammatory response network, A2M and GFAP, are up-regulated (red). (d) Transcriptional activation and repression concordances among Log Fold Changes of the genes in common (Swaroop et al., 2010) between ZF6-DB and ZF6-KRAB (Pearson Correlation Test; PC=0.9787; p value << 1x10-5). (e) Venn Diagrams, pairwise intersection of the 2 sets of Differentially Expressed Genes (DEGs). An adjusted p value (False Discovery Rate; FDR ≤ 0.1), without filtering on fold change levels, resulted in 19 and 222 DEGs, in ZF6-DBD and ZF6-KRAB treated retina, respectively. The intersection resulted significant by hypergeometric test (p value << 1x10-5).
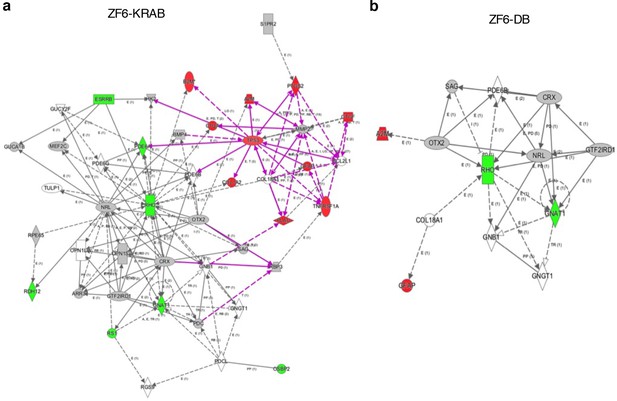
Ingenuity Pathway Analysis on DEGs of ZF6-KRAB treated retina.
(a) Delivery of AAV8-CMV-ZF6-KRAB resulted in up-regulation of 10 genes associated with inflammatory responses (red, up-regulation) and the down-regulation (green) of 7 genes associated with the rod phototransduction cascade. (b) the ZF6-DB pathway analysis (Figure 2) is reported for comparison.
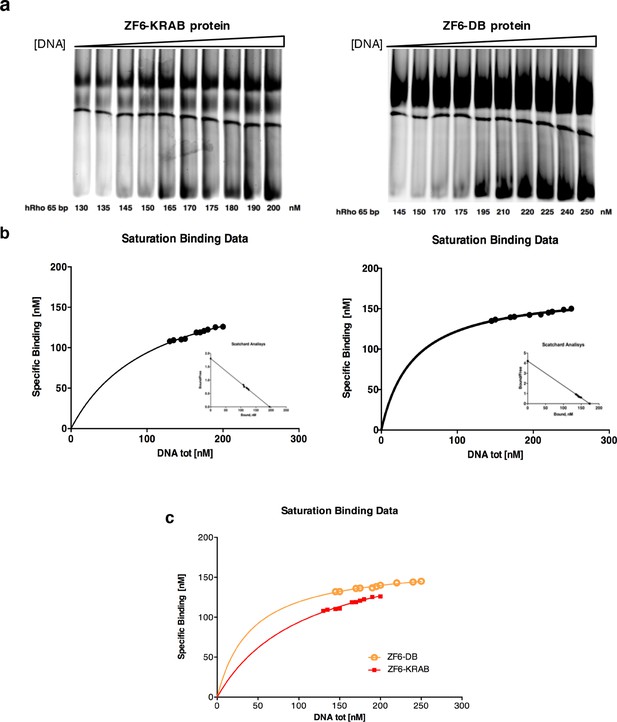
Determination of the binding constants of ZF6-KRAB and ZF6-DB.
(a) Gel mobility shift titrations of ZF6-KRAB and ZF6-DB with the hRHO 65 bp oligonucleotide (see 'Materials and methods'). (b) In the saturation binding experiments the nanomolar concentration of specific binding data was plotted against of nanomolar increasing concentration (130, 135, 145, 150, 165, 170, 175, 180, 190, and 200 nM, respectively) and (145, 150, 170, 175, 195, 210, 220, 225, 240, and 250 nM, respectively) of DNA ligand and Scatchard analysis of the gel shift binding data. The ratio of bound to free DNA is plotted versus the nanomolar concentration of bound DNA in the reaction mixture. The ZF6-KRAB and ZF6-DB apparent dissociation constants (Kd ZF6-KRAB = 108.00 ± 11.78 nM (R2 = 0.97) and Kd ZF6-DB = 41.94 ± 3.45 nM, R2 = 0.96), respectively) were determined. (c) combination of the a and b panels.
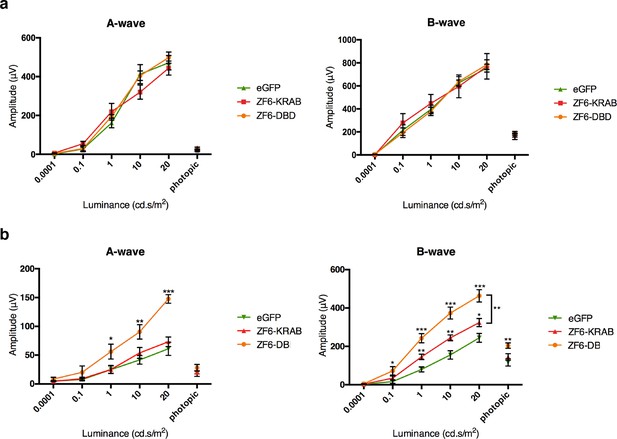
ZF6-DB DNA-binding protein preserves retinal function of the P347S adRP mouse model.
(a) Electroretinography (ERG) analysis on P347S mice mice subretinally injected at post natal day 14 (PD14) with AAV8-CMV-ZF6-DB (n=10), AAV8-CMV-ZF6-KRAB (n=10), or AAV8-CMV-eGFP (n=10) and analysed at P30. Retinal responses in both scotopic (dim light) and photopic (bright light) showed that both A- and B-waves amplitudes, evoked by increasing light intensities, were preserved in both AAV8-CMV-ZF6-DB and ZF6-KRAB compared to eGFP control (b) A- and B-wave are shown for injected C57Bl/6 mice with ZF6-DB (n=4), ZF6-KRAB (n=4) and eGFP (n=4), independently. No functional impairment is observed for each construct. Error bars, means +/- s.e.m. *p<0.05, **p<0.01, ***p<0.001; two-tailed Student’s t test.
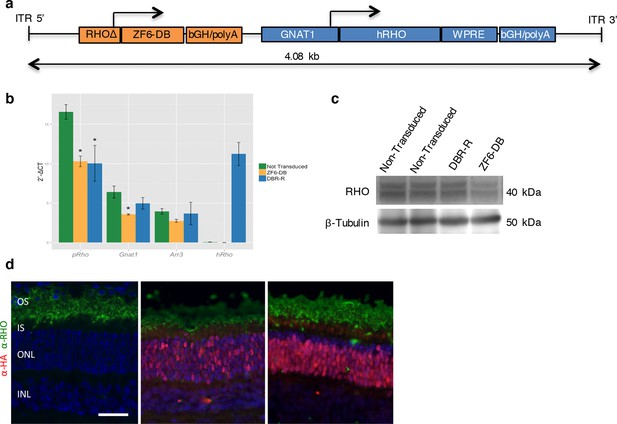
DNA-binding repression-replacement (DBR-R) of Rho in the porcine retina.
(a) AAV8-RHOΔ-ZF6-DB-GNAT1-hRHO DBR-R construct features, including the two expression cassettes, RHOΔ-ZF6-DB encoding for both the DNA-binding repressor ZF6-DB (orange), and GNAT1-hRHO for human RHO for replacement (blue). The size (kb) of the construct is indicated as a bar. (b) qReal Time PCR, mRNA levels (2^-ΔCT) 2 months after vector delivery of either AAV8-CMV-ZF6-DB (DBR; orange bars) or AAV8-RHOΔ-ZF6-DB-GNAT1-hRHO (DBR-R, blue bars) and non-transduced controls (green bars). pRho, porcine Rhodopsin; Gnat1, Guanine Nucleotide Binding Protein1; Arr3, Arrestin 3; hRHO, human Rhodopsin. The result is representative of two independent experiments. Error bars, means +/- s.e.m. ; *p<0.05, **p<0.01, ***p<0.001; two-tailed Student’s t test. (c) Western blot analysis on the retina showed in b, c and d. (d) Immunofluorescence double staining with Rho (green) and HA-ZF6-DB (red) antibodies. Left panel, non-transduced control retina; middle panel, AAV8-CMV-ZF6-DB treated retina; left panel, AAV8-RHOΔ-ZF6-DB-GNAT1-hRHO DBR-R treated retina. OS, outer segment; IS, inner segment; ONL, outer nuclear layer; INL, inner nuclear layer; scale bar, 100 µm.
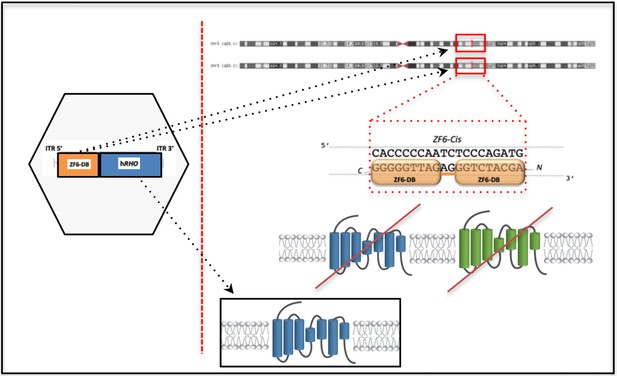
Outline of the DNA-binding repressor-replacement (DBR-R) strategy.
The DNA-binding protein, ZF6-DB, for transcriptional silencing of RHO is coupled to replacement, R, in the same AAV vector (left hexagon) to ensure simultaneous transduction of photoreceptors. The DNA-binding protein ZF6-DB operates on the regulatory region of the RHO promoter, in an allele and mutation independent manner i.e. ZF6 DNA binding on the endogenous promoter represses RHO transcription irrespectively of the mutated and WT alleles, preventing RHO expression (mutated RHO, green; and WT, blue). This strategy is designed to overcome the high heterogeneity of RHO mutations. Highlighted as a 'chromosome zoom-in' [Genome Browser] the ZF6-DB (orange squares) bound to the regulatory DNA-target sequence. Exogenously AAV vector delivered RHO (blue) for replacement is shown in the black box.
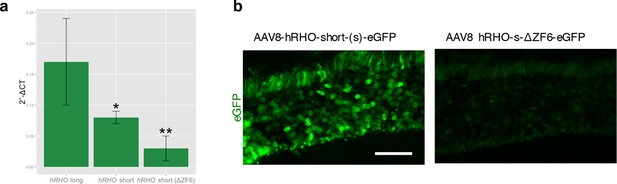
Strength and tissue specificity of RHOΔ promoter elements in murine retina.
(a) qReal Time PCR, mRNA levels (2^-ΔCT) on the adult murine retina 15 days after vector delivery of either AAV8-hRHO short-eGFP or AAV8-hRHO-s-ΔZF6-eGFP subretinally administered at a dose of 1x109, showed that AAV8-hRHO-s-ΔZF6-eGFP resulted in decreased transduction (about ten fold) compared with hRHO long. Error bars, means +/- s.e.m.; *p<0.05, **p<0.01; two-tailed Student’s t test. (b) Histology demonstrated maintenance of rod-specific expression by AAV8-RHOΔ-eGFP. Scale bar, 50 µm.
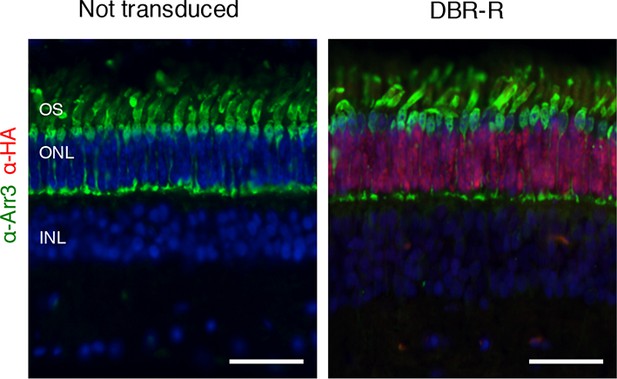
Cone morphological integrity after DNA-binding repression-replacement (DBR-R) subretinal delivery.
Rod-specific expression of DBR-R (AAV8-RHOΔ-ZF6-DB-GNAT1-hRHO) 2 month after vector delivery. Immunofluorescence double staining with human cone Arrestin 3 (hCAR; green) and HA-ZF6-DB (red) antibodies, showed rod specific transduction. Left panel, non-transduced control retina; right panel, AAV8-RHOΔ-ZF6-DB-GNAT1-hRHO DBR-R treated retina. OS, outer segment; IS, inner segment; ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar, 50 µm.





