Waves of actin and microtubule polymerization drive microtubule-based transport and neurite growth before single axon formation
Figures
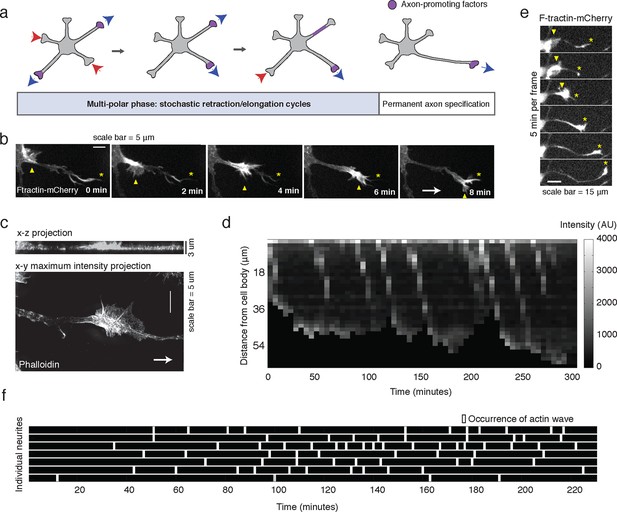
Stochastically-generated actin waves correlate with neurite extensions.
(a) Schematic showing two stages of symmetry breaking. The multi-polar phase, where the neuron experiences fluctuating neurite outgrowth and retraction and fluctuating microtubule-based transport, is highlighted. (b) Timelapse images of a F-tractin-mCherry-expressing primary hippocampal neurite showing actin wave propagation. Images were taken every 2 min. Yellow arrowheads mark the actin wave, yellow asterisks mark the neurite tip, white arrow marks direction of actin wave progression. Scale bar = 5 μm. (c) Structured illumination images of a phalloidin-stained neuron showing an actin wave. Top image is a x-z projection of a z-stack of images taken every 0.125 μm, bottom image is a maxiumum intensity projection of the z-stack. White arrow marks direction of actin wave progression. (d) Kymograph generated from a timelapse of a F-tractin-mCherry expressing neurite. Source images were acquired every 5 min. (e) Timelapse images of a F-tractin-mCherry-expressing neurite undergoing a growth spurt as the actin wave impacts the growth cone. Images were acquired every 5 min. Yellow arrowheads mark actin waves, yellow asterisks mark neurite tips. Scale bar = 15 μm. (f) Actin waves are stochastically generated in different neurites over time. Actin wave generation was assessed by eye in all neurites of a single neuron over time. Horizontal bars mark individual neurites, white dashes mark actin waves. Source images were acquired every 5 min.
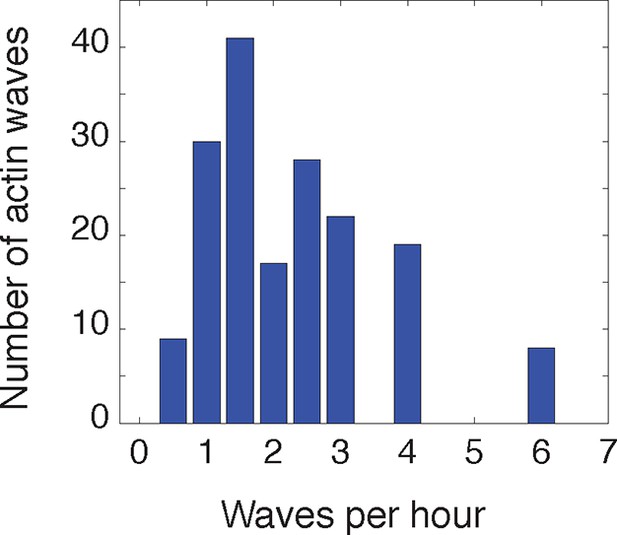
Frequency of actin waves.
https://doi.org/10.7554/eLife.12387.004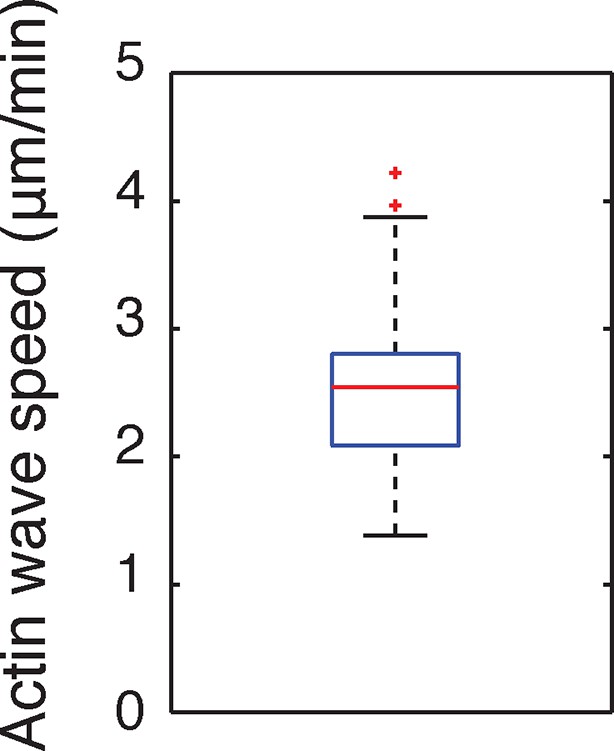
Speed of actin waves.
https://doi.org/10.7554/eLife.12387.005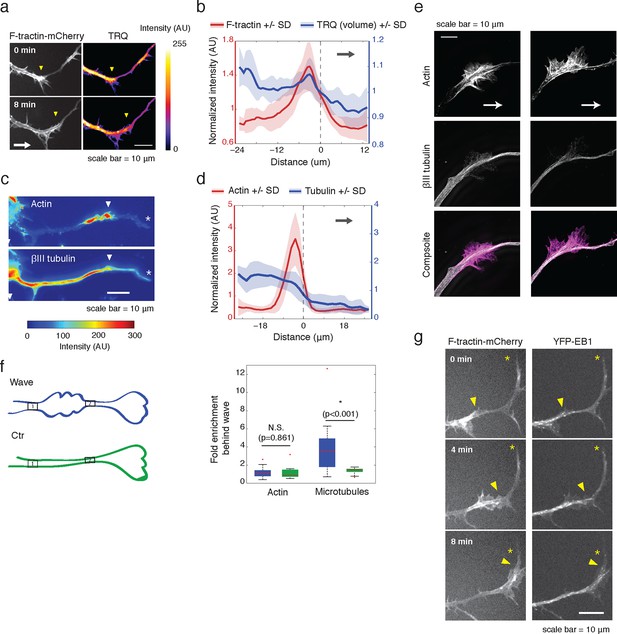
Actin waves contain more polymerizing microtubules in widened neurites.
(a) The volume marker cytoplasmic Turquoise shows an increase in volume in and behind the wave. Images acquired every 8 min. Yellow arrowheads mark actin waves. White arrow denotes direction of wave movement. Scale bar = 10 µm. (b) Averaged line scans show increased volume in and behind actin wave. Measurements are taken from cytoplasmic Turquoise and F-tractin-mCherry expressing cells. Gray arrow denotes direction of wave movement. Dashed line indicates alignment at half max of actin wave. All traces were normalized by mean intensity then smoothed before averaging. Error is standard deviation. N = 14 neurites. (c) Fixed hippocampal neurons stained with phalloidin (actin) and anti-βIII tubulin (neuronal microtubules) show enrichment of microtubules in and behind wave. White arrowheads mark actin waves, white asterisks mark neurite tips. Scale bar = 10 μm. (d) Quantification of (c) confirmed enrichment of microtubules in and behind wave. Averaged line scans of phalloidin signal and anti-βIII tubulin signal were obtained for neurites containing waves. See 2b for methodology. N = 27 neurites. (e) Structured illumination microscopy on phalloidin and anti-βIII tubulin-stained neurons shows enrichment of microtubules behind wave with single-microtubule resolution. White arrow marks direction of actin wave propagation. Scale bar = 10 μm. (f) Fold enrichment of phalloidin and anti-βIII tubulin intensity behind the wave was calculated by taking the ratio of intensities in an area behind the actin wave to an area in front of the actin wave (depicted on left: region1/region2). Fold enrichment was calculated for neurites containing waves (“Wave”, n = 20) and neurites lacking waves (“Ctr”, n = 12). Tubulin enrichment was statistically higher in waves compared to the control (two-sided Wilcoxon rank sum test). (g) Hippocampal neurons expressing F-tractin-mCherry and YFP-EB1 show enrichment of EB1 puncta in and behind actin wave. Yellow arrowheads mark front edge of actin waves. Yellow asterisks mark neurite tips. Scale bar = 10 μm.
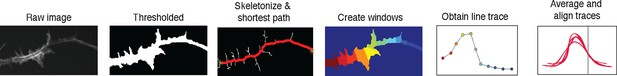
2D line scan analysis method.
https://doi.org/10.7554/eLife.12387.010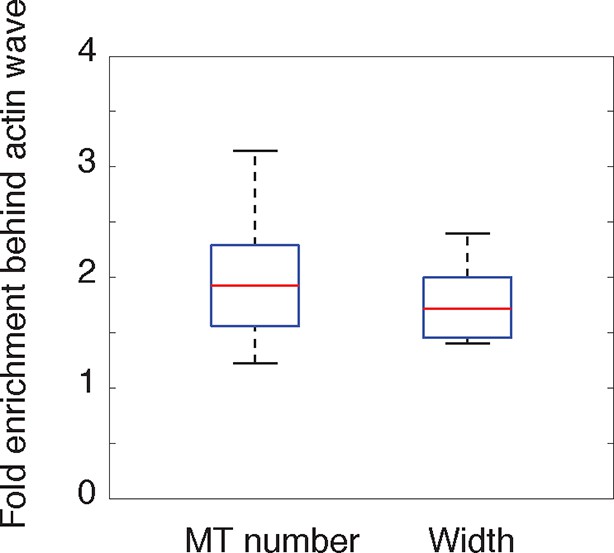
Single microtubule enrichment behind actin wave.
https://doi.org/10.7554/eLife.12387.011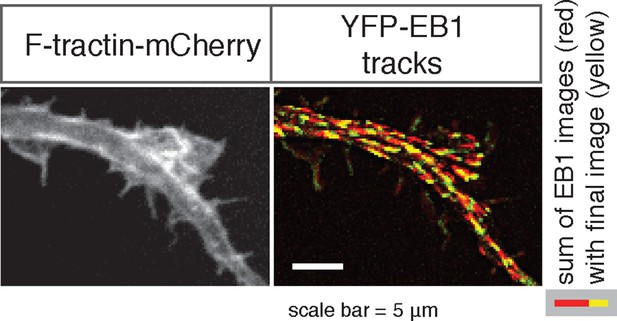
EB1 puncta move in an anterograde fashion.
https://doi.org/10.7554/eLife.12387.012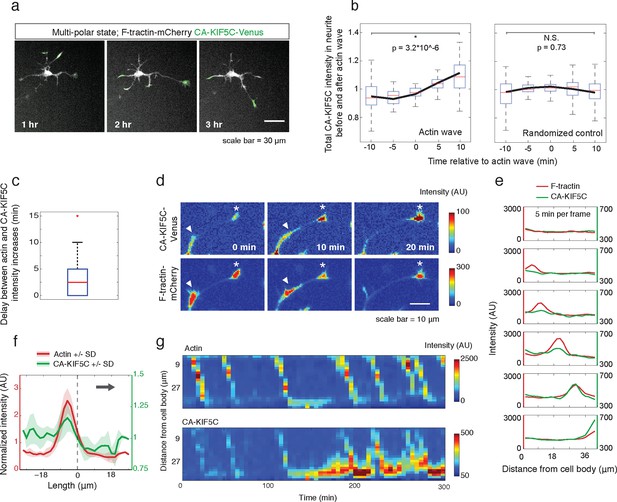
Actin waves coordinate with pulsatile transport of Kinesin-1 motor domain.
(a) Live cell images of a neuron expressing CA-KIF5C-Venus (green) and F-tractin-mCherry (white) exhibiting fluctuating CA-KIF5C localization and neurite lengths characteristic of the multi-polar stage. Images were acquired every hour. Scale bar = 30 μm. (b) For 45 visually-identified actin waves, total CA-KIF5C intensity in the neurite was measured before and after generation of the actin wave. A significant increase in CA-KIF5C is observed relative to a control. Control traces were obtained by randomly selecting 45 points in time and assessing CA-KIF5C intensity before and after each time point. The black line signifies the mean of the CA-KIF5C traces. For each time point the data is also represented with standard box plots with outliers not shown. Significance between the -10 and 10 min set of points was assessed using a two-sided Wilconox rank-sum test. (c) Increase in actin intensity precedes increase in CA-KIF5C intensity. Total intensities of actin and CA-KIF5C before, during, and after entry of CA-KIF5C was assessed and the delay between actin and CA-KIF5C intensity increase was noted. (28 entry events). Source images were acquired every 5 min. (d) Timelapse images of a CA-KIF5C-Venus and F-tractin-mCherry expressing neurite show that CA-KIF5C transports in pulses which coincide with actin waves. Images were taken every 10 min. White arrowheads mark position of actin waves. White asterisks mark neurite tips. Scale bar = 10 μm. (e) CA-KIF5C moves with an actin wave as illustrated by single cell successive line scans taken from a neurite with a traveling actin wave. Image data is in Figure 3—figure supplement 2. Frames were acquired every 5 min. (f) Averaged line scans show enrichment of CA-KIF5C in and behind the actin wave. See 2b for methodology. (g) Kymographs generated from timelapse images of a F-tractin-mCherry and CA-KIF5C-Venus expressing neurite show that CA-KIF5C travels with actin waves and accumulates in the growth cone. Source images were acquired every 5 min.
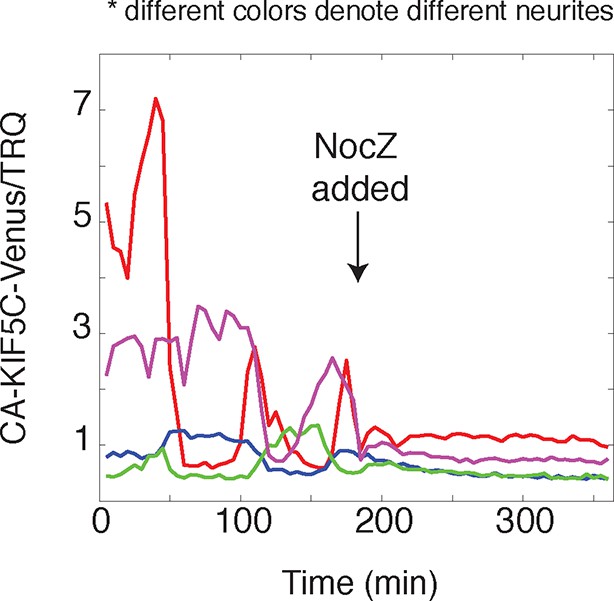
CA-KIF5C dynamic localization is dependent on microtubules.
https://doi.org/10.7554/eLife.12387.014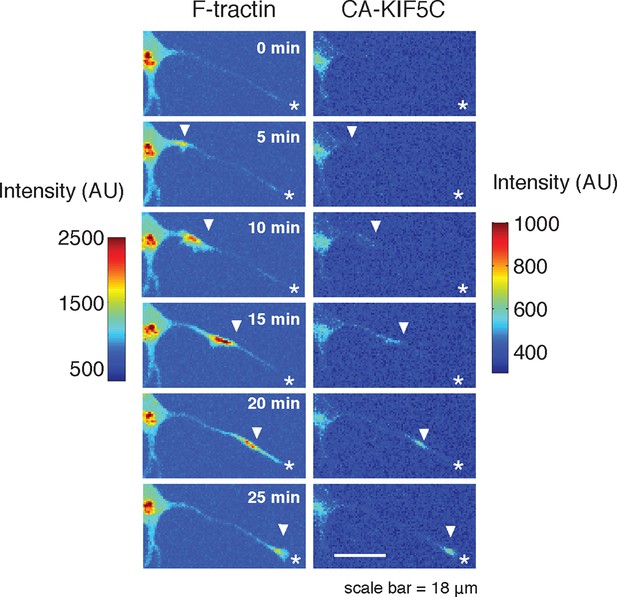
CA-KIF5C travels with actin waves.
https://doi.org/10.7554/eLife.12387.015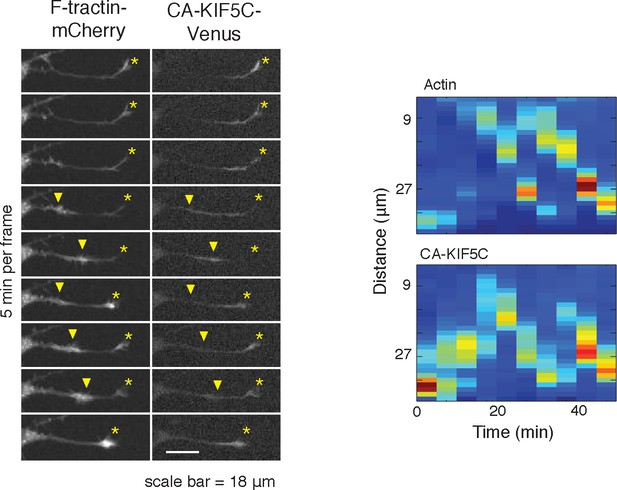
Actin waves can reverse retrograde CA-KIF5C movement.
https://doi.org/10.7554/eLife.12387.016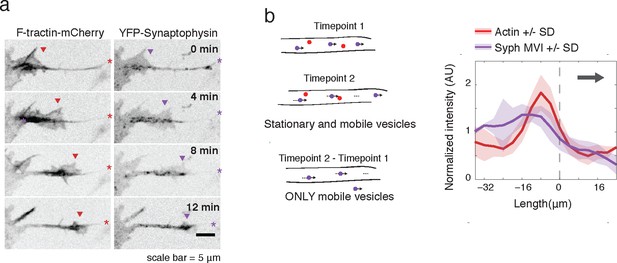
Actin waves contain Synaptophysin-positive vesicles.
(a) Timelapse imaging of a neurite expressing F-tractin-mCherry and Citrine-Synaptophysin shows Synaptophysin positive vesicles enriched in and behind wave. Images displayed with inverted grayscale. Frames were taken every 4 min. Red and purple arrowheads mark front edge of actin waves. Red and purple asterisks mark neurite tips. Half asterisks mark neurites continuing out of frame. Scale bar = 5 μm. (b) Difference imaging of images acquired every 600 ms (schema left) taken as average line scans shows enrichment of mobile vesicles in and behind actin waves (right). Grey arrow denotes direction of wave movement. Dashed line indicates alignment at half max of actin wave. All traces were normalized by mean intensity then smoothed before averaging. Error is standard deviation. N = 12 neurites.
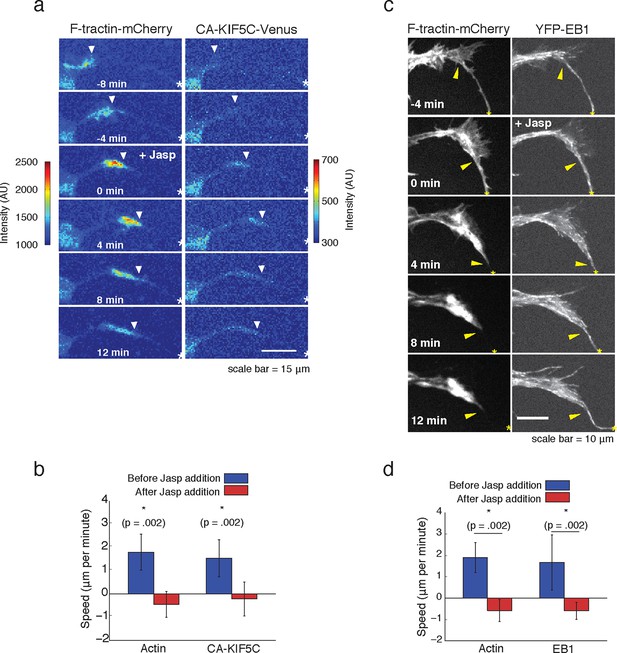
Forward advance of microtubule polymerization and Kinesin-1 is dependent on actin wave progression.
(a) Wave of CA-KIF5C does not advance independently of actin wave advancement. Addition of 10 nM Jasplakinolide stalls actin wave and movement of CA-KIF5C. Frames were acquired every 4 mins. White arrowheads mark actin waves. White half asterisks mark neurites continuing out of frame. Scale bar = 15 μm. (b) Quantification of (a). Speeds of actin waves and CA-KIF5C waves were measured before and after Jasplakinolide addition. Error bars represent standard deviation. N = 6 neurites. Statistical significance assessed with a two-sided Wilcoxon rank-sum test. (c) Wave of polymerizing microtubules does not advance independently of actin wave. Addition of 50 nM Jasplakinolide freezes actin wave and prevents wave of EB1 puncta from moving forward. Images were taken every 4 min. Yellow arrowheads mark front edge of actin waves. Yellow asterisks mark neurite tips. Half asterisks mark neurites continuing out of frame. Scale bar = 10 μm. (d) Quantification of (c). Speeds of actin waves and waves of EB1 puncta were measured before and after Jasplakinolide addition. Error bars representation standard deviation. N = 6 neurites. Statistical significance assess with a two-sided Wilcoxon rank-sum test.
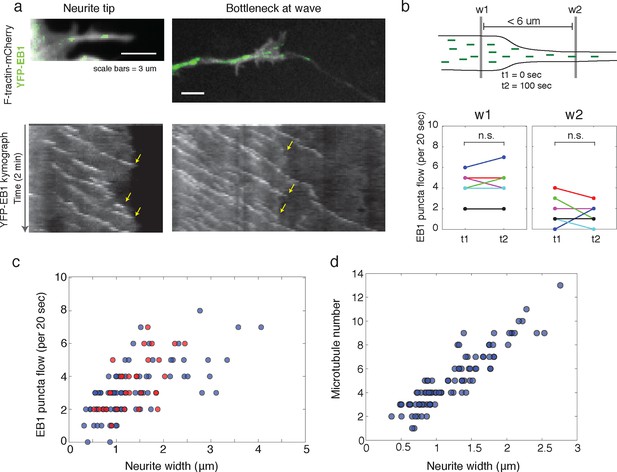
Structural bottleneck provided by actin waves inhibits progression of polymerizing microtubules.
(a) Image depicting EB1 puncta at a neurite tip (top left) and at a bottleneck provided by an actin wave (top right). Accompanying kymographs illustrating EB1 puncta disappearing at the growth cone tip (bottom left) and the bottleneck of an actin wave (bottom right) are below. For better signal, the actin image of the neurite tip was constructed using a maxiumum intensity projection. Images were acquired every 2 sec for 2 min. Scale bars are both 3 μm, with the horizontal axis of the kymographs matching the spatial scale of the images. (b) Flow of EB1 puncta is restricted by a bottleneck. Flow (number of EB1 puncta through a plane over 20 sec) was assessed in two windows <6 μm apart (w1 and w2) on each side of a structural bottleneck at two time points separated by 100 sec. Flow through each window does not significantly increase or decrease between t1 and t2, however the number of EB1 puncta moving through w1 was significantly higher than the number moving through w2 at each time point. Different colors denote different neurites. Signicance was assessed using a two-sided sign test (testing difference between measurement in t1 and t2 (p = 1 for w1 and p = 0.6 for w2) and between w1 and w2 (p = 0.03, for both t1 and t2)). Colors represent distinct neurites. (c) Flow of EB1 puncta (defined in (b)) assessed in neurites with waves (red) and without waves (blue) of varying widths. Both sets (red and blue) display a linear correlation between neurite width and puncta flow. Flows per unit width for neurites bearing waves falls within the distribution for neurites lacking waves. Pearson’s correlation coefficients are 0.60 for wave case and 0.68 for smooth neurite case. (d) Analysis of individual number of microtubules (visualized with SIM) assessed in neurites of varying widths shows a positive correlation. Measurements made in 9 distinct neurites.
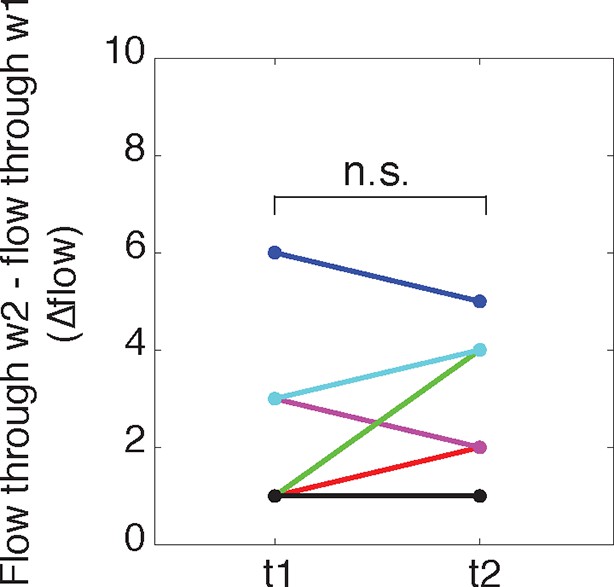
The difference in flow between windows 1 and 2 does not change over time.
https://doi.org/10.7554/eLife.12387.023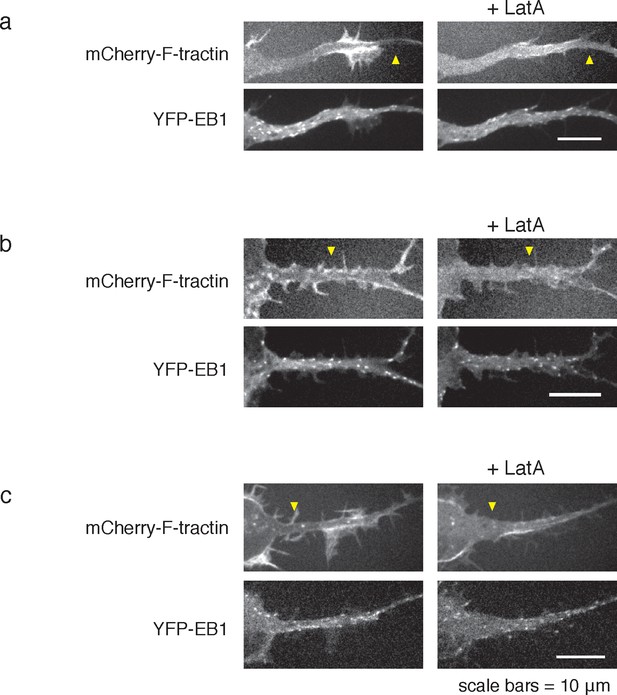
LatA treatment can cause neurite widening.
https://doi.org/10.7554/eLife.12387.024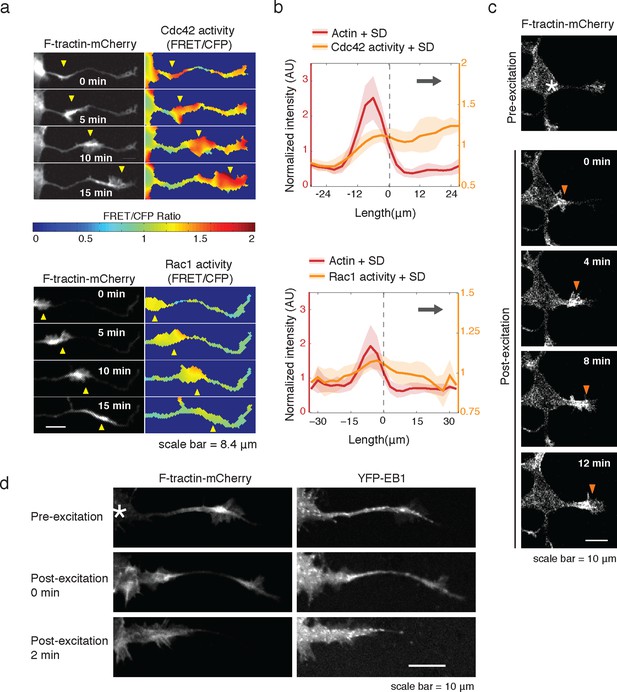
Rac1 activity is sufficient to generate actin waves enriched in polymerizing microtubules.
(a) Actin waves are high in Cdc42 and Rac1 activity. Neurons are expressing F-tractin-mCherry (left) and FRET sensors for Cdc42 (top) and Rac1 activity (bottom), images were taken every 5 min. Scale bar = 8.4 μm. (b) Averaged line scans show enrichment of Cdc42 activity in and in front of the wave (top) and enrichment of Rac1 activity in the wave (bottom). Methodology in 2b. N = 22 neurites (Cdc42), n = 19 neurites (Rac1). (c) Neuron expressing F-tractin-mCherry and Cerulean-PA-Rac1 generates actin wave upon local excitation. White asterisk marks excitation area. Excitation protocol is described in Materials and Methods. Images were acquired every 4 min. Scale bar = 10 μm. (d) Neuron expressing F-tractin-mCherry, Cerulean-PA-Rac1 and YFP-EB1 shows stereotypical widening and increase in EB1 puncta upon excitation of actin wave. White asterisk marks excitation area. Scale bar = 10 μm.
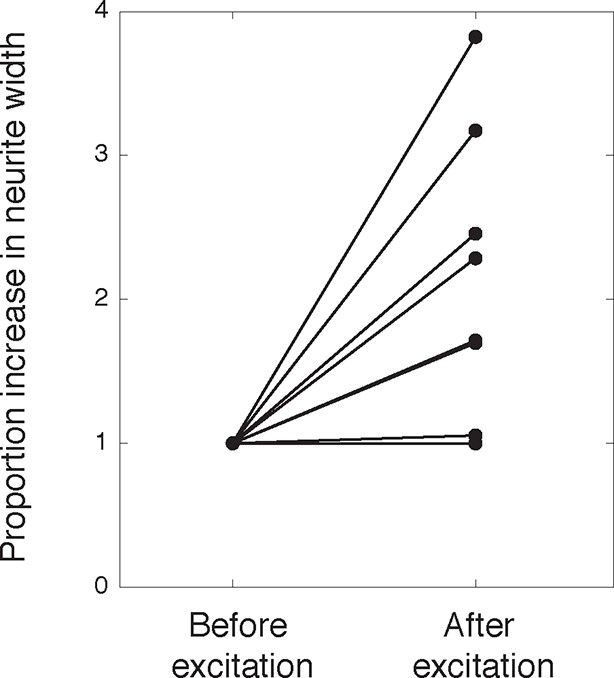
Activation of PA-Rac1 leads to neurite widening.
https://doi.org/10.7554/eLife.12387.026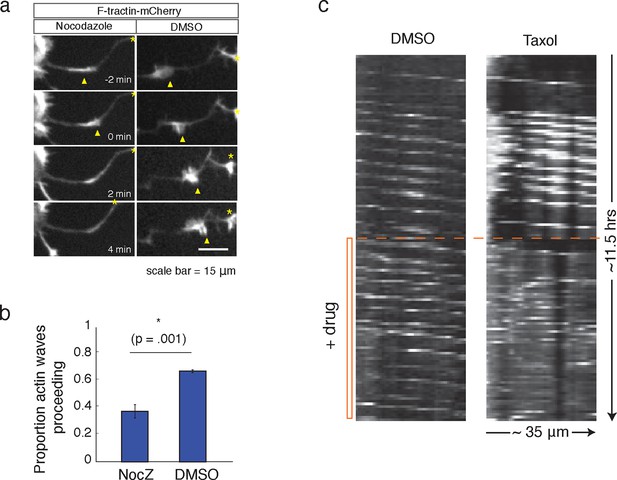
Microtubules are necessary to drive actin wave progression.
(a) Actin waves are dependent on polymerizing microtubules. Addition of 1 μM Nocodazole dissolves an actin wave. Frames were taken every 2 min. Yellow arrowheads mark front edge of actin waves. Yellow asterisks mark neurite tips. Half asterisks mark neurites continuing out of frame. Scale bar = 15 μm. (b) Quantification of (a), showing that addition of Nocodazole dissolves a significantly greater proportion of actin waves than addition of a control. Data averaged from 3 experiments. Statistical significance assessed with 2 sample t-test. (c) Addition of 50 nM Taxol causes non-processive actin polymerization. Neurons were imaged for 69 frames before addition of DMSO or Taxol, then imaged for another 69 frames (5 min per frame). Neurons were expressing F-tractin-Citrine. Kymographs were constructed in Fiji.
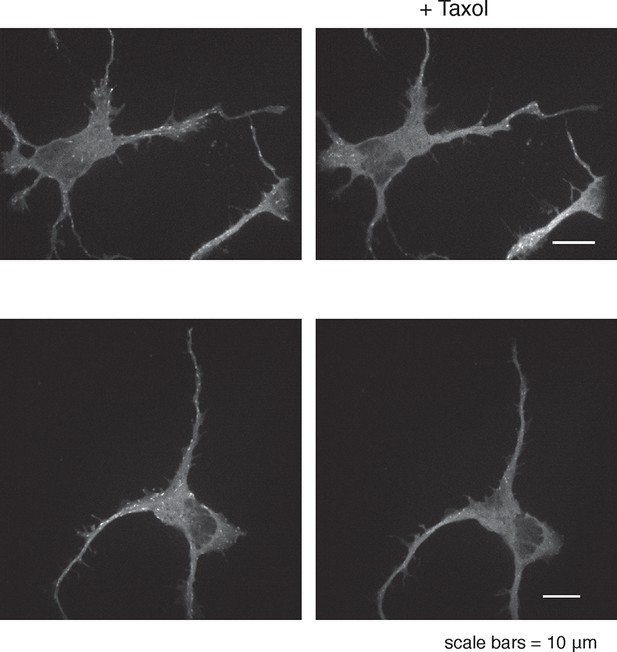
50 nM Taxol can affect microtubule polymerization.
https://doi.org/10.7554/eLife.12387.028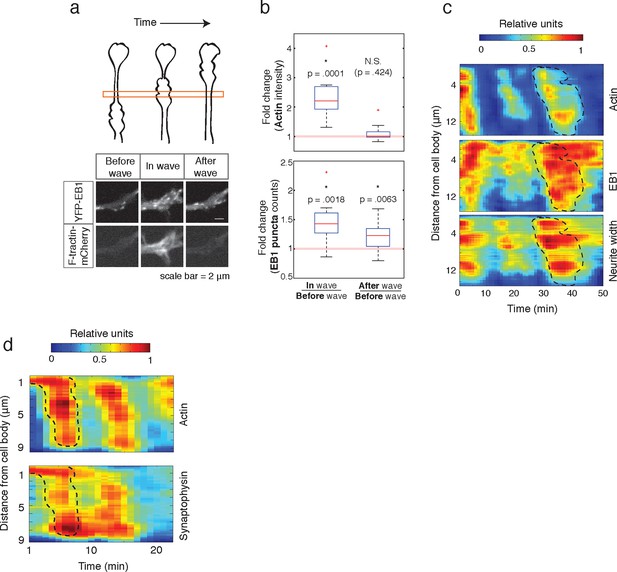
Individual actin waves drive transient increases in neurite width, microtubule polymerization, and microtubule-based transport.
(a) EB1 puncta observed in a single section of neurite shaft before, during, and after wave progression (schema, top). Images show lingering EB1 puncta after wave has passed (bottom). Scale bar = 2 μm. (b) Quantification of (a). Fold change of EB1 puncta counts and actin intensity in the wave versus before the wave, and after the wave versus before the wave, show increased numbers of EB1 puncta in the wave and lingering enrichment of EB1 puncta after the wave has passed. N = 14, a two-sided sign test was used to asses statistical significance of a set of ratios distinct from 1. (c) 2D kymograph were generated from timelapse images of hippocampal neurons expressing F-tractin-mCherry and YFP-EB1 (Video 7). Width was calculated from segmenting summed actin and EB1 image. Region containing actin wave was marked with a dashed line and superimposed on width and EB1 kymographs. Each kymograph was normalized from 0 to 1. (d) 2D Kymograph shows transient enrichment of Synaptophysin vesicles in actin waves. Kymograph generated from timelapse imaging data (Video 8). Region containing an actin wave was marked with a dashed line and superimposed on the Synaptophysin kymograph. Each kymograph was normalized from 0 to 1.
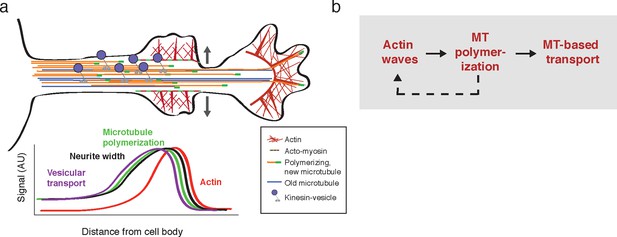
Model illustrating the co-regulation of actin and microtubules that drive microtubule-based transport.
(a) Schematic of changes caused by the actin wave. Actin waves cause transient increases in cargo delivery by increasing microtubule polymerization. We propose that actin waves aid microtubule polymerization by widening the neurite, allowing more space for microtubules to polymerize within the shaft and leading to an increase in microtubule-based transport. However, the changes are transient and will fade in time. (b) Flow chart depicts working model: positive feedback between actin waves and microtubule polymerization increases microtubule-based transport.
Videos
Actin waves are widespread and move in an anterograde fashion.
This movie shows timelapse images from the entire frame of acquisition for F-tractin-mCherry expressing neurons. Images were collected every 5 min and the movie was generated at 5 frames per second. Scale bar = 100 μm.
Actin waves are generated in a seemingly stochastic fashion and move anterogradely through neurites to cause neurite extension.
This movie shows timelapse images of an F-tractin-mCherry-expressing neuron producing actin waves. Images were collected every 5 min and the movie was generated at 5 frames per second. Scale bar = 30 μm.
Actin waves are observed in neurons expressing a membrane marker.
This movie shows timelapse images of a Lyn-mCherry-expressing neuron producing actin waves. Neuron was imaged on DIV2 under CO2 in standard culturing Neurobasal Media. Images were collected every 5 min and the movie was generated at 5 frames per second. Scale bar = 40 μm.
CA-KIF5C switches between neurites before localizing into a single neurite.
This movie shows timelapse images from a neuron expressing F-tractin-mCherry (white) and CA-KIF5C-Venus (green). Images were collected every 15 min and the movie was generated at 5 frames per second. Scale bar = 30 μm.
Pulsatile CA-KIF5C transport coincides with moving actin waves.
This movie shows timelapse images of the F-tractin-mCherry- and CA-KIF5C-Venus-expressing neurons displayed in Figure 3d. Images were collected every 5 min and the movie was generated at 5 frames per second. Scale bar = 30 μm.
Synaptophysin-positive vesicles in actin wave do not experience Brownian motion.
This movie shows timelapse images of an F-tractin-mCherry and Citrine-Synaptophysin expressing neurite with an actin wave. A single F-tractin-mCherry image was taken to identify the actin wave, followed by timelapse imaging of Citrine-Synaptophysin. Images were acquired every 600 ms and the movie was generated at 5 frames per sec. Scale bar = 10 μm.
Actin waves coincide with transiently increased microtubule polymerization and neurite width.
This movie shows timelapse images of an F-tractin-mCherry and YFP-EB1 expressing neuron generating actin waves. As actin waves moves through, increases in neurite width and EB1 puncta number were observed. Spatially cropped images were used to generate Figure 9c kymograph. Images were acquired every min and the movie was generated at 5 frames per sec. Scale bar = 8 μm.
Actin waves coincide with transiently increased numbers of Synaptophysin-positive vesicles.
This movie shows timelapse images of an F-tractin-mCherry and Citrine-Synaptophysin expressing neuron generating actin waves. Actin waves coincide with increased numbers of vesicles. Spatially cropped images were used to generate Figure 9d kymograph. Images were acquired every min and the movie was generated at 5 frames per sec. Scale bar = 10 μm.
Actin waves are observed in post-polarized neurons.
This movie shows timelapse images of a polarized Lyn-mCherry-expressing neuron producing actin waves. Neuron was imaged on DIV2 under CO2 in standard culturing Neurobasal Media. Images were collected every 5 min and the movie was generated at 5 frames per second. Scale bar = 50 μm.





