Transcranial magnetic stimulation (TMS) inhibits cortical dendrites
Figures
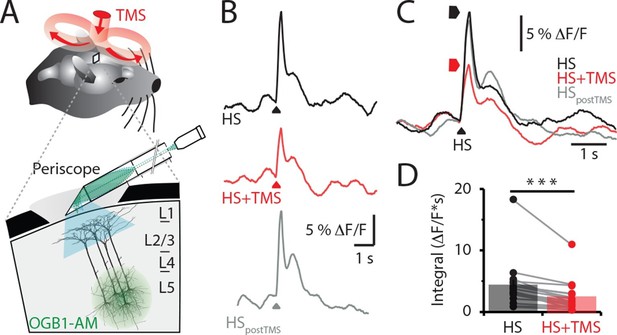
TMS inhibits sensory evoked Ca2+ activity in layer 5 dendrites.
(A) Schematic of the experimental design. Layer 5 pyramidal neurons were bulk loaded with OGB1-AM and dendritic Ca2+ activity was recorded using a flat-periscope configured horizontally and inserted underneath the TMS coil from the side. The TMS coil was placed above the dendrites in the hindpaw region of the somatosensory cortex. (B) Typical dendritic Ca2+ response to hindpaw stimulation (HP) alone (black) and during a single TMS pulse (red) and HP alone post-experiment (grey). (C) Overlay of traces in (b) and (D) graph illustrating the decrease in Ca2+ response during TMS (n=17). p<0.001 (***). TMS, transcranial magnetic stimulation.
-
Figure 1—source data 1
Integral and amplitude of evoked calcium transient.
- https://doi.org/10.7554/eLife.13598.004
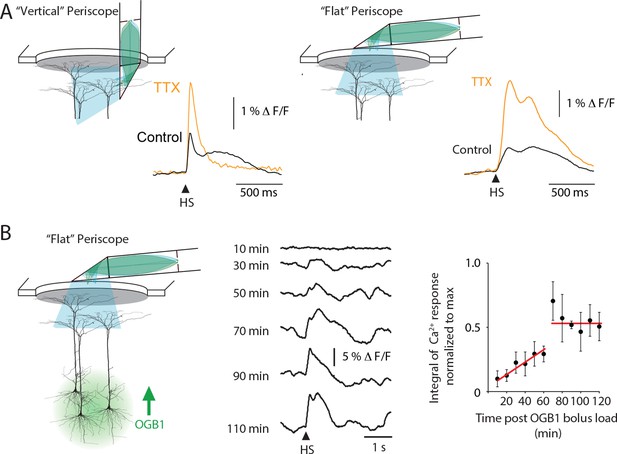
Periscope position and temporal characteristics of sensory-evoked Ca2+ responses in layer 5 pyramidal neuron dendrites.
(A) Similar to the ‘vertical’ periscope, the ‘flat’ periscope recorded an increase in Ca2+ activity during TTX application into layer 5 which is caused by TTX blocking layer 5 Martinoti cells that normally inhibit sensory evoked dendritic Ca2+ influx. These results illustrate both ‘periscopes’ are able to reliably record dendritic Ca2+ dynamics. (B) A large Ca2+ response was recorded after 70min using the ‘flat’ periscope indicating the length of time required for OGB1-AM to diffuse into the dendrites of layer 5 pyramidal neurons. (left) Schematic representation of the experimental setup. The Ca2+ indicator OGB1-AM was bulk loaded into layer 5 and the fiber optic ‘flat periscope’ was positioned horizontally above the craniotomy. (middle) Ca2+ responses to hindpaw stimulation (HS) recorded 10, 30, 50, 70, 90, 110 min after OGB1-AM loading. (right) Integral of Ca2+ response normalized to the maximum at different times after OGB1-AM load. The increase over time is assumed to be due to diffusion of the indicator in the dendrites and corresponds to the time needed for stable recordings using the vertical periscope configuration.
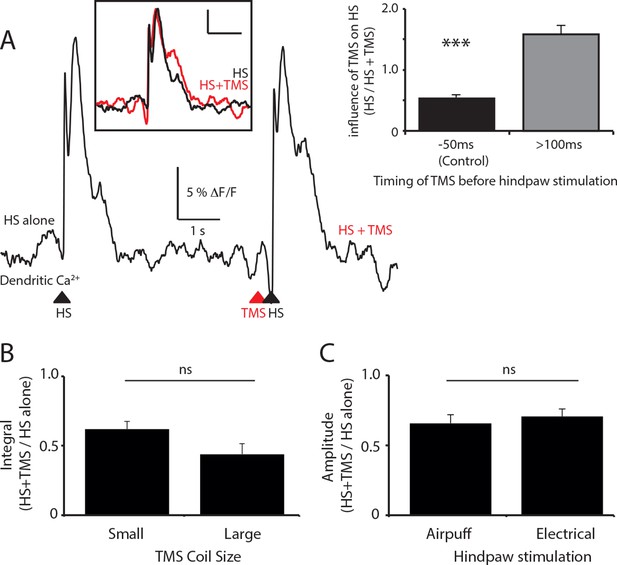
The effect of TMS timing, coil size and stimulation paradigm on layer 5 dendritic sensory responses.
(A) Layer 5 (L5) pyramidal neurons were bulk loaded with the calcium indicator OGB1 AM and the effect of the timing of TMS on the dendritic response during hindpaw stimulation was investigated. Example Ca2+ response to hindpaw stimulation alone (HS) and during TMS generated 200 ms before HS. Inset (left): overlay of HS alone (black) and HS+TMS (red). Scale bar: 5% △F/F, 1 s. Inset (right): Compared to control (TMS 50 ms before HS) which causes a 45 ± 5% decrease in the evoked dendritic Ca2+ response to HS (see Figure 1; solid; n=17), on average, TMS did not significantly influence the dendritic response to HS when evoked greater than 100 ms before HS (lines; n=6). (B) L5 pyramidal neurons were bulk loaded with the Ca2+ indicator OGB1 AM and the effect of TMS on hindpaw sensory-stimulation was tested using different sized coils. Both coils were positioned the same distance from the region of interest. There was no significant difference between the TMS evoked inhibition of the HS dendritic response using either the small (25 mm; n=10) or large (70 mm; n=7) TMS coil. (C) The effect of TMS on hindpaw sensory-stimulation was tested using different hindpaw stimulation protocols. The hindpaw was stimulated by either a triggered brief airpuff (400 ms; n=12) or electrical stimulation (100 V; n=9) delivered to the pad of the paw. There was no significant difference between the TMS evoked inhibition of the HS dendritic response using either hindpaw stimulation technique. TMS, transcranial magnetic stimulation.
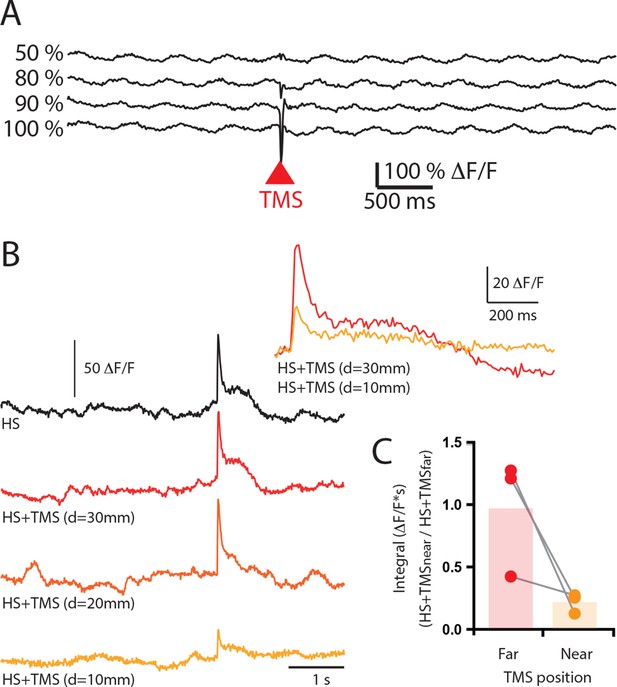
Increasing TMS strength did not elicite an excitatory response in layer 5 pyramidal neuron dendrites.
(A) Ca2+ transients in a population of layer 5 pyramidal neuron dendrites during TMS delivered at increasing strengths (50%, 80%, 90%, 100% ). Note, there was no excitatory Ca2+ response to TMS. (B) Sensory evoked Ca2+ transients in a population of layer 5 pyramidal neuron dendrites during hindpaw stimulation (HS, black) and combined HS and TMS delivered at decreasing distances from the craniotomy (10–30 mm). Inset, overlay of Ca2+ transient during HS+TMS at far (30 mm) and near (10 mm) TMS coil distances. (C) Integral of the sensory evoked Ca2+ transients during HS+TMS when the TMS coil is positioned is at different distances from the craniotomy. TMS, transcranial magnetic stimulation.
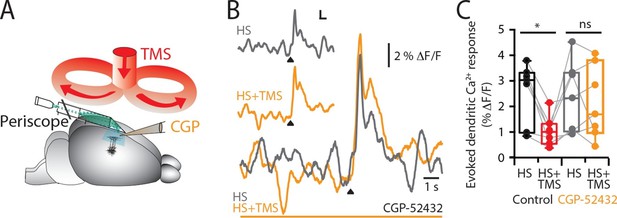
TMS causes GABAB-mediated inhibition of layer 5 dendrites.
(A) Schematic of the experimental design illustrating the application of the GABAB antagonist CGP52432 on the cortical surface. (B) Typical dendritic Ca2+ response to hindpaw stimulation (HS) alone (grey) and during a single TMS pulse (orange, HS+TMS) during cortical CGP. (C) Block of TMS-evoked inhibition of the dendritic sensory response in the presence of CGP52432 compared with control (prior to CGP52432; HS, black; HS+TMS, red; n=7). p<0.05 (*).
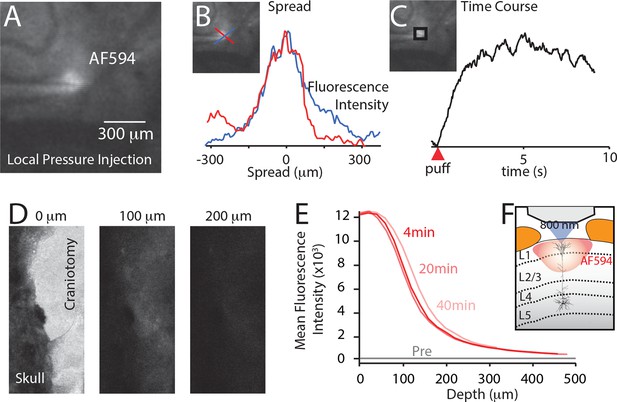
Spread of localized injection (A–C) and cortical surface application (D–F).
Drug application characteristics were measured using fluorescent indicator, AlexFluor594 (AF594; 50 μM) and two-photon imaging (800 nm). (A) To test localized injection, AF594 was included in the drug application pipette and was puffed into the upper cortical layers at approximately 200 μm deep. (B) The lateral spread of the dye was approximately 300 μm. (C) The fluorescence reached maximal intensity within 5 s. (D) AF594 was placed onto the cortical surface. Two photon images of the fluorescence measured at the same laser intensity at 0, 100 and 200 μm. (E) The fluorescence was only measureable at a maximal distance of 200 μm. (F) Therefore, cortical application of fluorophores penetrates the cortex to layer 2/3.
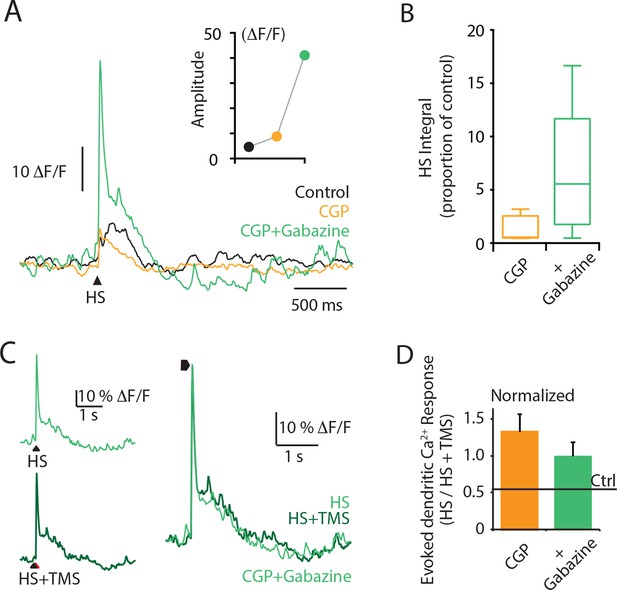
Cortical application of the GABAA antagonist Gabazine causes a dramatic increase in the dendritic response to hindpaw stimulation.
Layer 5 (L5) pyramidal neurons were bulk loaded with the Ca2+ indicator OGB1 AM, and the evoked Ca2+ response was recorded using a fiber optic. (A) An example L5 dendritic Ca2+ response to hindpaw stimulation (HS) during control (black), CGP (orange) and CGP+Gabazine (green) cortical application. (B) Integral of the HS-evoked Ca2+ response as a proportion of the control response during cortical application of CGP+gabazine (green) and CGP (orange). (C) An example L5 dendritic Ca2+ response to hindpaw stimulation (HS, light green) and hindpaw stimulation during TMS (HS+TMS, dark green) during CGP+Gabazine cortical application. Individual traces are overlaid on right. (D) Evoked dendritic Ca2+ response during TMS (HS+TMS) normalized to HS alone during CGP (orange) and CGP + Gabazine (green). TMS, transcranial magnetic stimulation.
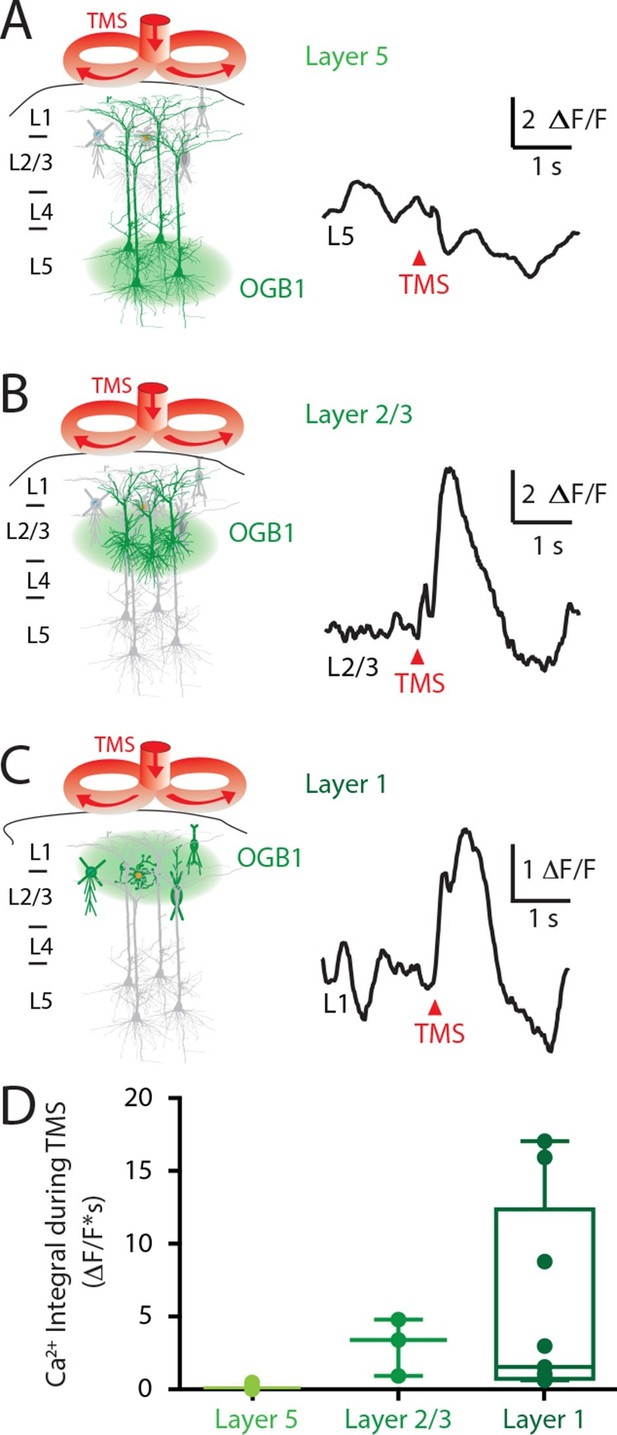
Upper layers of the cortex have Ca2+ transients in response to TMS.
(left) Schematic diagram illustrating Ca2+ indicator loaded into (A) layer 5, (B) layer 2/3 and (C) layer 1. For each cortical depth, the Ca2+ indicator loading location (green circle) and target neurons (green) are indicated. (right) Ca2+ activity was recorded in response to a single TM pulse. (D) Comparison of the integrals of the TMS-evoked Ca2+ responses recorded at the different cortical depths. TMS, transcranial magnetic stimulation.
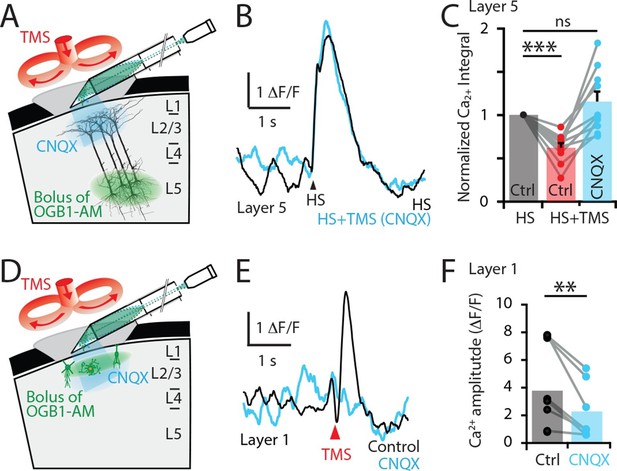
TMS directly activates cells in the upper cortical layers.
(A) Schematic diagram of the experimental design. Layer 5 pyramidal neurons were bulk loaded with OGB1-AM and dendritic Ca2+ activity was recorded using a side-on (horizontal) periscope during application of CNQX to the upper cortical layers. (B) Typical dendritic Ca2+ response to hindpaw stimulation (HS) alone (black) and during a single TMS pulse in the presence of cortical CNQX (blue). (C) Ca2+ responses (integrals) during HS+TMS in the presence (blue) and absence (red) of CNQX normalized to control HS (black; n=10). (D) Schematic diagram of the experimental design. Layer 1 neurons were bulk loaded with OGB1-AM and dendritic Ca2+ activity was recorded during TMS using the side-on periscope during application of CNQX into the upper cortical layers. (E) Dendritic Ca2+ response to a single TMS pulse (black) and in the presence of cortical CNQX (blue). (F) Amplitude of the TMS-evoked Ca2+ responses in L1 neurons during control (black) and CNQX (blue) (n=8). p<0.005 (**), p<0.001 (***).
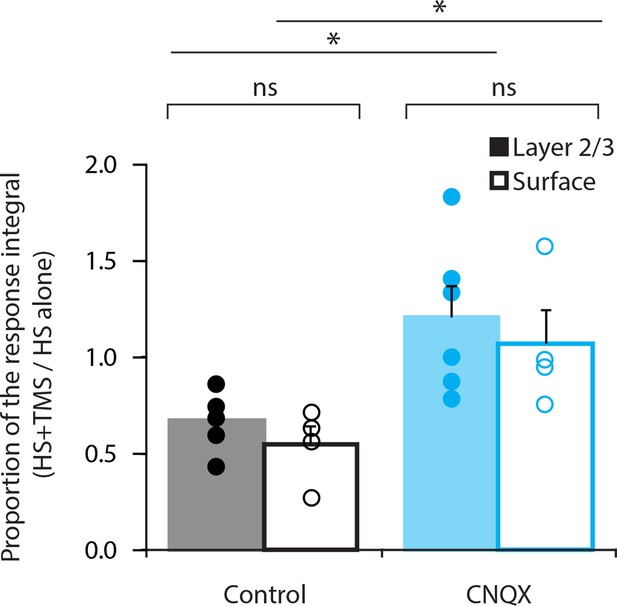
Comparison of CNQX application.
Layer 5 pyramidal neurons were bulk loaded with the Ca2+ indicator OGB1-AM and the effect of TMS on the dendritic response during hindpaw stimulation (HS+TMS) was compared in control and during CNQX application. CNQX was either applied locally into layer 2/3 (L2/3) by a puff pipette (n=6; solid) or topically onto the pia surface (n=4; empty). During both application methods, CNQX caused significant increase in the integral of the Ca2+ response during HS+TMS (p < 0.05). There was no significant difference in the Ca2+ response between local or topical application of CNQX (Control, p = 0.27; CNQX, p = 0.59).
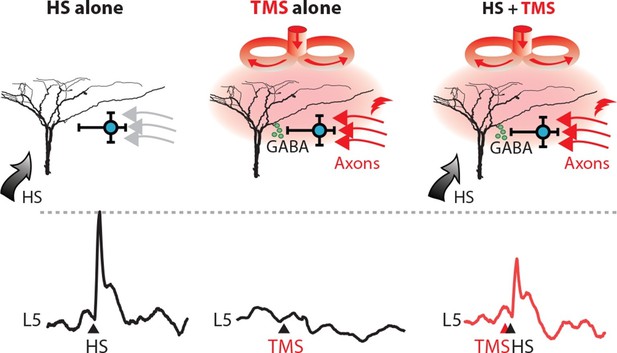
TMS activates an inhibitory microcircuit in the upper cortical layers.
Hypothesized effect of TMS on cortical processing; TMS activates axons (red) which excite upper layer interneurons (blue) causing GABA neurotransmitter release (green) which provides GABA-mediated inhibition to layer 5 pyramidal neuron dendrites. (left) Hindpaw stimulation (HS) causes large Ca2+ responses in layer 5 pyramidal neuron dendrites. (middle) TMS directly activates upper layer neurons but does not cause a Ca2+ response in layer 5 dendrites. However, (right) TMS paired with HS causes a large decrease in the HS Ca2+ response. TMS, transcranial magnetic stimulation.





