Spatial signals link exit from mitosis to spindle position
Figures
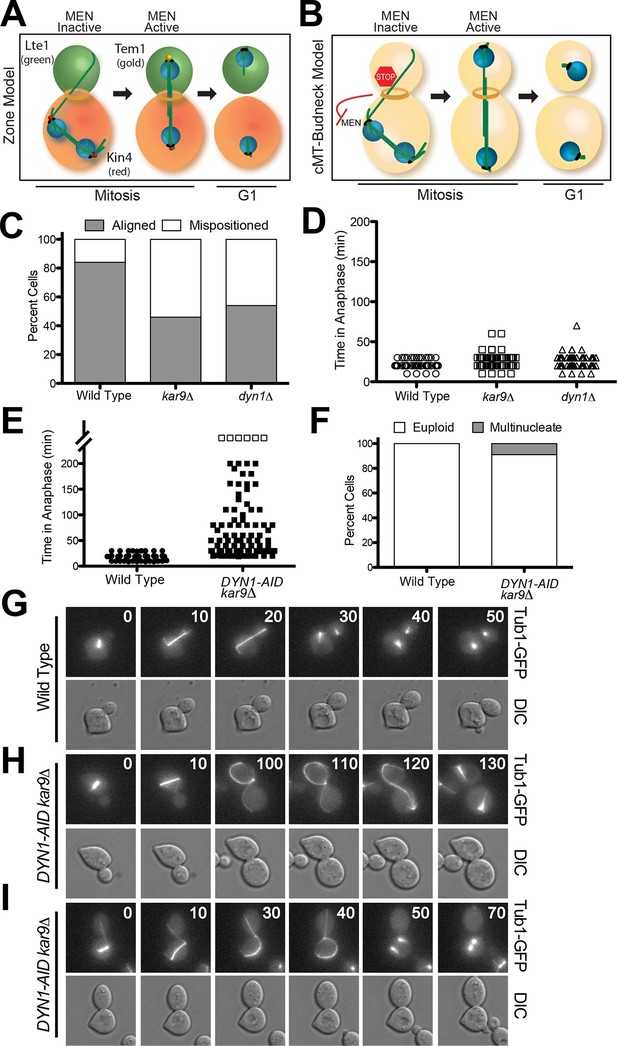
A system to induce spindle misposition.
(A) Zone model of exit from mitosis. Yeast cells are partitioned into two zones: a MEN inhibitory zone in the mother cell compartment (red) and a MEN activating zone in the bud cell compartment (green). If the spindle becomes misaligned in the inhibitory zone, MEN inhibitors such as Kin4 prevent Tem1 enrichment on SPBs thereby inhibiting exit from mitosis. It is only once one SPB escapes the MEN inhibitory zone and moves into the bud cell compartment that Tem1 can become enriched at the daughter-bound SPB and the cell can exit mitosis. Note that in this model, Tem1 is shown not to localize to SPBs in cells with mispositioned spindles. This is based on the observation that Tem1-13MYC does not localize to SPBs in cells with mispositioned spindles (D’Aquino et al., 2005). (B) cMT - budneck model of exit from mitosis. If the spindle becomes misaligned in the mother compartment, cytoplasmic microtubules activate a checkpoint response through their interactions with factors at the bud neck. Once the spindle has realigned, the cytoplasmic microtubules are no longer in contact with the bud neck, the checkpoint signal is eliminated and cells exit from mitosis.(C–D) Wild type (A33138), kar9Δ (A33729) and dyn1Δ (A32922) cells harboring GFP-tagged α-tubulin were grown to mid-log in YEPD and arrested in G1 with 10 μg/mL of the α-factor pheromone at 25°C. The cultures were released into the cell cycle in YEPD and then loaded onto a Y04C CellASIC flow cell. Cells were imaged on the flow cell in synthetic complete pH 6.0 medium. (C) Quantification of the percent of anaphase cells which misposition their anaphase spindle. Anaphase was defined as any spindle measuring >2 μm. Aligned spindles were defined as those that entered anaphase with one spindle pole in the bud cell compartment. Mispositioned spindles were defined as those that entered anaphase with both spindle poles the mother cell compartment. (D) Time-lapse analysis of anaphase length. n =100 cells for each strain (E–I) osTIR1 (A35699) and osTIR1 DYN-AID kar9Δ (A35707) cells expressing GFP-tagged α-tubulin were grown in YEPD medium at 25°C and arrested in the G1 phase of the cell cycle with 10 μg/mL α-factor pheromone. Cells were released into the cell cycle in YEPD pH 6.0 medium and then monitored by live cell microscopy. Depletion of dyn1-AID was induced on a Cellasic flow cell with 100 μM auxin in SC pH 6.0 medium at 25°C. (E) Time-lapse analysis of anaphase length. Open squares indicate cells arrested in anaphase for more than 200 min. (F) Analysis of ploidy. Cells that were arrested and contained a misaligned spindle or cells that exited mitosis that contained an aligned spindle were categorized as 'euploid'. Cells that inappropriately exited mitosis and broke down the spindle in the mother cell compartment were categorized as 'multinucleate'. n=100 cells. (G–I) Montage of representative time-lapse images. The numbers at the top of the GFP images are time in minutes.

Analysis of cytoplasmic microtubules in the bud neck.
Cells harboring osTIR1 DYN-AID kar9Δ and expressing GFP-labeled tubulin (A35707) were grown and imaged as descried in Figure 1E–I. A table summarizing cMT-bud neck contact for cells that contained a mispositioned spindle for 60-min (cells 1–30) or exited mitosis within that time frame (cells 31–40) is shown. Each row shows the color-coded fate of one cell for the given time period, as well as whether it had a cMT in contact with the bud neck. cMT analysis was performed by assessing whether a cMT was present or absent from the bud neck. Cells in which the tip of a cMT interacted with the bud neck or where the cMT traversed the bud neck was categorized as 'cMT in bud neck' (grey boxes) Cells lacking any cMT in the bud neck are described as 'cMT absent from bud neck' (black boxes). Movement of one spindle pole into the bud is described as 'spindle pole movement into bud' (blue boxes). Inappropriate exit from anaphase was determined by the spindle morphology and is described as 'spindle breakdown' (red boxes). A second category of inappropriate exit from mitosis was scored based on whether the cell rebudded without spindle collapse or cytokinesis (yellow boxes). Due to the low frequency of inappropriate spindle breakdown in the mother compartment, this table shows all cells that inappropriately exit mitosis from 2 experiments (cells 31–40). The cells that remain euploid (cells 1–30) are from experiment 1.
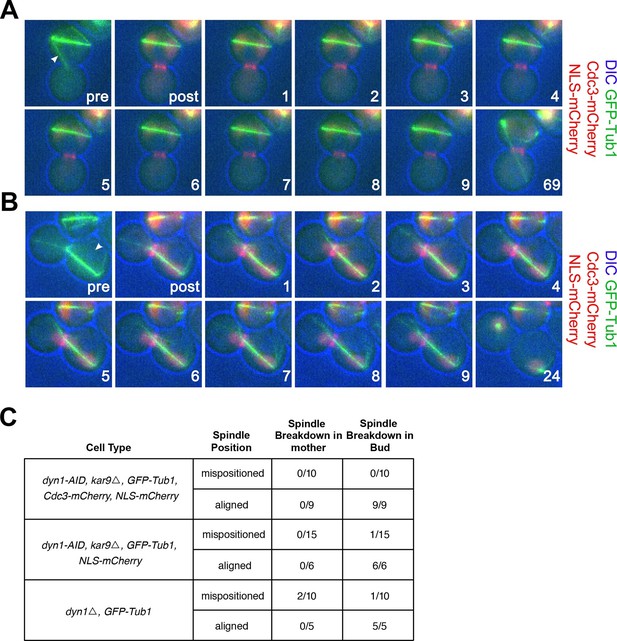
cMT laser ablation does not promote exit from mitosis in cells with mispositioned spindles.
(A-B) Cells harboring the osTIR1 DYN-AID kar9Δ constructs that also expressed GFP-tagged α-tubulin, mCherry-tagged Cdc3 and an NLS-mCherry (A35143) were grown overnight to mid-log in YEPD at 25°C. Cells were then resuspended in synthetic complete medium supplemented with 100 μM IAA and incubated for 2–3 hr at 25°C. The cells were prepared on an agar pad for live cell microscopy. Two pre-ablation images were taken (only one is shown) before the cMT was cut. Post ablation cells were monitored for 9 min at 1-min intervals for cMTs and then 1 hr at 15-min intervals to follow cell cycle progression. The arrowheads indicate the approximate laser targeting site. (A) A montage of a cell with a mispositioned spindle where cMT bud neck interactions were disrupted due to microtubule severing. The DIC channel is optimized to enhance contrast. (B) A montage of a control cell with an aligned spindle where the laser was targeted to the cytoplasm. The DIC channel is optimized to enhance contrast. (C) Table summarizing cell cycle stage of aligned and misaligned spindles 69 min post ablation. The culturing conditions for cells in the first (A35143) and second rows (A34832: the same as A35143 but lacking Cdc3-mCherry) of the table are the same as described above. In the third row, cells lacking DYN1 and expressing GFP-tagged α-tubulin and NLS-mCherry (A34722) were grown overnight at 25°C to mid-log and then shifted to 16°C for 2–5 hr to enrich for cells with mispositioned spindles. The cells were then mounted on an agar pad for live cell microscopy. One set of pre-ablation images was taken before the cMT was cut. Post ablation cells were monitored as described above.
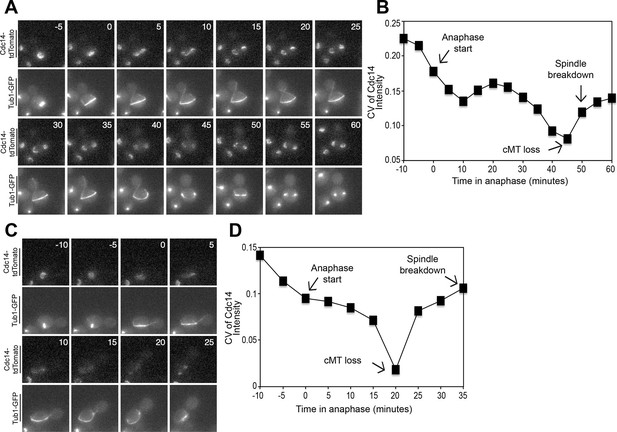
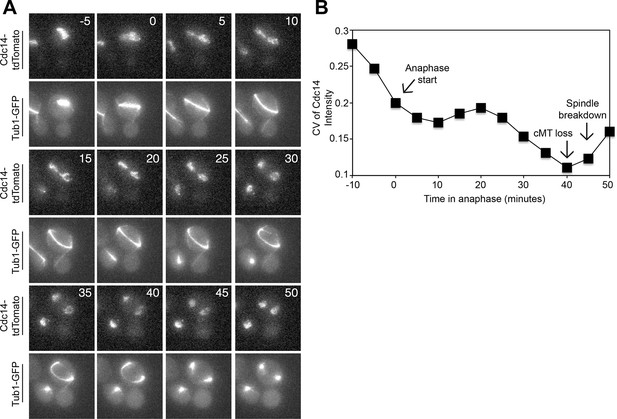
Cdc14 Release from the nucleolus precedes cMT retraction in cells that inappropriately breakdown their anaphase spindles in the mother cell compartment.
(A–D) A diploid strain (A37463) homozygous for osTIR1 dyn1-AID kar9Δ and heterozygous for GFP-Tub1 and Cdc14-tdTomato was grown to midlog in Synthetic Complete medium. Cycling cells were imaged on a flow cell in Synthetic Complete medium supplemented with 100 μM IAA. (A) Representative images are shown for the anaphase release of Cdc14-tdTomato with respect to GFP-Tub1 cMT retraction. (B) The coefficient of variation (the standard deviation divided by the mean) was measured for Cdc14 pixel intensity for the cell pictured in the Figure 4A montage. Time (in minutes) is displayed on the X-axis and the zero time point reflects anaphase onset. (C) Representative images are shown for a cell with a mispositioned spindle in which complete Cdc14-td Tomato release from the nucleolus was observed at anaphase onset. cMTs are also shown for that cell during the same time interval. (D) The coefficient of variation (the standard deviation divided by the mean) was measured for Cdc14 pixel intensity for the cell pictured in the Figure 4C montage. Time (in minutes) is displayed on the X-axis. The zero time point reflects anaphase onset. See also: Figure 4—figure supplement 1–4.
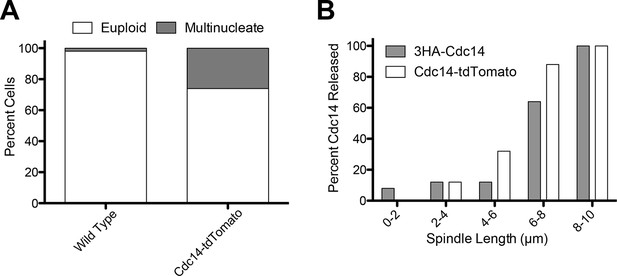
Analysis of the CDC14-tdTomato allele.
(A) Cells homozygous for osTIR1 dyn1-AID kar9Δ and heterozygous for either GFP-Tub1 (A38232) or GFP-Tub1 and Cdc14-td Tomato (A37464) were grown overnight in YPD to mid-log and then loaded onto a flow cell and imaged in synthetic complete medium containing 100 μM auxin. Degree of ploidy was analyzed 3 hr after the addition of auxin. (B) Cells harboring Cdc14-3HA (A1411) or Cdc14-tdTomato and GFP-Tub1 (A38243) were grown overnight to mid-log in YEPD and then arrested in G1 with 10 μg/mL of the α-factor pheromone. Cells were then released into the cell cycle, fixed and Cdc14 localization and mitotic spindle length was analyzed.
Analysis of Cdc14 Release in cells with mis-positioned spindles.
The diploid strain (A37463) homozygous for osTIR1 dyn1-AID kar9Δ and heterozygous for GFP-Tub1 and Cdc14-tdTomato was grown to midlog in synthetic complete medium. Cycling cells were imaged as in Figure 4. (A) Representative images are shown for Cdc14-tdTomato release and GFP-Tub1 cMT retraction in anaphase. (B) The coefficient of variation (the standard deviation divided by the mean) was measured for Cdc14 intensity for the cell pictured in (A). Time (in minutes) is displayed on the X-axis and the zero time point reflects anaphase onset.
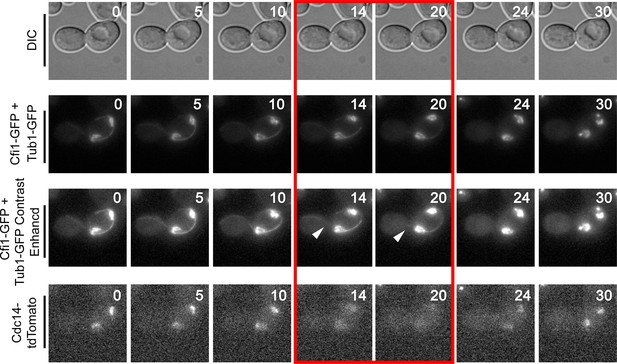
Montage of Cdc14 Release in cells with mis-positioned spindles containing the nucleolar marker Cfi1.
The diploid strain (A38233) homozygous for osTIR1 dyn1-AID and kar9Δ and heterozygous for GFP-Tub1, Cfi1-GFP and Cdc14-tdTomato was grown overnight in synthetic complete medium and then imaged as in Figure 4. Depicted are time-lapse images showing GFP-Tub1 cMT retraction and Cdc14-tdTomato release with respect to Cfi1-GFP. The red-boxed time points highlight the time frame when Cdc14-tdTomato intensity decreases in the nucleolus yet the cell still harbors a cMT in contact with the bud neck (arrowheads). Note that Cfi1-GFP intensity remains largely unchanged throughout the time course. Time (in minutes) is shown in the upper hand corner of each DIC image.
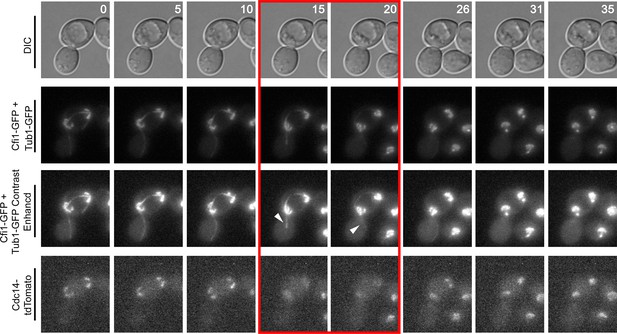
Montage of Cdc14 Release in cells with mis-positioned spindles containing the nucleolar marker Cfi1.
The diploid strain (A38233) homozygous for osTIR1 dyn1-AID and kar9Δ and heterozygous for GFP-Tub1, Cfi1-GFP and Cdc14-tdTomato was grown overnight in synthetic complete medium and then imaged as in Figure 4. Depicted are time-lapse images showing GFP-Tub1 cMT retraction and Cdc14-tdTomato release with respect to Cfi1-GFP. The red-boxed time points highlight the time frame when Cdc14-tdTomato intensity decreases in the nucleolus yet the cell still harbors a cMT in contact with the bud neck (arrowheads). Note that Cfi1-GFP intensity remains largely unchanged throughout the time course. Time (in minutes) is shown in the upper hand corner of each DIC image.
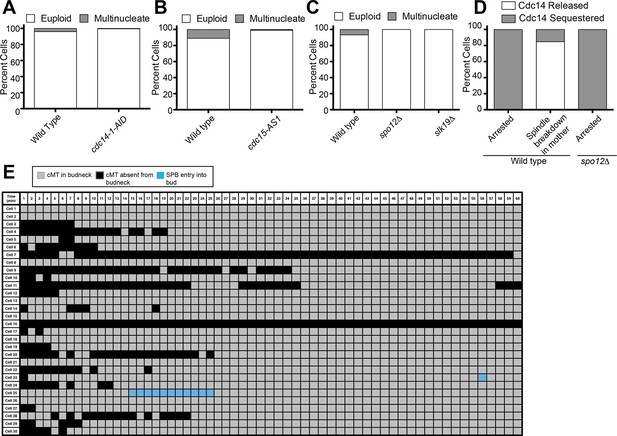
Inhibition of Cdc14, the MEN and the FEAR Network prevents Spindle Breakdown in the mother cell compartment.
(A) osTIR1 dyn1-AID kar9Δ (A35707) and osTIR1 dyn1-AID kar9Δ cdc14-1-AID (A37895) cells harboring GFP-tagged α-tubulin were grown as described in Figure 1E–I. Cells were monitored by live cell microscopy and scored for inappropriate spindle breakdown in the mother cell compartment. n=100 for osTIR1 dyn1-AID kar9Δ and 225 for osTIR1 dyn1-AID kar9Δ cdc14-1-AID. (B) osTIR1 dyn1-AID kar9Δ (A35707), osTIR1 dyn1-AID kar9Δ cdc15-AS1 (A36264) expressing GFP-tagged α-tubulin, were analyzed as in Figure 5A but with the addition of 20 μM NAPP1 to the medium. Cells were imaged in a Lab-Tek II chamber. n=100 for each strain. (C) osTIR1 dyn1-AID kar9Δ (A35707), osTIR1 dyn1-AID kar9Δ spo12Δ (A35700), osTIR1 dyn1-AID kar9Δ slk19Δ (A36028) expressing GFP-tagged α-tubulin, were analyzed as in Figure 5A. n ≥ 284 for each strain. (D) Wild type (A37753) or spo12Δ (A37610) diploid strains homozygous for osTIR1 dyn1-AID kar9Δ and GFP-Tub1 and heterozygous for Cdc14-tdTomato were grown to mid log in synthetic complete medium. Cells with mispositioned spindles that had two distinct nucleoli were scored based on whether Cdc14-tdTomato was released from the nucleolus in late anaphase. Cells that did not exit mitosis in the mother cell compartment were monitored for 60 min. n≥22 cells. (E) cMT analysis was performed on osTIR1 dyn1-AID kar9Δ spo12Δ (A35700) as described in Figure 2. Each row shows the color-coded fate of one cell for the given time period, as well whether it had a cMT in contact with the bud neck. cMT analysis was performed by assessing whether a cMT was present or absent from the bud neck. Cells with a cMT end that was in the bud neck or in the bud was categorized as 'cMT in bud neck' (grey boxes) Cells lacking any cMT in the bud neck are described as 'cMT absent from bud neck' (black boxes). Movement of one spindle pole into the bud is represented by the 'spindle pole movement into bud category (blue boxes). Note: Cell #25 transiently moves a spindle pole into the bud cell compartment but does not exit from mitosis. This is most likely due the inefficient activation of the MEN in cells lacking the FEAR network.
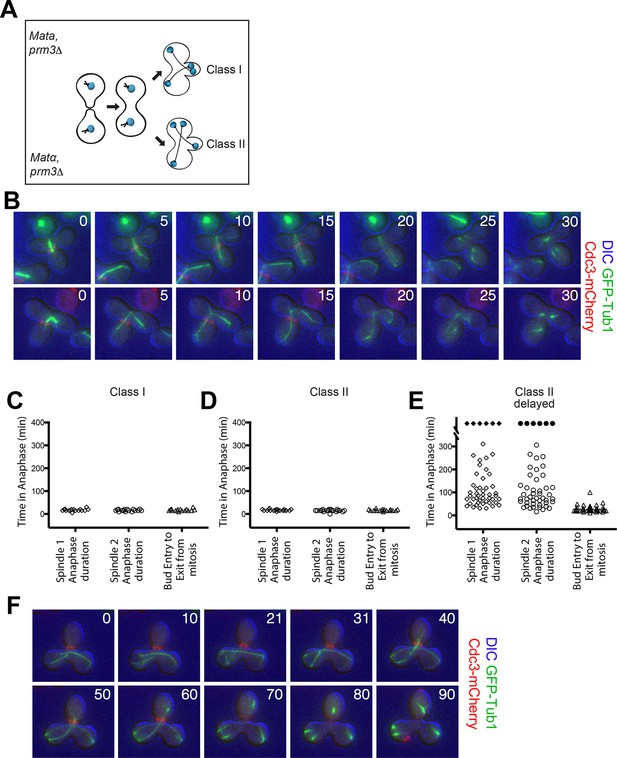
Analysis of Exit from Mitosis in prm3Δ heterokaryons.
(A) Cartoon of the prm3Δ heterokaryon system showing the two main classes of heterokaryons obtained. (B–F) Heterokaryons were obtained by mating cells lacking PRM3 (see Materials and methods for details). Briefly, G1 cells isolated by centrifugal, elutriation were mated at 30° for 2 hr and then loaded onto a Y04D CellASIC flow cell for imaging. Synthetic complete pH 6.0 medium was used in the flow cell during imaging and was supplemented with 100 μM IAA to induce spindle mispositioning. (B) Montages of binucleate zygotes created by mating cells lacking PRM3. Binucleate diploids are homozygous for prm3Δ and osTIR1 and heterozygous for DYN-AID, kar9Δ, mCherry-labeled Cdc3 and GFP-labeled α-tubulin (A37892 x A35570). The DIC channel was adjusted to maximize contrast. (C–D) Analysis of anaphase kinetics of cells described in Figure 6B. Class 1: n= 16. Class II: n= 21 (E) Binucleate diploids that were homozygous for prm3Δ, osTIR1, DYN-AID, kar9Δ and heterozygous for mCherry-labeled Cdc3 and GFP-labeled α-tubulin (A35570 x A35571) were analyzed for anaphase duration. Black diamonds indicated permanently arrested cells (permanently arrested ≥320 min) n=51 cells. (F) Montage of cells from Figure 6E. The DIC channel was adjusted to maximize contrast.
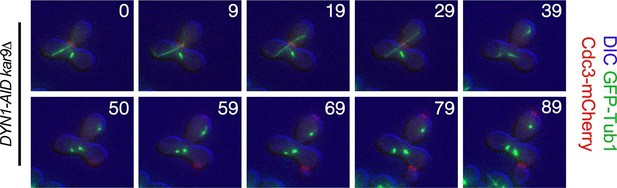
Exit from Mitosis in a prm3Δ heterokaryon with one aligned anaphase spindle and one metaphase spindle.
Montage of binucleate zygotes created by mating homozygous for prm3Δ, osTIR1, DYN-AID, kar9Δ and heterozygous for mCherry-labeled Cdc3 and GFP-labeled α-tubulin (A35570 x A35571). The montage depicts a zygote with one aligned anaphase spindle and a second spindle in metaphase in the mother compartment. Both spindles exit mitosis at the same time with the metaphase spindle never going through anaphase. The DIC channel was adjusted to maximize contrast.
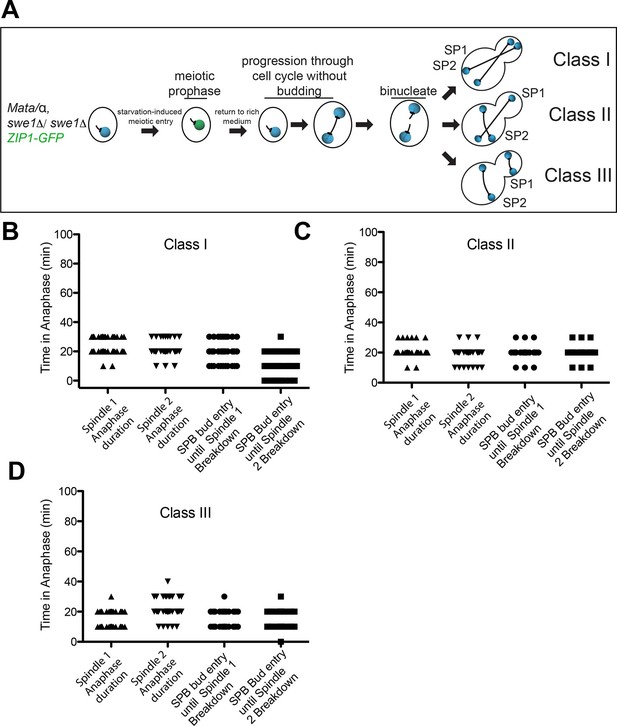
Analysis of Exit from Mitosis in swe1Δ heterokaryons.
(A) Cartoon of swe1Δ return to growth heterokaryon system and depictions of cell type classes that were analyzed. Briefly, diploid cells lacking SWE1, also harboring the meiotic prophase marker Zip1, tagged with GFP as well as GFP-tagged Tub1 (LY1043), were induced to enter meiotic prophase through nutrient starvation. Upon entry into meiotic prophase (as judged by the presence of Zip1-GFP positive cells), cells were returned to glucose-containing complete medium in microfluidic chambers and thus induced to grow mitotically. These cells were monitored by live cell microscopy. (B) Anaphase kinetics of Class I cells (depicted in Figure 8A). Anaphase duration is classified as the time the cell spends with a spindle >2 μm to spindle breakdown. 'Bud entry to exit from mitosis' is defined as the time from when at least one spindle pole is in the bud to anaphase spindle disassembly. (C) Anaphase kinetics of Class II cells (depicted in Figure 8A). Anaphase duration and bud entry to exit from mitosis are define as in Figure 8B. (D) Anaphase kinetics of Class III cells (depicted in Figure 8A). Anaphase duration and bud entry to exit from mitosis are define as in Figure 8B. n=50 cells for each class.
Videos
A cell with a mispositioned spindle that has a cMT in the bud neck.
osTIR1 DYN-AID kar9Δ cells expressing GFP-labeled α-tubulin (A35707) were grown and imaged as described in Figure 1E-I. Depicted is a cell that mis-positions its spindle and displays cMTs that protrude into the bud. Time in minutes is show in the upper left-hand corner of the time-lapse image. A merged image of the DIC and GFP channels is shown on the left. The GFP channel alone is shown on the right. The DIC image was adjusted to maximize contrast.
A cell with a mispositioned spindle that inappropriately exits mitosis in the mother cell compartment.
osTIR1 DYN-AID kar9Δ cells expressing GFP-labeled α-tubulin (A35707) were grown and imaged as described in Figure 1E-I. Depicted is a cell that mis-positions its spindle and then inappropriately exits mitosis in the mother cell. Time in minutes is show in the upper left-hand corner of the time-lapse image. A merged image of the DIC and GFP channels is shown on the left. The GFP channel alone is shown on the right. The DIC image was adjusted to maximize contrast.
A cell with a mispositioned spindle that inappropriately exits mitosis but does not complete spindle disassembly or cytokinesis.
osTIR1 DYN-AID kar9Δ cells expressing GFP-labeled α-tubulin (A35707) were grown and imaged as described in Figure 1E-I. Depicted is a cell that mispositions its spindle and then inappropriately exits mitosis in the mother cell but does not complete cytokinesis or spindle disassembly. Time in minutes is show in the upper left-hand corner of the time-lapse image. A merged image of the DIC and GFP channels is shown on the left. The GFP channel alone is shown on the right. The DIC image was adjusted to maximize contrast.
A cell with a mispositioned spindle that lacks cMT-bud neck interactions but arrests in anaphase.
osTIR1 DYN-AID kar9Δ cells expressing GFP-labeled α-tubulin (A35707) were grown and imaged as described in Figure 1E-I. Depicted is a cell that arrests in late anaphase with a mispositioned spindle. This cell lacks cMTs in the bud neck for a substantial time period in anaphase. Time in minutes is show in the upper left-hand corner of the time- lapse image. A merged image of the DIC and GFP channels is shown on the left. The GFP channel alone is shown on the right. The DIC image was adjusted to maximize contrast.
Additional files
-
Supplementary file 1
Yeast Strain Table.
The strains listed in Supplementary file 1 are all derivatives of W303. The table shows the relevant genotype for each yeast strain and the associated strain number.
- https://doi.org/10.7554/eLife.14036.019





