Conditional deletion of WT1 in the septum transversum mesenchyme causes congenital diaphragmatic hernia in mice
Figures
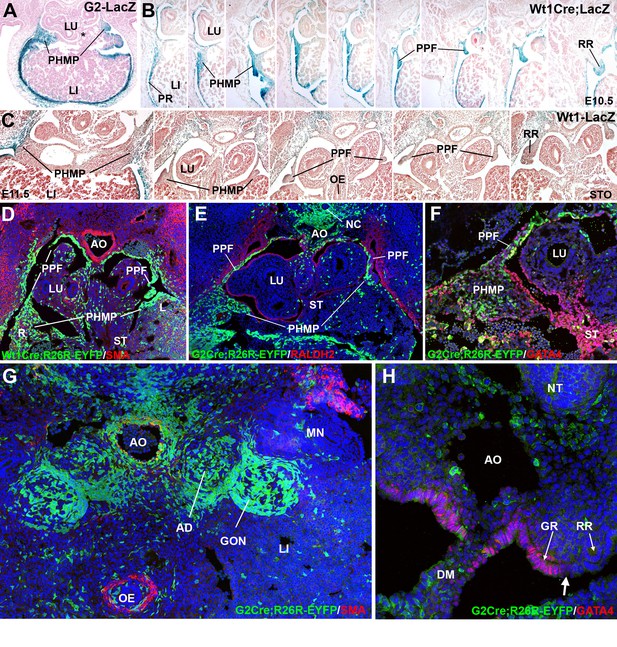
Cell lineages in the septum transversum (ST), posthepatic mesenchymal plate (PHMP) and pleuroperitoneal folds (PPF).
(A) G2-LacZ embryo at the stage E11.5. The G2 enhancer is active in the liver (LI) mesothelium and posthepatic mesenchymal plate (PHMP). However, the central area of the septum transversum shows no activity of this enhancer (asterisk). (B) Wt1Cre;R26RLacZ embryo, E10.5. Cells from the WT1 lineage appear in blue. Series of transverse sections from cephalic (left) to caudal (right) levels. The anterior peritoneal recess (PR) is splitting the liver from the body wall. The PHMP is continuous with the PPF at cephalic levels. Note the abundance of mesenchymal cells in the anterior PHMP and how this mesenchyme is less abundant in posterior levels. The PPF is continuous with the renal ridge (RR). The mesenchymal cells of all these structures belong to a WT1-expressing cell lineage. (C) Wt1LacZembryo, E11.5. Activation of the Wt1 reporter can be seen in the liver mesothelium. PHMP, PPF and RR. The RRs appear at the level of the stomach (STO). OE: oesophagus. (D) Wt1Cre;R26RYFP embryo, E11.5. Cells from the WT1-expressing cell lineage (green) are more abundant at the right PHMP (R) as compared with the left one (L). Note the lack of YFP+ cells in the central area of the ST. AO: aorta; LU: lung. (E) G2-Gata4Cre; R26RYFP embryo, E11.5. Cells from the lineage expressing GATA4 under the control of the G2 enhancer are stained in green, and RALDH2 is stained in red. Most cells in the PHMP and PPFs belong to the G2-Gata4 lineage, but they are very scarce in the central area of the ST. Abundant YFP+ cells are present dorsally to the aorta, and around the notochord (NC). (F) G2-Gata4Cre; R26RYFP embryo, E11.5. GATA4 immunostaining in red. Colocalization of GATA4 and YFP is observed in the PHMP and PPF, but the central area of the ST shows GATA4 expression not driven by the G2 enhancer. (G) G2-Gata4Cre; R26RYFP embryo, E11.5. Smooth muscle alpha-actin is stained in red. Gonads (GON) and adrenals (AD) contain a large number of G2-Gata4 lineage cells, but they are scarcer into the mesonephros (MN). Data reused, with permission, from Figure 4A, Muñoz-Chápuli et al. Developmental Dynamics, Special Issue: Mechanisms of Morphogenesis, 245:307–322 (2016). © 2015 Wiley Periodicals, Inc. (H) Immunolocalization of GATA4 (red) in an E10.5 G2-Gata4Cre; R26RYFP embryo. The G2 lineage is shown in green. There is a sharp boundary (arrow) between the mesothelial cells expressing GATA4 of the genital ridge (GR), close to the dorsal mesentery (DM), and the mesothelial cells of the renal ridges (RR), which are GATA4-. AO: aorta; OE: oesophagus.
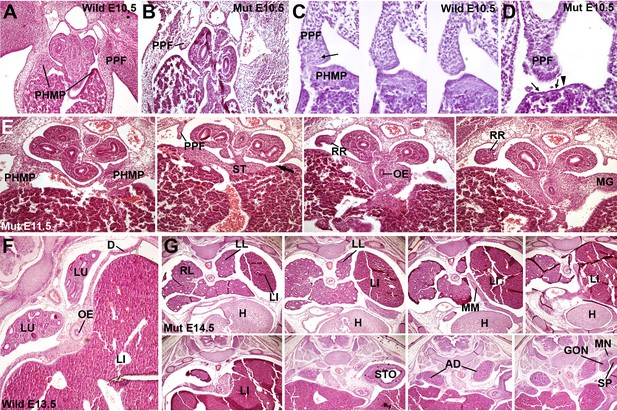
Phenotype of the G2-Gata4Cre; Wt1fl/fl embryos.
(A–D) Wildtype (A,C) and mutant (B,D), E10.5 littermates. The larger amount of mesenchymal cells in the posthepatic mesenchymal plate (PHMP) is evident in the wildtype, especially in the right side. Serial sections corresponding to the connection between the PHMP and the pleuroperitoneal folds (PPF) are shown in C. Note the presence of compact mesenchyme in the PHMP and also in the closest part of the PPF (arrow in C). A corresponding section of the mutant in shown in D. Note the lack of PHMP, the coelomic epithelium lying directly on the hepatic tissue (arrows in D) and the limit of the septum transversum (ST) mesenchymal cells (arrowhead in D), which do not extend laterally. (E) Serial sections of a G2-Gata4Cre; Wt1fl/fl E11.5 embryo at levels equivalent to those shown in Figure 1C. Despite the presence of normal PHMP in the anterior part, the posterior areas of the liver lack of lateral mesenchymal cells (arrow). The mesenchyme is restricted to the central ST. Renal ridges (RR) appear at a level corresponding to the entrance of the oesophagus (OE) into the ST. MG: mesogastrium. (F) Wildtype E13.5 embryo showing complete isolation of the pleural cavities by the pleuroperitoneal membranes that constitute the main part of the diaphragm (D). (G) G2-Gata4Cre; Wt1fl/fl E14.5 embryo at eight different levels showing left diaphragmatic defect with herniation of the left liver lobe (LI) into the pleural cavity and severe hypoplasia of the left lung (LL). Ectopic muscle appear in the mediastinum (MM). Adrenals (AD), mesonephros (MN), gonads (GON) and spleen (SP) appear normal. LU: lungs; H: heart; RL: right lung; STO: stomach.
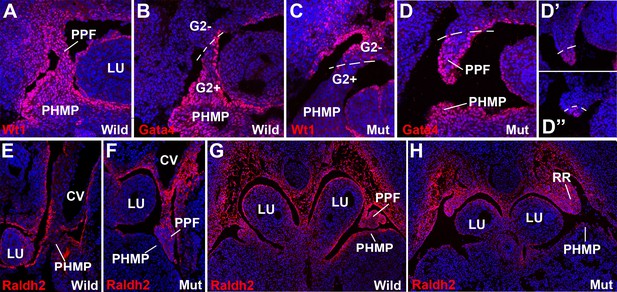
WT1, GATA4 and RALDH2 expression in G2-Gata4Cre; Wt1fl/flembryos.
(A,B) Immunolocalization of WT1 (A) and GATA4 (B) in wildtype E11.5 embryos. The posthepatic mesenchymal plate (PHMP) and pleuroperitoneal folds (PPF) show abundant positive cells. However, the WT1 immunoreactive cells are present in the base of the PPF and the adjacent body wall, where GATA4+ cells are absent. This difference defines the G2+ and the G2- domains. Data in A reused, with permission, from Figure 3E, Muñoz-Chápuli et al. Developmental Dynamics, Special Issue: Mechanisms of Morphogenesis, 245:307–322 (2016). © 2015 Wiley Periodicals, Inc. (C–D) Immunolocalization of WT1 (C) and GATA4 (D) in G2-Gata4Cre; Wt1fl/fl E11.5 embryos. The PHMP and the G2+ domain of the PPF show GATA4+ cells but no WT1+ cells due to the conditional deletion of this gene in the G2+ domain. WT1 expression remains in the G2- domain of the base of the PPF and body wall. GATA4 expression is normal in most cephalic PPF, but immunoreactivity disappears in more caudal areas of PPF, as shown in D’ and D’’. (E–H) Immunolocalization of RALDH2 in E10.5 (E,F) and E11.5 (G,H) wildtype and G2-Gata4Cre; Wt1fl/fl mutant embryos. RALDH2 immunoreactivity is high in PPF and the adjacent body walls. Note strong immunoreactivity in the renal ridges (RR) of the E11.5 mutant embryo. However, RALDH2 immunoreactivity is reduced or absent in the PHMP and liver mesothelium of the mutant embryos, as compared with the controls. CV: cardinal veins; LU: lungs.
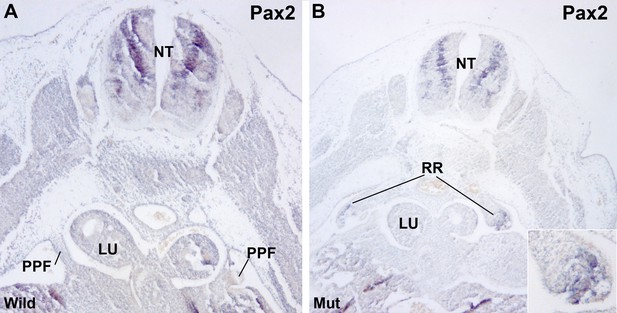
Localization of Pax2 expression by in situ hybridization.
(A) Control E11.5 embryo. Pax2 is expressed in the neural tube (NT). The pleuroperitoneal folds (PPF) lack of Pax2 expression. (B) G2-Gata4Cre; Wt1fl/fl E11.5 embryo. Pax2 is expressed in the PPF at the level of the lung buds (LU). The expression is stronger in the tubular structures that are developing in the left PPF (insert).
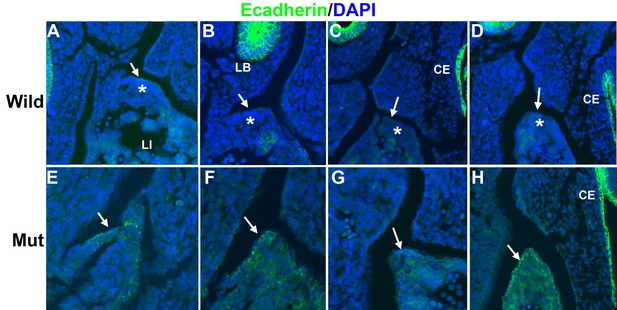
Immunolocalization of E-cadherin in the left posthepatic mesenchymal plate (PHMP) of four E11.5 control embryos (A–D) and four E11.5 G2-Gata4Cre; Wt1fl/fl embryos (E–H).
E-cadherin appears upregulated in the coelomic epithelium of the PHMP of the mutant embryos (arrows in E–H). In wildtype embryos the coelomic epithelium of the PHMP lacks of E-cadherin expression (arrows in A–D) and covers a layer of E-cadherin-negative mesenchymal cells (asterisks).
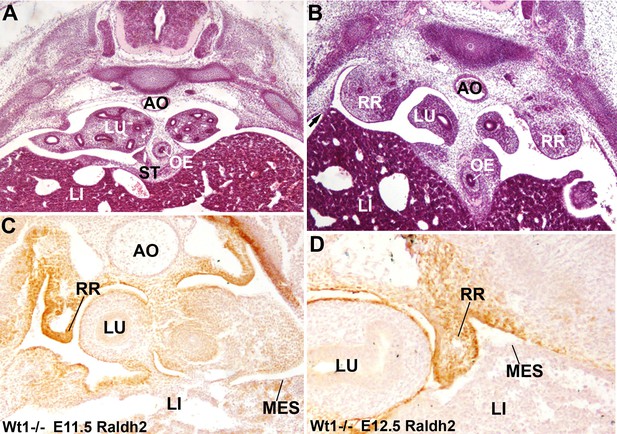
Diaphragmatic phenotype in systemic WT1 loss of function.
(A,B) WT1-/- E13.5 embryos. The complete lack of posthepatic mesenchymal plate is evident, mesenchymal cells are absent from the lateral ends of the liver lobes and remain restricted to the central area of the septum transversum (ST), close to the oesophagus (OE). The pleural cavities are continuous with the peritoneal cavity. Note the renal ridges (RR) persisting at thoracic level, and the abnormal adhesion of the right liver lobe with the body wall (arrow in B). AO: aorta; LI: liver; LU: lungs. (C,D) Immunolocalization of RALDH2 in WT1-/- embryos, E11.5 (A) and E12.5 (B). RALDH2 immunoreactivity is strong in the renal ridges (RR), but weak or absent in the liver (LI) mesothelium (MES). AO: aorta; LU: lung.
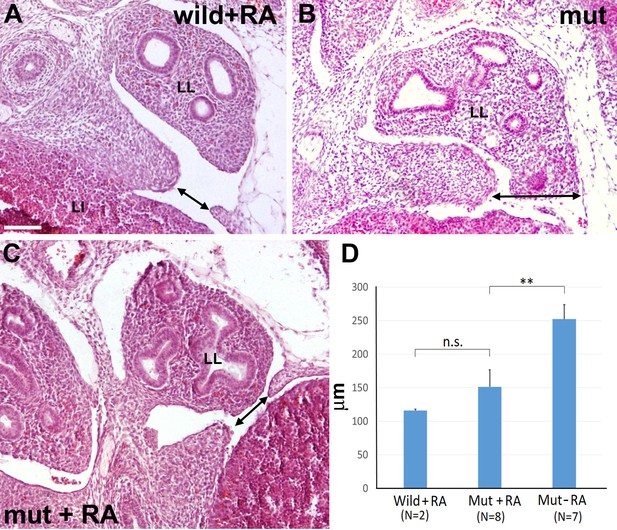
G2-Gata4Cre; Wt1fl/fl mutant embryos and wildtype littermates collected at stage E13.5 from females fed with a retinoic acid (RA) supplemented diet or with control diet by days E8.5-E10.5 of pregnancy.
(A) Wildtype embryo treated with RA. A small discontinuity appears in the left pleuroperitoneal septum (arrow). (B) Mutant embryo not treated with RA. A wide defect is evident in the left pleuroperitoneal septum. (C) Mutant embryos treated with RA and showing a discontinuity similar to that described in A. (D) Width of the discontinuity of the pleuroperitoneal opening in mutant and control embryos treated with RA compared with mutant embryos not treated with RA. The latter embryos show significantly wider openings than the mutant embryos treated with RA. LI: liver; LL: left lung. Scale bar = 100 µm.
-
Figure 7—source data 1
Measurements of the pleuropericardial opening used for Figure 7D.
- https://doi.org/10.7554/eLife.16009.010
Tables
Number of embryos studied and % of G2-Gata4Cre;Wt1fl/fl found. The five E12.5 embryos showed abnormal posthepatic mesenchymal plates at the left side, but they were not computed for the diagnosis of the diaphragmatic hernia, that was performed only on the 23 embryos at the stages E13.5 or older.
| Stage | Number of embryos studied | Number and% G2Cre+;Wt1fl/fl | Embryos analyzed for phenotype | No defect | Mild defect | Diaphragmatic hernia |
|---|---|---|---|---|---|---|
| E10–10.5 | 42 | 11 (26,2%) | 3 | |||
| E11.5 | 89 | 21 (23,6%) | 5 | |||
| E12.5 | 76 | 21 (27,6%) | 5 | (5) | ||
| E13,5 | 65 | 19 (27,1%) | 13 | 1 | 1 | 11* |
| E14,5 | 53 | 17 (32,1%) | 4 | 2 | 2 | |
| E15,5 | 60 | 4 (6,7%) | 4 | 2 | 2 | |
| E16,5 | 21 | 4 (19,0%) | 1 | 1 | ||
| E17,5–18,5 | 21 | 5 (23,4%) | 1 | 1 | ||
| Total | 427 | 102 (23,6%) | 36 | 4 (17,4%) | 3 (13,0%) | 16 (69,6%) |
-
*We have included seven embryos from females fed with control diet in the RA-rescue experiment. One embryo showed CDH only at the right side.
Additional files
-
Supplementary file 1
Image analysis data of the volume taken up by the YFP+ cells in the right and left PHMP.
This experiment is described at the end of the first section of the results.
- https://doi.org/10.7554/eLife.16009.011





