Calcium imaging with genetically encoded indicators in behaving primates
Figures
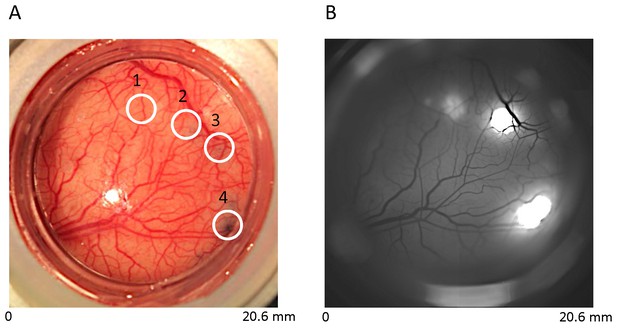
Construct-specific EGFP expression in V1.
(A) Cortical vasculature with four injection sites in one cranial window over V1 of a macaque monkey: 1, rAAV9:mSYN-EGFP; 2, rAAV8:CaMKII-EGFP; 3, rAAV8:mSYN-EGFP; 4, rAAV1:CaMKII-EGFP. Blue stain at sites 2, 3 and 4 reflect the location of viral injections with trypan blue (see Experimental Procedures). Viral injection at site 1 was performed three months earlier, and hence shows minimal blue stain. (B) In vivo epifluorescence image three months post-injection at sites 2–4. Fluorescence at sites 1, 2 and 4 was prominent, but not at site 3. Fluorescence using the human synapsin promoter construct is not shown.
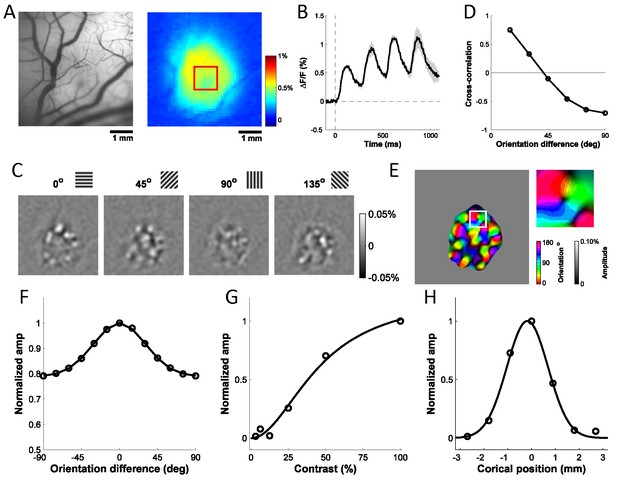
Columnar-scale GCaMP signals from V1 of a behaving macaque.
(A) Vasculature (left) and GCaMP signal (right) at one injection site. (B) Average time course of GCaMP response to a flashed grating. Shaded area ± SEM. (C) Spatial pattern of orientation selective GCaMP signals obtained by bandpass filtration of the response maps to 4 orientations (out of 12 evenly spaced orientations). (D) Pairwise correlations between all 12 orientation maps as a function of stimulus orientation difference. (E) Orientation map with an insert showing a pinwheel. (F–H) Examples of GCaMP orientation tuning (F), contrast tuning (G) and position tuning on the cortex (negative towards fovea) (H).
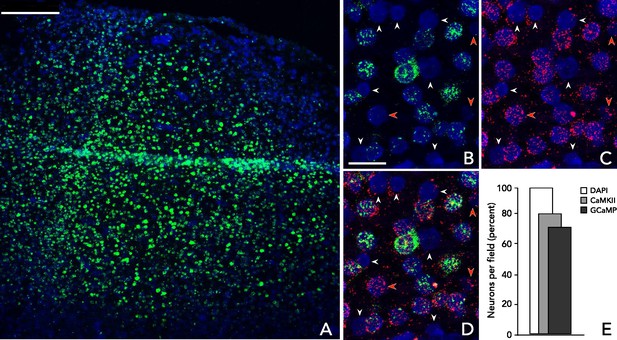
Analysis of virus-mediated GCaMP expression.
Multiplexed in situ hybridization using probes to GFP (green, to detect GCaMP) and CaMKIIα (red) in tissue samples from a GCaMP6f expressing site in a third macaque 10 weeks after viral injection. Cells are identified using DAPI nuclear stain. (A) Low magnification image of a representative section within rAAV:CaMKII-GCaMP6f injection site. (B–C) Example GCaMP and CaMKIIα expression patterns, respectively. Red arrows: CaMKIIα(+)/GCaMP(-) cells; white arrows: CaMKIIα(-)/GCaMP(-) cells. (D) Overlayed hybridization patterns: 24 of 31 cells are CaMKIIα positive (77%), 20 of 24 CaMKIIα positive cells co-express GCaMP (83%). (E) Aggregate results. In a field of view containing 232 cells, 185 (80%) were CaMKIIα(+); of these, 168 (91%) were GCaMP(+) and 17 (9%) were GCaMP(-); none was CaMKIIα(-)/GCaMP(+). Scale bars: (A) 250 μm; (B–D) 25 μm.
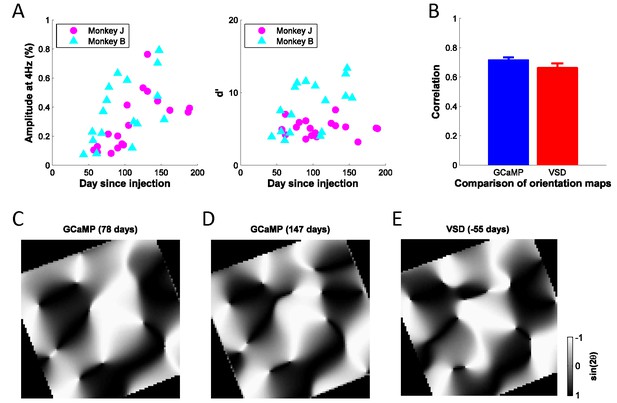
Stability of GCaMP signals over time and cortical map.
(A) Signal amplitudes and d’ (signal amplitude over signal standard deviation) to large flashed gratings over a period of ~200 days after viral injection for two monkeys. Fluctuations in signal amplitude and quality are mainly due to variations in the clarity of the aCSF fluid in the chamber. (B) Average correlation coefficients between any two GCaMP orientation maps (N = 8, blue bar), or between one GCaMP map (N = 8) and one VSD map (N = 2, red bar), where 'N' indicates separate experiments. Error bars ± SEM. Prior to computing correlations, maps were aligned based on the vasculature. (C), (D) Two examples of GCaMP orientation maps at the same site, but measured at different times after viral injection. The preferred orientation at each pixel is converted to the range of [−1, 1] by taking the sine of twice the preferred orientation. The correlation coefficient between the two gray-scale maps is 0.80. (E) One example of VSD orientation map at the same site as (C) and (D), but 55 days before viral injection. The correlation coefficients between the map in (E) and the maps in (C) and (D) are 0.71 and 0.78, respectively.
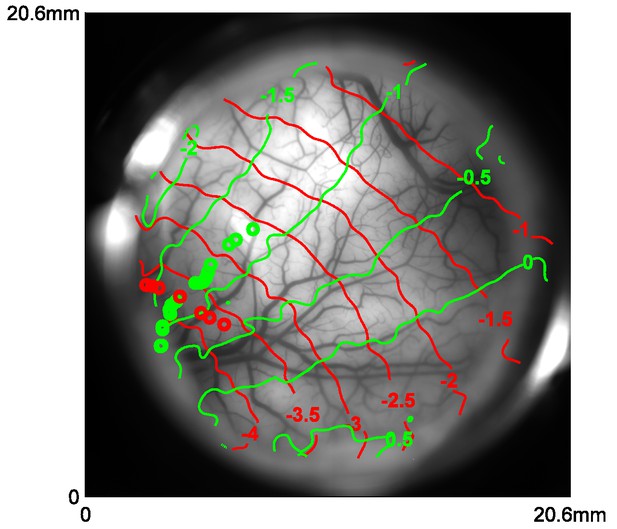
Retinotopic map obtained with VSD imaging and corresponding cortical locations of the centers of Gabor patches used in position tuning.
Red and green curves represent the coordinates of X and Y in visual field in one monkey, respectively. Red and green circles represent the corresponding locations of the Gabors in vertical and horizontal trajectories, respectively.
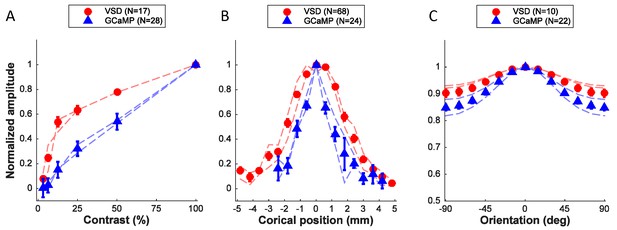
Quantitative comparison of neural population responses measured with GCaMP and VSD imaging.
Summary of responses as a function of stimulus contrast (A), position (B), and orientation (C). Data points are normalized amplitudes averaged across multiple experiments. 'N' refers to experiments, with the exception of the VSD signals in panel B, where several position tuning curves were obtained simultaneously in the same experiment. In each panel results from the two animals that provided the majority of the data are indicated by the thin dashed curves separately for each animal (red – VSD; blue – GCaMP). Error bars ± SEM.
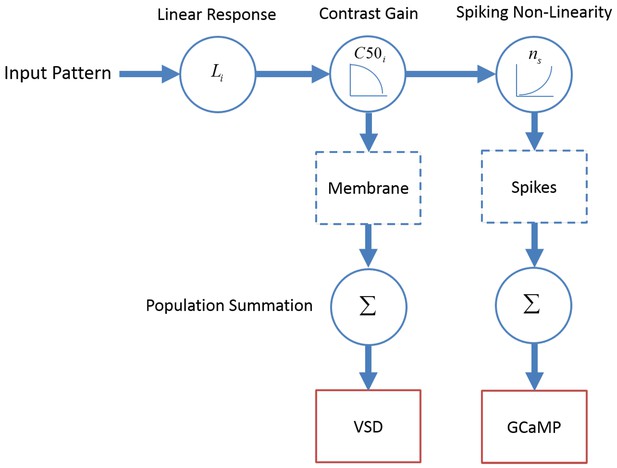
A computational framework relating GCaMP and VSD signals to single neuron membrane potential and spiking activity.
Membrane potential of V1 neurons reflect a dot product of the visual input with the linear receptive field followed by contrast gain control. The neural mechanism producing spikes from membrane potential is described by a power-law non-linearity. As a first approximation, GCaMP and VSD signals reflect spiking activity and membrane potential, respectively, pooled over a local population of neurons with heterogeneous tuning properties.
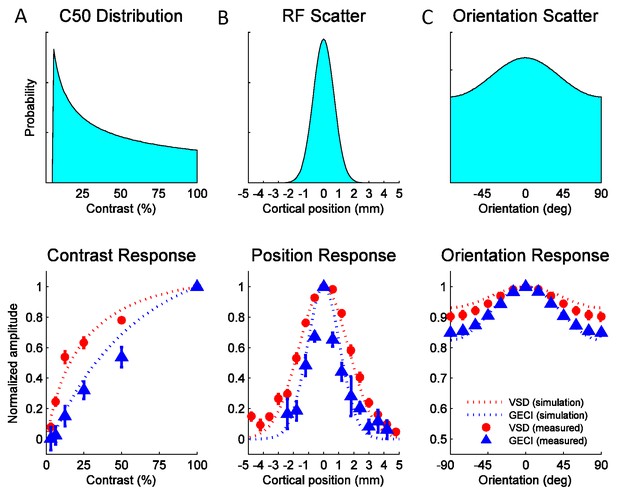
Widefield GCaMP signals in V1 of behaving macaques are consistent with the summed spiking activity.
Simulated and observed tuning curves for stimulus contrast (A), position (B) and orientation (C). The computational framework in Figure 7 was simulated with typical values for V1 receptive field (RF) parameters from the literature (see Table 1). Top panels show distributions of tuning properties while bottom panels show model predicted and observed tuning curves for GCaMP and VSD signals. Population pooling was made across neurons with different contrast semi-saturation (A), RF center position (B) and preferred orientation (C). In all stimulus dimensions (contrast, position and orientation), the simulation predicted GCaMP and VSD responses closely approximate the measured responses.
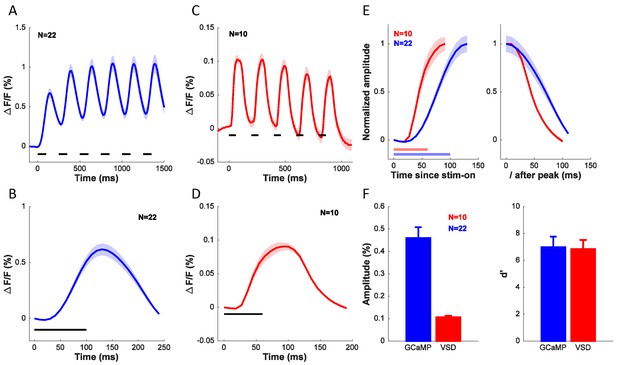
Comparison of dynamics, signal amplitude and signal sensitivity between GCaMP and VSD.
(A) Time course of GCaMP signal in response to a grating flashed for 6 cycles at 4 Hz. (B) Average response from (A) collapsed across the 6 cycles. (C) Time course of VSD signal in response to a grating flashed for 5 cycles at 5 Hz. (D) Average response from (C) collapsed across the 5 cycles. (E) Dynamics of rising edge (left) and falling edge (right) of GCaMP and VSD. (F) Average amplitude (left) and sensitivity (right) of GCaMP and VSD signals. Horizontal lines in A–E indicates timing of stimulus presentation. When collapsing the response across cycles in A–E, the responses to each cycle were first anchored by subtracting the mean response in the first 36 ms (4 frames) for VSD and 50 ms (1 frame) for GCaMP after stimulus onset. Results from the same experiments as in Figure 6C.
Tables
Computational model parameters, values and literature references.
| Description | Symbol | Mean value | Refs. |
|---|---|---|---|
| Receptive field size full width half max | 2.0 mm | Hubel and Wiesel, 1974; Van Essen et al., 1984; Read and Cumming, 2003; Chen et al., 2012 | |
| Orientation tuning bandwidth full width half max | 40 deg | De Valois etal., 1982; Vogels and Orban, 1990; Ringach et al., 2002; Nauhaus et al., 2008; Nowak and Barone, 2009; Palmer et al., 2012 | |
| Fraction of tuned response | 0.9 | Ringach et al., 2002; Finn et al., 2007; Nowak and Barone, 2009; Palmer et al., 2012 | |
| Spiking non-linearity exponent | 3.0 | Anderson et al., 2000; Hansel and van Vreeswijk, 2002; Miller and Troyer, 2002; Priebe et al., 2004; Tan et al., 2014 | |
| Contrast semi-saturation distribution constant | 0.5 ( = 30%) | Albrecht and Hamilton, 1982; Sclar et al., 1990; Geisler and Albrecht, 1997 |





