AMPK acts as a molecular trigger to coordinate glutamatergic signals and adaptive behaviours during acute starvation
Figures
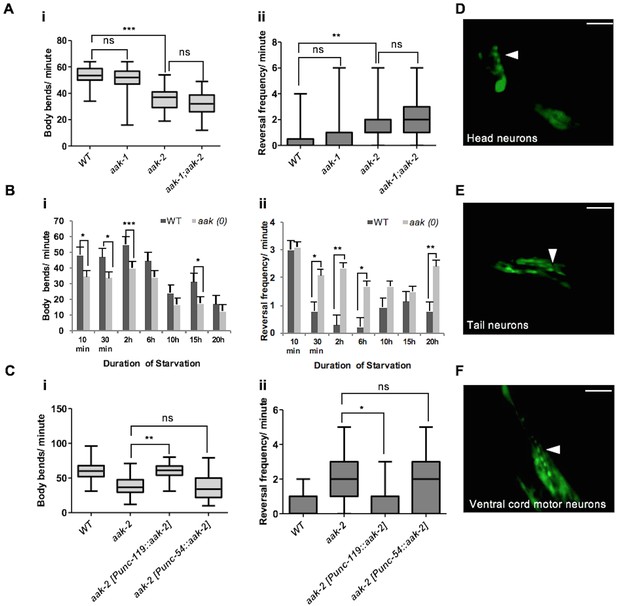
Neuronal AMPK regulates distal exploratory behaviour in starved animals.
(A) Starved aak-2 mutants display defective transition from local to distal exploration indicated by (i) decreased forward locomotion and (ii) increased reversal rate during acute bouts of starvation. These phenotypes are not dependent on aak-1 (n>40), (one-way ANOVA **p<0.001, ***p<0.0001). (B) AMPK mutants display persistent decreased forward locomotion rate (i) and increased reversal frequency (ii) during periods of acute starvation (n>10), Error bars represent ± SEM (Student's t-test, *p<0.05, **p<0.001, ***p<0.0001). (C) aak-2 reconstitution within the nervous system using the pan-neural [Punc-119::aak-2] transgene rescues the defective distal exploratory behaviour in starved aak-2 mutants while its reconstitution within the body wall muscle using [Punc-54::aak-2] transgene does not improve the distal exploratory behaviour of aak-2 mutants (n>15), (one-way ANOVA *p<0.05, **p<0.001). (D,E,F) aak-2 is highly expressed throughout the nervous system. Scale bars are 40 μm. In the box and whisker plots (A,C) the central line is the median, the edges of the box are the 25th and 75th percentiles, and the whiskers extend to the most extreme data points.
-
Figure 1—source data 1
Locomotory behaviour in AMPK mutant animals.
- https://doi.org/10.7554/eLife.16349.004
-
Figure 1—source data 2
Food and stimuli-related behaviours in AMPK mutants.
- https://doi.org/10.7554/eLife.16349.005
-
Figure 1—source data 3
Locomotory behaviour upon depletion of aak-2 with 1535 RNAi.
- https://doi.org/10.7554/eLife.16349.006
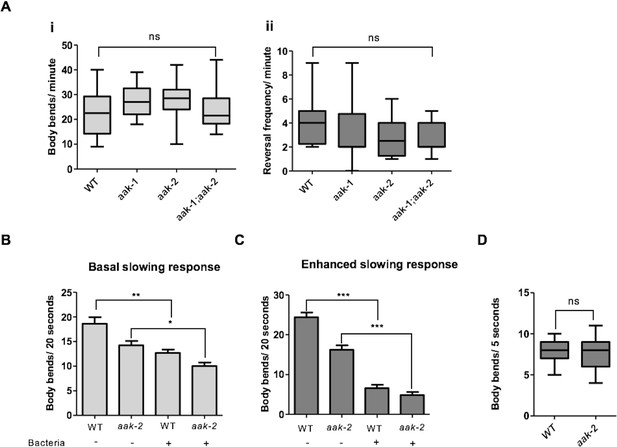
AMPK function is not required for other food-related behaviours rather than distal exploratory behaviour.
(A) AMPK mutants display normal locomotory behaviour in the presence of food (i and ii) (n>20), (one-way ANOVA). In the box and whisker plots the central line is the median, the edges of the box are the 25th and 75th percentiles, and the whiskers extend to the most extreme data points (B) Well-fed aak-2 mutants display normal basal locomotory behaviour once transferred to food (n>10). (C) Starved aak-2 mutants show normal enhanced slowing response once returned to food after 30 min of starvation (n>15). (D) aak-2 mutants display normal locomotory speed upon exposure to a gentle touch in the posterior part of their body in the absence of food (n>15). In (B,C) Error bars represent SEM (two-way ANOVA *p<0.05, **p<0.001, ***p<0.0001).
Reduced forward locomotion rate in aak-2 mutants is not a consequence of developmental defect in their nervous system.
(A) AMPK depletion in the late L2 stage by knocking down aak-2 in the nervous system resulted in a decreased forward locomotion rate and increased reversal frequency under starvation condition (n>15), (one-way ANOVA *p<0.05, **p<0.001). In the box and whisker plots the central line is the median, the edges of the box are the 25th and 75th percentiles, and the whiskers extend to the most extreme data points.
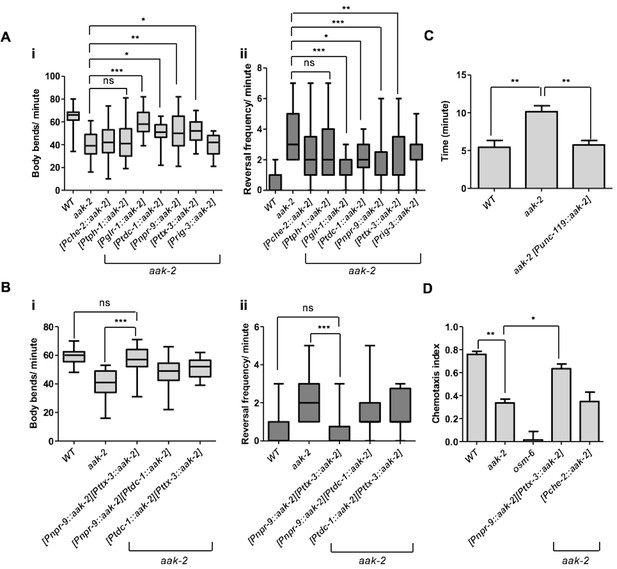
AMPK acts in the AIB and AIY interneurons to integrate chemosensory signals and trigger distal exploratory behaviour in starved animals.
(A) Targeted expression of aak-2 within the GLR-1-expressing neurons [Pglr-1::aak-2], RIM neurons [Ptdc-1::aak-2], AIB interneurons [Pnpr-9::aak-2] and AIY interneurons [Pttx-3::aak-2], but not chemosensory neurons [Pche-2::aak-2], serotonergic neurons [Ptph-1::aak-2] or AVA neurons [Prig-3::aak-2] partially restores the distal exploratory defect in starved aak-2 mutants by affecting both forward locomotion (i) and reversal frequency (ii) (n>20), (one-way ANOVA *p<0.05, **p<0.001, ***p<0.0001). (B) Simultaneous rescue of aak-2 in the AIB and AIY using [Pnpr-9::aak-2][Pttx-3::aak-2] transgenes is sufficient to completely restore the defective exploratory behaviour typical of starved aak-2 mutants (n>20), (one-way ANOVA ***p<0.0001). (C) Starved worms were placed 1.5 cm away from a spot of food (fresh OP50) and the time required to encounter the food was monitored for the worms that found food within 16 minutes. aak-2 mutants display defective food detection indicated by increased time spent to find food and this defect can be rescued by specific expression of aak-2 throughout the nervous system using [Punc-119::aak-2] transgene (n>10). (D) Targeted expression of aak-2 within the AIB and AIY interneurons, but not the chemosensory neurons rescue the defective chemosensation of aak-2 mutants toward IAA (n>300). Error bars in (C,D) represent ± SEM (one-way ANOVA *p<0.05, **p<0.001).
-
Figure 2—source data 1
AMPK reconstitution in different neurons to restore the defective distal exploratory behaviour and defective chemotaxis typical of aak-2 mutants.
- https://doi.org/10.7554/eLife.16349.010
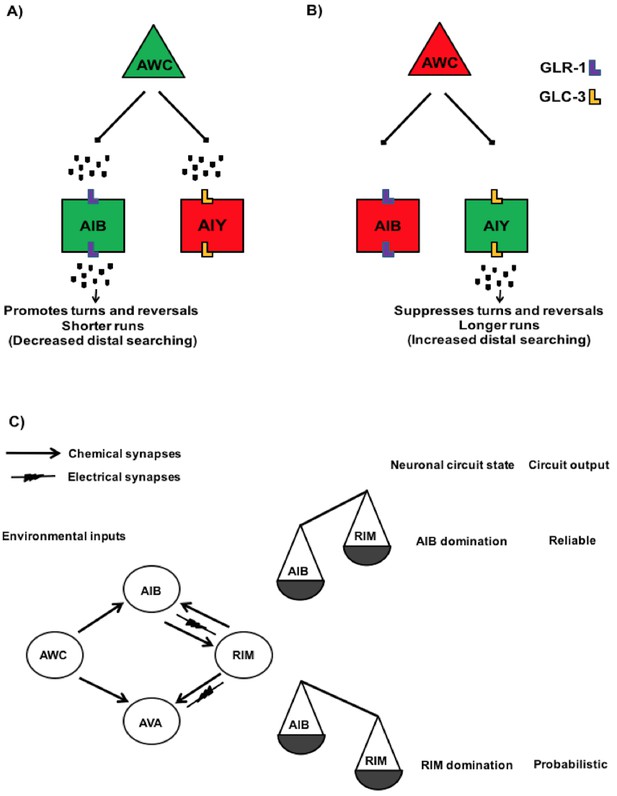
Neural circuits engaged in the regulation of locomotory behaviour in C. elegans.
(A) Schematic showing the proposed circuitry that regulates locomotory behaviour upon food removal. Green and red colos show depolarized and hyperpolarized neurons, respectively. This circuitry consists of AWC sensory neurons and AIB and AIY interneurons. Food or odour removal results in AWC depolarization. Upon its depolarization, AWC releases glutamate at AWC/AIB and AWC/AIY synapses. The released glutamate then eventually acts on AMPA-type glutamate receptor GLR-1 in the AIB or glutamate-gated chloride channel GLC-3 in the AIY resulting in their depolarization and hyperpolarization, respectively. This neuronal pattern ultimately promotes reversals and turns resulting in shorter runs and increased local exploration (Chalasani et al., 2007). (B) Once AWC neuronal activity reduces, it however results in a reduction in the AIB neuronal activity and AIY depolarization that collectively leads to suppression of reversals and turns and inducing longer runs and distal exploration (Chalasani et al., 2007; Gordus et al., 2015). (C) Simplified wiring diagram showing the synaptic connection between AWC sensory neurons and three AIB, RIM and AVA interneurons in a circuit linking AWC to reversal behaviour. RIM, AIB and AVA participate in the overall network states. Once RIM is dominated, the circuit output is toward more probabilistic and upon domination of AIB the circuit output is more reliable (Model proposed by Gordus et al., 2015).
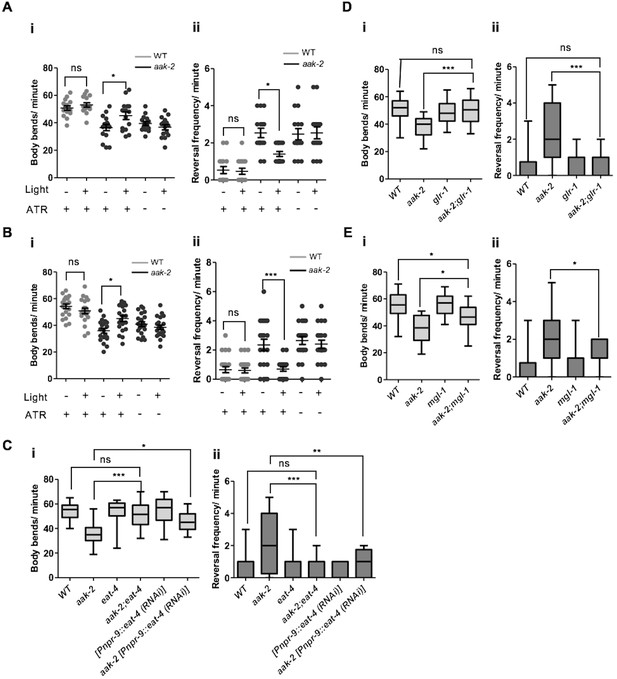
AMPK regulates glutamatergic signalling.
(A,B) ChR2-mediated activation of AIY interneurons (A), or ARCH -mediated silencing of AIB interneurons (B) in starved aak-2 mutants grown in the presence of all-trans retinal (ATR) induces distal exploration by suppressing reversals and inducing forward locomotion (n>15), Error bars represent ± SEM (two-way ANOVA *p<0.05, ***p<0.0001). (C,D,E) Compromised eat-4, glr-1 or mgl-1 function rescues the defective exploratory behaviour of starved aak-2 mutants by inducing forward locomotion (i) and suppressing reversals (ii) (n>20), (one-way ANOVA *p<0.05, **p<0.001, ***p<0.0001). In the box and whisker plots (C,D,E) the central line is the median, the edges of the box are the 25th and 75th percentiles, and the whiskers extend to the most extreme data points.
-
Figure 3—source data 1
Optogenetics and epistatic analysis.
- https://doi.org/10.7554/eLife.16349.013
-
Figure 3—source data 2
The analysis of locomotory behaviour of potential AMPK phospho targets.
- https://doi.org/10.7554/eLife.16349.014
-
Figure 3—source data 3
Potential AMPK phosphorylation targets expressed within the AIB and AIY interneurons.
- https://doi.org/10.7554/eLife.16349.015
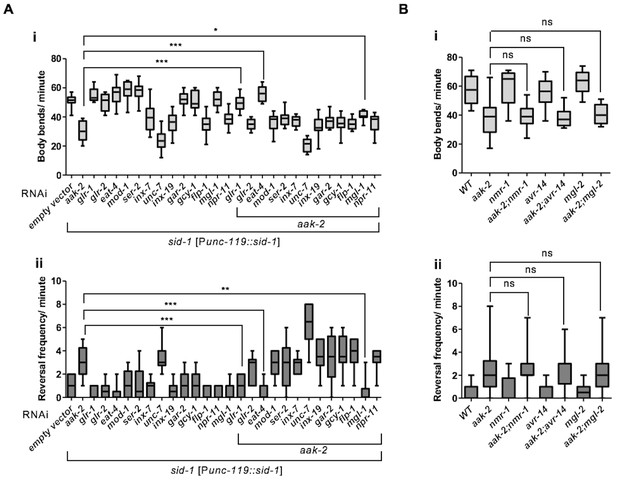
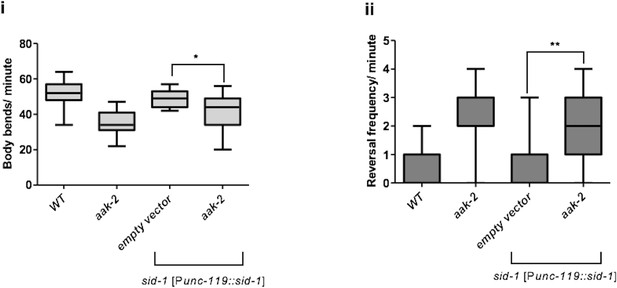
glr-1, eat-4 and mgl-1 are epistatic to aak-2.
(A) sid-1 [Punc-119::sid-1+ Pmyo-2::mCherry] or aak-2; sid-1 [Punc-119::sid-1+ Pmyo-2::mCherry] young adult animals grown on HT-115 E. coli clones expressing double stranded RNAi targeting various RNAi clones were starved for 1 hr and their locomotory behaviour was assessed (n>10). Among all the potential phosphorylation targets of AMPK within the AIB and AIY interneurons, glr-1, eat-4 and mgl-1 displayed epistatic relationship with aak-2 in both forward (i) and backward locomotion (ii). (B) Depletion of nmr-1, avr-14 and mgl-2 in aak-2 background did not result in any additive or epistatic relationship with aak-2 (n>10). Error bars represent SEM (one-way ANOVA *p<0.05, **p<0.001, ***p<0.0001).
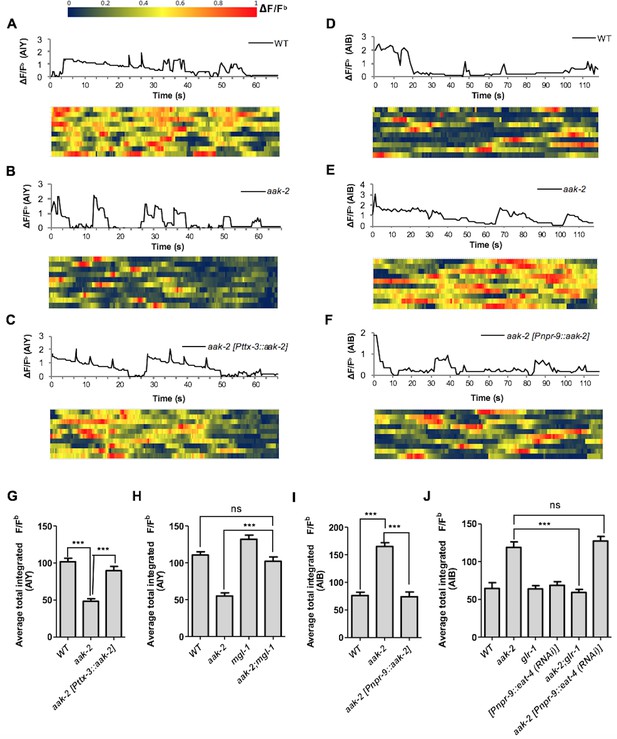
AMPK regulates AIB and AIY neuronal activity.
(A,B,C,D,E,F) A sample ΔF/Fb plot showing spontaneous changes in the AIY and AIB neuronal activity in starved individuals during the course of a 65 and 120 s imaging window, respectively. These plots are the basis of the data in G, H, I and J. The heatmaps show normalized G-CaMP1 and G-CaMP3 traces in AIY and AIB, respectively in multiple animals. (G) The average total integrated fluorescence intensity (ΔF/Fb) over the course of a 65 s window. AIY neuronal activity is reduced in starved aak-2 mutants and this reduction in the AIY neuronal activity is restored by targeted expression of aak-2 within the AIY interneurons using the AIY-specific [Pttx-3::aak-2] transgene (n>10). (H) Removal of mgl-1 in aak-2 mutants rescues the reduced AIY neuronal activity observed in starved aak-2 mutants (n>10). (I) The average total integrated fluorescence intensity (ΔF/Fb) in AIB is increased upon removal of aak-2 in starved animals, and this increase is reversed by expression of aak-2 within the AIB using the AIB-specific [Pnpr-9::aak-2] transgene (n>10). (J) Removal of glr-1, but not eat-4, restores the increased AIB neuronal activity in starved aak-2 mutants (n>10). Error bars represent ± SEM (one-way ANOVA ***p<0.0001).
-
Figure 4—source data 1
Calcium imaging in the AIB and AIY interneurons in WT, aak-2, mgl-1, glr-1 and eat-4 and double mutants.
- https://doi.org/10.7554/eLife.16349.018
-
Figure 4—source data 2
Calcium imaging in freely WT and aak-2 behaving animals.
- https://doi.org/10.7554/eLife.16349.019
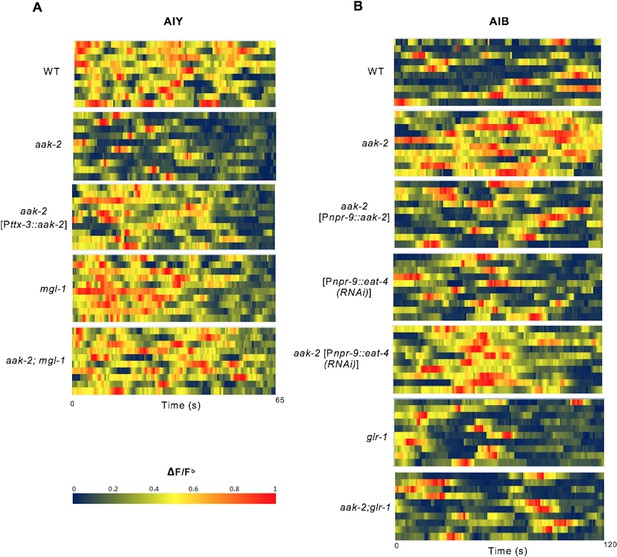
Heat maps showing AIY and AIB spontaneous neuronal activity in starved animals.
(A,B) Starved animals were placed on 10% agarose pads and the AIY and AIB neuronal activity was measured for 65 and 120 s respectively (n>10). G-CaMP imaging was performed on a Zeiss microscope. Images were captured by AxioVision software at 3Hz for AIY and 2Hz for AIB and were analyzed by imageJ. Baseline F (Fb) was measured as the global minima for 5–10 frames over the duration of each time-lapse sequence and the ΔF/Fb results were normalized between 0 and 1 for each animal.
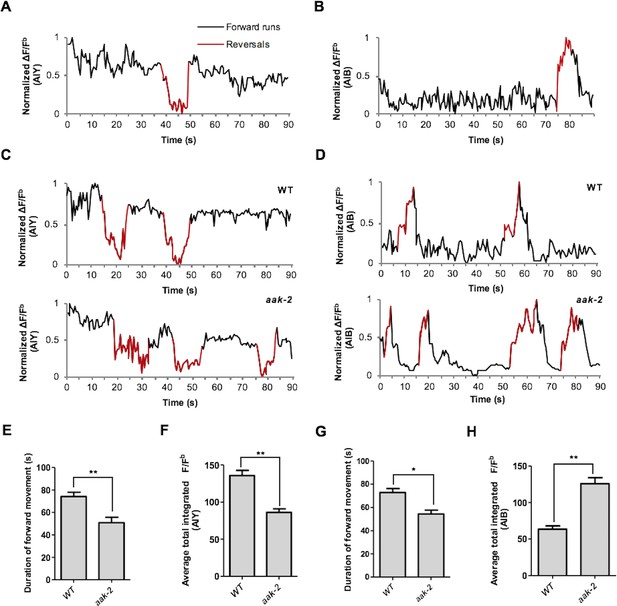
AIY and AIB spontaneous neuronal activity in starved freely behaving animals.
(A,B) A sample normalized ΔF/Fb plot showing spontaneous changes in the AIY and AIB neuronal activity in starved individuals during the course of a 90 s imaging window. AIY calcium levels decreases at the onset of reversals and reach the maximum levels at the onset of forward locomotion and it remains consistently high during forward runs (n>6) (A). AIB calcium level increases during reversals and it gradually decreases and return to baseline values with the resumption of forward movement (n>6) (B). (C,D) aak-2 mutants display higher reversal frequency and decreased duration of forward runs which is correlated with increased AIB calcium levels and decreased AIY neuronal activity (n>6). (E,F) The decreased forward locomotion rate and increased reversal frequency in aak-2 mutants are consistently correlated by increased AIB neuronal activity and decreased AIY activity (n>6). Error bars represent ± SEM (two-way ANOVA *p<0.05, **p<0.001).
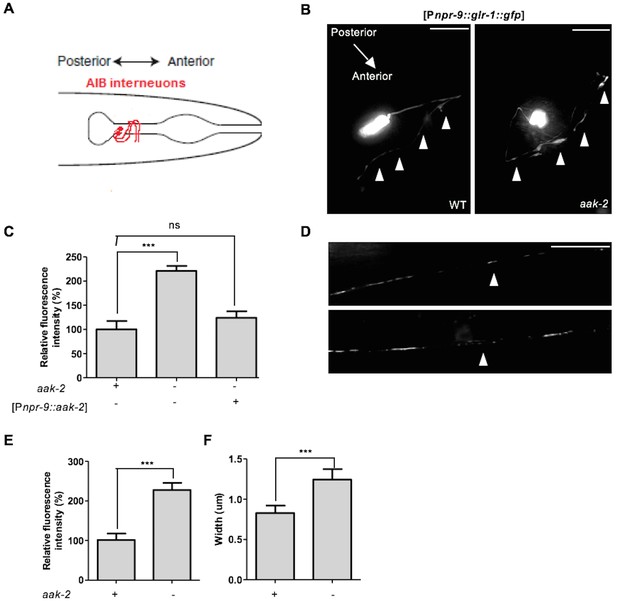
AMPK regulates GLR-1 abundance in the AIB neuronal process and the ventral nerve cord.
(A) Schematic representation showing AIB interneurons that extend their processes into the nerve ring. (B,C) Representative images of [Pnpr-9::glr-1::gfp] in the AIB neuronal processes in starved WT and aak-2 mutants (B). GLR-1::GFP level is significantly increased in the AIB neuronal processes in the aak-2 mutants compared to WT animals under starvation condition. Targeted expression of aak-2 within the AIB interneurons rescues the increased GLR-1::GFP level in starved aak-2 mutants (C). At least 3 [Pnpr-9::glr-1::gfp] transgenic lines were separately examined for this experiment (n>20), Error bars represent ± SEM (one-way ANOVA ***p<0.0001). Scale bars are 10 μm. (D,E,F) Representative images of [Pglr-1::glr-1::gfp] in the ventral nerve cord (D). GLR-1::GFP level (E) and puncta width (F) are significantly increased in the ventral nerve cord of starved aak-2 mutants compared to WT animals. (n>20), Error bars represent ± SEM (Student's 2-tailed t test ***p<0.0001). Scale bars are 10 μm.
-
Figure 5—source data 1
Measurement of GLR-1 abundance in the AIB neuronal process and ventral nerve cord of WT and aak-2 mutants.
- https://doi.org/10.7554/eLife.16349.023
-
Figure 5—source data 2
The extent of GLR-1 and EAT-4 colocalization in WT and aak-2 mutants.
- https://doi.org/10.7554/eLife.16349.024
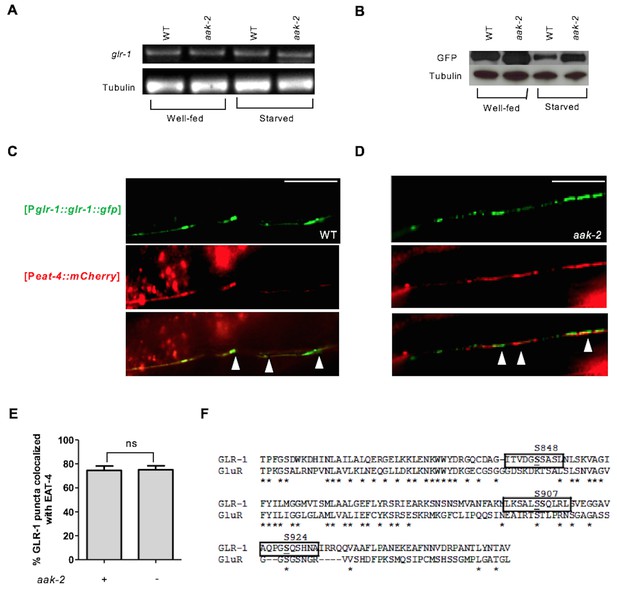
AMPK regulates GLR-1 abundance by affecting steady state protein levels.
(A) Relative messenger RNA levels were analyzed with semi-quantitative RT-PCR in well-fed and starved animals. glr-1 mRNA levels were similar in well-fed and starved WT and aak-2 mutants. (B) Western blot analysis of GLR-1 levels in Well-fed and starved WT and aak-2 mutants expressing GLR-1::GFP using GFP antibody. GLR-1 protein levels decrease upon starvation in WT animals and this is less pronounced in aak-2 mutants. (C,D,E) Co-localization (arrows) of EAT-4 with GLR-1 is shown in the merged images. GLR-1 puncta were closely opposed to EAT-4 puncta in both WT (C) and aak-2 mutants (D) (n>7), Error bars represent ± SEM (Student's two-tailed t test). (F) A schematic showing the 3 AMPK phosphorylation sites in the cytoplasmic tail of the GLR-1 protein two of which are conserved between C. elegans and rat glutamate receptor GluR1.
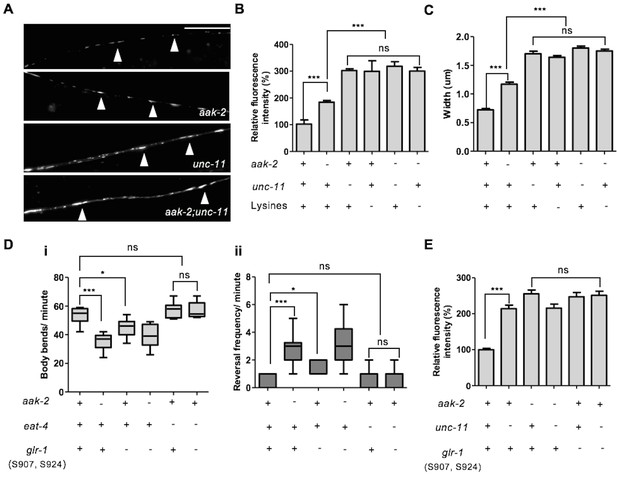
AMPK directly regulates GLR-1 abundance through endocytic pathway.
(A) Representative images of [Pglr-1::glr-1::gfp] in the ventral nerve cord in starved WT, aak-2, unc-11 and aak-2; unc-11 mutants. Scale bars are 10 μm. (B,C) Similar to starved aak-2 mutants, GLR-1::GFP level (B) and puncta width (C) are increased upon removal of unc-11 or mutation of four lysines required for GLR-1 ubiquitination and endocytosis [Pglr-1::glr-1(4KR)::gfp]. Increased GLR-1::GFP level and enhanced puncta width are not further enhanced upon depletion of aak-2 in GLR-1 endocytosis defective mutants (n>25), Error bars represent ± SEM (one-way ANOVA ***p<0.0001). (D) Mutating AMPK phosphorylation sites S907 and S924 to non-phosphorylable alanine residues present in the cytoplasmic domain of GLR-1 (S907A, S924A) results in increased reversal frequency compounded with reduced forward locomotion and this defect can be rescued by introducing eat-4 mutations (n>10), (one-way ANOVA *p<0.05, **p<0.001, ***p<0.0001). In the box and whisker plots the central line is the median, the edges of the box are the 25th and 75th percentiles, and the whiskers extend to the most extreme data points. (E) Expression of the non-phosphorylable variant of [Pnpr-9::glr-1(S907A, S924A)::gfp] results in increased GLR-1::GFP level in the AIB neuronal process and it is not further increased upon disruption of endocytosis in unc-11 mutants. At least 3 transgenic lines expressing non-phosphorylable variant of GLR-1 (S907A, S924A) were separately examined for this experiment (n>15), Error bars represent ± SEM (one-way ANOVA ***p<0.0001).
-
Figure 6—source data 1
Analysis of GLR-1 abundance in endocytosis defective mutants.
- https://doi.org/10.7554/eLife.16349.027
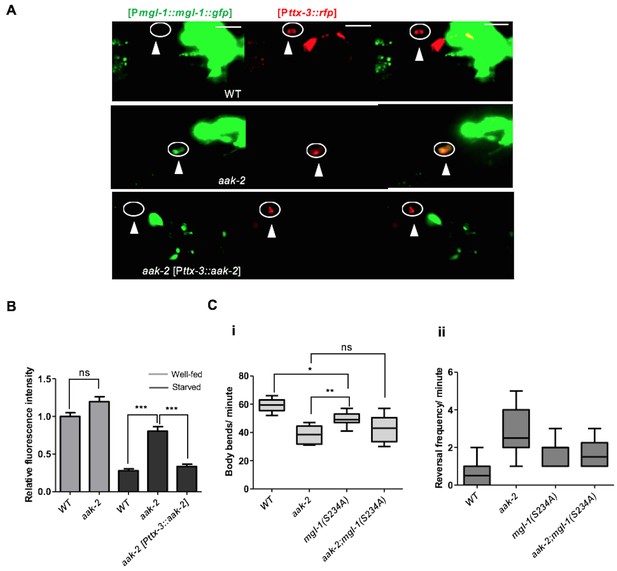
AMPK regulates the abundance of MGL-1 levels, while direct phosphorylation may affect MGL-1 function.
(A,B) Starvation reduces [MGL-1::GFP] levels significantly in the AIY [Pttx-3::RFP] of WT animals and to a lesser extent in aak-2 mutants (AIY neuron is shown in the circle). Increased [MGL-1::GFP] levels in starved aak-2 mutants can be reversed by targeted expression of aak-2 in the AIY interneurons using [Pttx-3::aak-2] transgene (n>15), Error bars represent ± SEM (two-way ANOVA ***p<0.0001). Scale bars are 20 μm. (C) Mutating the consensus AMPK phosphorylation site at serine 234 to a non-phosphorylable alanine in MGL-1 (S234A) resulted in a reduced forward locomotion rate that is not further reduced in aak-2; mgl-1 (S234A) double mutants (i) (n>10), (one-way ANOVA *p<0.05, **p<0.001). In the box and whisker plots the central line is the median, the edges of the box are the 25th and 75th percentiles, and the whiskers extend to the most extreme data points.
-
Figure 7—source data 1
Analysis of MGL-1 abundance in WT and aak-2 mutants.
- https://doi.org/10.7554/eLife.16349.029
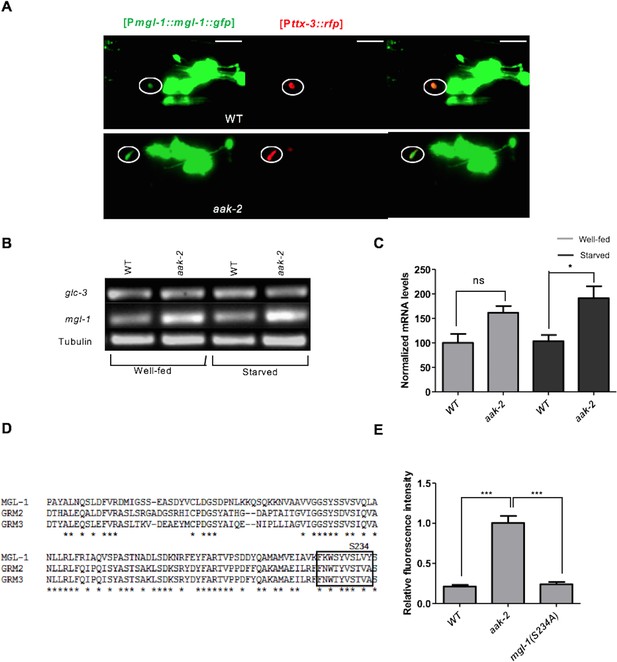
AMPK regulates MGL-1 by modulating its steady state mRNA levels while also affecting MGL-1 function through direct phosphorylation.
(A) [MGL-1::GFP] levels are unchanged in well fed WT and aak-2 mutants in the AIY interneurons (shown in the circle) indicated by [TTX-3::RFP]. Scale bars represent 20 μm. (B,C) Relative messenger RNA levels were analyzed with semi-quantitative RT-PCR in well-fed and starved animals. mgl-1 mRNA levels (normalized to WT) are significantly higher in starved aak-2 mutants compared to starved WT animals. Error bars represent ± SEM (two-way ANOVA *p<0.05). (D) A schematic showing the conserved AMPK phosphorylation site in C. elegans MGL-1 and human GRM2 and GRM3 group II metabotropic glutamate receptors. (E) Normalized [MGL-1::GFP] levels are unchanged upon mutating serine 234 in mgl-1 (n>10), Error bars represent ± SEM (one-way ANOVA ***p<0.0001).
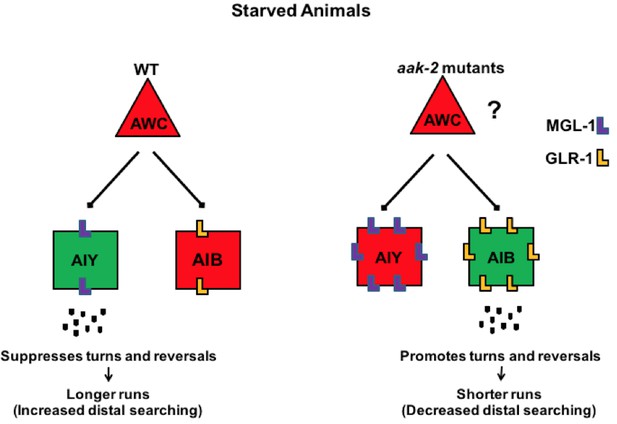
AMPK coordinates neuronal activity of the AIB and AIY interneurons by regulating their glutamatergic synaptic inputs in response to acute starvation.
The schematic showing the proposed role of AMPK in the AIB and AIY interneurons for regulation of appropriate transition between local to distal exploration during starvation conditions. Green and red colos show depolarized and hyperpolarized neurons, respectively. AMPK regulates GLR-1-mediated synaptic inputs into AIB interneurons by directly phosphorylating GLR-1 and subsequently modulating its endocytosis and potentially its degradation leading to decreased AIB activity in starved animals. AMPK also regulates the MGL-1-dependent synaptic inputs into the AIY interneurons, not only through its effects on mgl-1 mRNA levels but also by direct phosphorylation of MGL-1 protein. These functions of AMPK within the AIB and AIY interneurons collectively result in the transition between local exploration that occurs in well fed animals to distal exploration that occurs in starved animals, thus allowing them to explore their environment more extensively for energy resources. Legends to figure supplements
-
Figure 8—source data 1
glr-1 and mgl-1 over-expression data.
- https://doi.org/10.7554/eLife.16349.032
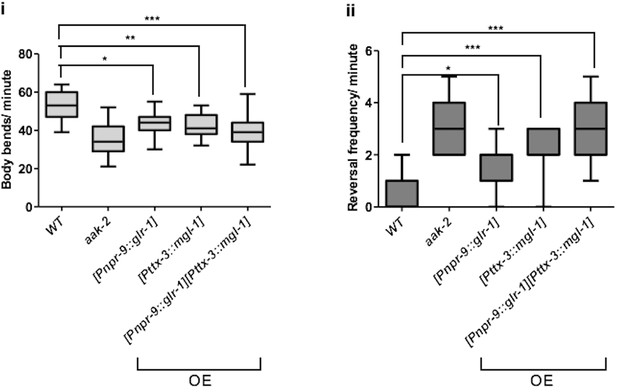
Overexpression of glr-1 and mgl-1 in the AIB and AIY, respectively results in the similar defect in distal locomotory behaviour as aak-2 mutants.
Overexpression (OE) of glr-1 or mgl-1 in the AIB and AIY interneurons, respectively results in decreased forward locomotion rate and increased reversal frequency similar to starved aak-2 mutants (n>15), (one-way ANOVA *p<0.05, **p<0.001, ***p<0.0001). In box and whisker plots, the central line is the median, the edges of the box are the 25th and 75th percentiles, and the whiskers extend to the most extreme data points.
Additional files
-
Supplemenmtary file 1
Complete list of strains used in this study.
- https://doi.org/10.7554/eLife.16349.034





